Abstract
The NADPH oxidases (Nox) are transmembrane proteins dedicated to producing reactive oxygen species (ROS), including superoxide and hydrogen peroxide, by transferring electrons from NAD(P)H to molecular oxygen. Nox2 and Nox4 are expressed in the heart and play an important role in mediating oxidative stress at baseline and under stress. Nox2 is primarily localized on the plasma membrane, whereas Nox4 is found primarily on intracellular membranes, on mitochondria, the endoplasmic reticulum or the nucleus. Although Nox2 plays an important role in mediating angiotensin II-induced cardiac hypertrophy, Nox4 mediates cardiac hypertrophy and heart failure in response to pressure overload. Expression of Nox4 is upregulated by hypertrophic stimuli, and Nox4 in mitochondria plays an essential role in mediating oxidative stress during pressure overload-induced cardiac hypertrophy. Upregulation of Nox4 induces oxidation of mitochondrial proteins, including aconitase, thereby causing mitochondrial dysfunction and myocardial cell death. On the other hand, Noxs also appear to mediate physiological functions, such as erythropoiesis and angiogenesis. In this review, we discuss the role of Noxs in mediating oxidative stress and both pathological and physiological functions of Noxs in the heart.
Keywords: oxidative stress, superoxide, hydrogen peroxide, mitochondria, cardiac hypertrophy, apoptosis, heart failure, oxygen sensing
Introduction
Oxygen derivatives with instabilities and an increased reactivity, including superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH−), are generically termed reactive oxygen species (ROS) [1]. Excess ROS production damages DNA, protein and lipids, and causes cell death in the heart and the cardiomyocytes (CMs) therein [2, 3]. Strictly regulated generation of ROS at low doses also mediates physiological functions, such as growth, differentiation, and metabolism in CMs [2, 3]. The NADPH oxidase (Nox) family proteins are enzymes that are dedicated themselves to producing O2− and/or H2O2, a property which distinguishes them from other enzymes producing ROS as a by-product [4, 5]. The prototype of Nox, gp91phox, also known as Nox2, in leukocytes is primarily responsible for host defense mechanisms through O2−-mediated killing of pathogens [4, 6]. However, the Nox family proteins are widely expressed in non-phagocytic cells, including CMs, where they mediate a wide variety of cellular functions. Thus far, seven members of the Nox family of proteins (Nox1-Nox5, Duox1 and Duox2) have been discovered [5, 7, 8] (Figure 1). Nox1-Nox4 have conserved structures, including 6 trans-membrane domains with FAD and NADPH-binding domains in their C-terminal regions and two hemes, and form a complex with p22phox, a membrane-integrated protein [4]. In the heart, CMs express primarily Nox2 and Nox4 [9, 10]. Despite the similarity in their structures, Nox2 and Nox4 have markedly different properties in terms of regulation and subcellular localization, and both play critical but distinct roles in mediating a wide variety of functions in the heart [11]. In this review, we summarize the latest findings regarding both physiological and pathological functions of Nox2 and Nox4 in the heart and discuss whether these Nox isoforms can be targets of treatment for heart failure.
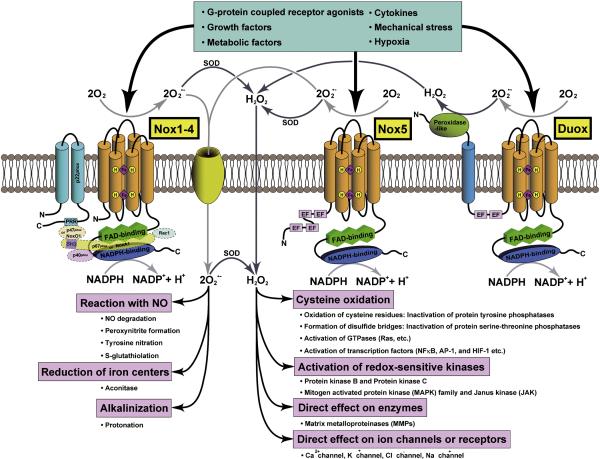
Figure 1.
Schematic representations of the structures of various subtypes of NADPH oxidase-family enzymes and their signaling pathways. Cylinders represent six transmembrane alpha-helices, PRR indicates a proline-rich region (PRR), and EF stands for Ca2+-binding EF-hand motif. Nox is either activated or upregulated by various stimuli, such as growth factors, cytokines, G-protein coupled receptor agonists, metabolic factors, mechanical stress, and hypoxia. The immediate product of NADPH oxidases is superoxide (OM2−). However, due to spontaneous and enzymatic dismutation, hydrogen peroxide (H2O2) can also be generated. O2− generation from NADPH oxidases occurs either in the extracellular or the cytosolic space. The negatively charged O2− does not permeate the lipid bilayer of biological membranes. However, it may pass through the pore of anion channels. Biological effects of Nox-derived O2− include: 1) reaction with nitric oxide (NO) leading to NO degradation, peroxynitrite formation, protein tyrosine nitration, and the addition of glutathione to thiols; 2) reduction of iron centers within enzymes; and 3) alkalinization of intracellular organelles. In most cases, however, biological effects of Nox are mediated through H2O2 after O2− is dismutated. H2O2 is a well-established signaling molecule that readily permeates biological membranes. We propose that Nox4 induces oxidation of many mitochondrial proteins due to its proximity to them.
Nox2 and Nox4 are major NADPH oxidases in the heart
The classical phagocyte oxidase (gp91phox or Nox2) is also expressed in non-phagocytic cells in the heart, such as CMs and fibroblasts [9, 12–22]. Activation of Nox2 requires stimulus-induced membrane translocation of cytosolic regulatory subunits, including p47phox, p67phox, p40phox, and Rac1, a small GTPase [23, 24]. In resting cells, p47phox, p67phox, and p40phox form a ternary complex in the cytoplasm, whereas Rac associates with Rho-GDP dissociation inhibitor. When cells are stimulated with agonists for G protein-coupled receptors, such as angiotensin II (Ang II) type 1 receptors, p47phox is phosphorylated by protein kinase C, which in turn undergoes conformational changes and allows the phox homology (PX) domain and the SH3 domain in p47phox to interact with phosphoinositides and p22phox in the membrane, respectively [25]. As p67phox and p40phox interact with p47phox, this process leads to membrane translocation of p67phox and p40phox. Rac1 translocates to the membrane independently of p47phox and p67phox, where they form a functional complex with the Nox2-p22phox heterodimer, followed by a transfer of electrons to molecular oxygen [5, 26, 27]. Thus, the activity of Nox2 is subjected to regulation through multiple mechanisms.
Nox4 was originally identified as a renal-specific oxidase [28, 29]. However, Nox4 is also expressed in CMs, endothelium, vascular smooth muscle cells, and fibroblasts [30–34]. Nox4 can form a heterodimer with p22phox and constitutively generates O2− [35–37]. Although Nox4 appears to generate O2− from NADH more efficiently than from NADPH in CMs [10], this may not be the case in other cell types [29]. It is believed that Nox4-mediated O2− generation does not require association with cytosolic factors, including p40phox, p47phox, p67phox, or Rac1 [28, 29, 38, 39]. Whether or not the activity of Nox4 is also regulated by posttranslational mechanisms is currently unknown. Poldip2 was identified as a novel binding partner with Nox4 and p22phox through yeast-two-hybrid screening. Expression of Poldip2 enhances the enzymatic activity of Nox4 [40]. However, the physiological function of Poldip2 remains to be elucidated. Expression of Nox4 is upregulated by hypertrophic stimuli and aging. However, the molecular mechanism mediating upregulation of Nox4 during cardiac hypertrophy and aging is currently unknown.
Although O2− is the primary product of Noxs, Nox4 also produces H2O2 [41]. Whether or not Nox4 produces O2− is a subject of debate because production of O2− from Nox4 is undetectable in some cell types [37]. O2− produced by Nox4 may be dismutated to H2O2 so rapidly that O2− cannot be detected with conventional methods [42]. Alternatively, Nox4 may directly produce H2O2 through an unknown mechanism, although the Nox4 amino acid structure alone does not clearly predict an ability to directly produce H2O2, such as an SOD like domain.
Activation of Noxs and downstream signaling mechanisms
ROS play an important role in mediating a wide variety of functions in cells under baseline conditions and in response to stress [43]. However, where and how ROS are produced in CMs is not well understood. Genetically altered animal models have shed light on the role of Nox isoforms in mediating the production of ROS in the heart and the CMs therein. Under baseline conditions, neither systemic Nox2 homozygous knock-out nor cardiac specific Nox4 homozygous knock-out mice show an obvious cardiac phenotype, suggesting that both Nox isoforms are dispensable, at least individually for basal cardiac function in adult mice [9, 31, 44]. Since baseline production of O2− in the myocardium is reduced in cardiac specific Nox4 knock-out mice, Nox4 at least partially mediates O2− production in the heart at baseline. Since marked reduction in O2− in transgenic mice with cardiac specific overexpression of dominant negative Nox4 also did not affect the cardiac phenotype at baseline, we predict that Nox-derived O2− may be dispensable for the adult heart at baseline.
Various stimuli, such as growth factors, cytokines, G-protein coupled receptor agonists, metabolic factors, mechanical stress, and hypoxia, activate Noxs in CMs [45–47] (Figure 1). As already discussed, the O2− producing activity of Nox2 is regulated by posttranslational modification of the cytosolic factors, whereas that of Nox4 is regulated primarily at the level of mRNA and/or protein expression. Thus, we predict that the activity of Nox2 may be subjected to rapid alteration in response to given stimuli, but that of Nox4 may not. However, further experiments with loss of function mouse models are needed to prove this hypothesis.
Since O2− is rapidly dismutated to H2O2 in the intracellular environment, H2O2, rather than O2−, may mediate the effect of Noxs upon signal transduction. However, since the intracellular environment has strong reducing mechanisms for H2O2, such as peroxiredoxin [48], we predict that subcellular localization still determines the downstream signaling effect of each Nox isoform [49]. Nox2 is expressed primarily on the plasma membrane. On the other hand, Nox4 appears to be localized on intracellular membranes in the perinuclear space, including those of mitochondria, endoplasmic reticulum and nucleus [10, 44]. Distinct localization of Nox2 and Nox4 suggests that each Nox isoform differentially affects the redox status of proteins in different subcellular locations.
Is Nox4 responsible for oxidative stress in the failing heart?
As CMs have a greater volume of mitochondria compared to other cell types, the mitochondrion is one of the most important sources of ROS in CMs [50] (Figure 2). Under physiological conditions, the small amount of ROS formed through mitochondrial respiration is effectively removed by endogenous antioxidants, such as MnSOD, which is located in the mitochondrial matrix and dismutates O2−. However, under pathological conditions in the heart, suppression of the mitochondrial electron transport chain at complex I facilitates electron leakage and accumulation of ROS [51]. Prolonged oxidative stress causes damage to both mitochondrial DNA and proteins, and further stimulates ROS generation, termed “ROS-induced ROS release”, mitochondrial dysfunction and cell death. Oxidative stress affects the mitochondrial permeability transition pore (mPTP), a putative conductance pore that spans the inner and outer mitochondrial membranes. An excessive amount of ROS induces high levels of Ca2+ within mitochondria, which causes the mPTP complex to form an open pore, allowing free diffusion of solutes across the membranes. Opening of the mPTP induces further depolarization of the mitochondrial membrane, which in turn reduces the production of ATP. Prolonged opening of the mPTP results in an efflux of Ca2+ and electrons, activation of Ca2+-dependent proteases, such as calpain, leakage of proapoptotic molecules, such as cytochrome-c and procaspases, mitochondrial swelling, rupture of the mitochondrial membrane, and catastrophic induction of apoptotic and/or necrotic cell death [52].
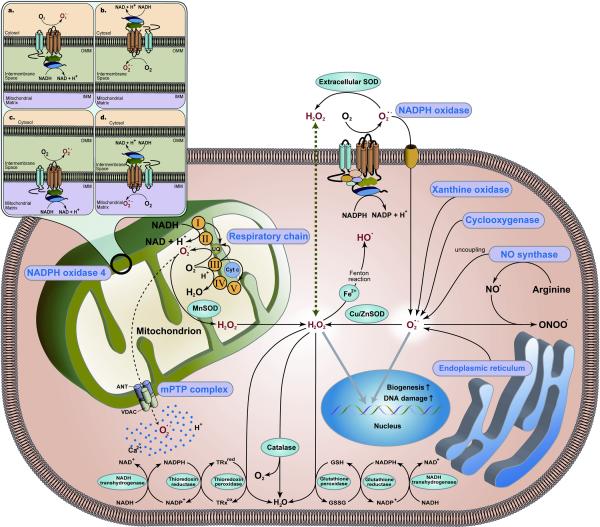
Figure 2.
Schematic representations of the sources of reactive oxygen species (ROS) in CMs. The precise localization and orientation of Nox4 on the mitochondrial membranes remain to be elucidated. Red characters indicate ROS; O2−, H2O2, and 2 hydroxyl radical (OH−). Blue characters indicate the sources of ROS. mPTP, mitochondrial permeability transition pore; SOD, superoxide dismutase; UQ, ubiquinone; Cyt c, cytochrome c; GSH, glutathione; GSSG, glutathione disulfide; TRxred, reduced thioredoxin; TRxox, oxidized thioredoxin.
We recently found that Nox4 is primarily expressed in mitochondria in CMs [10] (Figure 2). Nox4 has a putative mitochondrial localization signal (MLS), such that Nox4 [ΔN (75–578)] lacking the MLS is no longer localized in mitochondria in cultured CMs. The mitochondrial fraction isolated from CMs has the ability to produce O2− in a DPI (an inhibitor for flavin containing enzymes, such as Nox4)-sensitive manner, and O2− production is enhanced by overexpression of Nox4 and significantly reduced by downregulation of Nox4 in CMs. This is an unexpected finding because it has been believed that Noxs are unlikely to be expressed in mitochondria.
Consistent with the mitochondrial localization of Nox4 in CMs, cysteine residues in many mitochondrial proteins containing iron-sulfur clusters, including ANT1, a pore protein in the inner mitochondrial membrane, NADH dehydrogenase flavoprotein I, a component of complex I, and aconitase-2, a component of the TCA cycle, are oxidized and their function is inhibited in Nox4 transgenic mouse hearts, suggesting that cysteine residues in these proteins are oxidized by O2−/H2O2 generated by Nox4 [10].
Importantly, since expression of Nox4 in cardiac mitochondria is upregulated during cardiac hypertrophy, one might predict that Nox4 contributes to O2− production in mitochondria and oxidation/dysfunction of mitochondrial proteins during cardiac hypertrophy. In fact, an increase in O2− production in the mitochondrial fraction induced by pressure overload (PO) was abolished in cardiac specific Nox4 knock-out mice. Furthermore, cysteine oxidation of many mitochondrial proteins during PO was normalized in the cardiac specific Nox4 knock-out mice [44]. These results suggest that Nox4 plays an essential role in mediating increases in mitochondrial production of O2− and oxidation of mitochondrial proteins during PO. Upregulation of Nox4 may indirectly increase O2− generated by electron leakage by causing damages in the mitochondrial respiratory chain. However, PO increased a DPI-sensitive component of O2− production, rather than a rotenone-sensitive component that would reflect electron leakage from complex I, and Nox4 knock-out abolished the DPI-sensitive component of O2− production in PO hearts. We also found that PO-induced increases in cysteine oxidation of mitochondrial proteins, including ANT1 and aconitase, are significantly attenuated in cardiac specific Nox4 knock-out mice [44]. These results are consistent with the notion that PO increases O2− production directly from Nox4 localized in mitochondria and that Nox4 is an active source of oxidative stress during pathological hypertrophy. It should be noted that DPI has the ability to inhibit mitochondrial respiratory chain enzymes, as well as NOS. Thus, it remains possible that downregulation of Nox4 reduces the overall level of oxidative stress in the cell, which in turn reduces electron leakage from mitochondria through suppression of ROS-induced ROS release.
Since Noxs transfer electrons from NAD(P)H, the availability of NAD(P)H may influence the oxidase activity of Nox in some settings [53], and the activity of the TCA cycle, electron transport chain, and glucose-6-phosphate dehydrogenase, major regulators of the NAD(P)H levels in the cell, may indirectly affect ROS production by Nox. Interestingly, Nox4 preferentially uses NADH as an electron donor in our experiments [10, 44]. This raises a possibility that Nox4 in mitochondria may produce O2− as a negative feedback mechanism when more NADH is produced from the TCA cycle. Increased production of O2− may inhibit the function of mitochondrial proteins through cysteine oxidation. The physiological significance of such a mechanism is currently unknown. It should be noted that whether or not Nox4 preferentially utilizes NADH rather than NADPH is controversial, and, thus, further experimentation is needed to elucidate where and how Nox4 generates ROS in CMs.
Myocardial stress is often accompanied by increases in ER stress, which triggers ROS production through the unfolded protein response (UPR) in CMs. Major enzymatic mechanisms for ROS generation from ER during the UPR may be multiple thiol-disulfide exchanges involving oxidoreductases of ER, including flavooxidase Ero1 and protein disulfide isomerase [54]. The involvement of these mechanisms in mediating oxidative stress during heart failure remains to be elucidated.
Other ROS producing enzymes may also be involved in the increase in oxdative stress during cardiac hypertrophy and heart failure (Figure 2). Xanthine oxidase (XO) produces O2− as a by-product in the terminal step of purine catabolism [55, 56]. Expression and/or activity of XO are increased in response to hypoxia and during heart failure with Ca2+ overload [57]. Cyclooxygenase (COX) is a principal enzyme in the arachidonate metabolism. The arachidonate cascade catalyzed by COX contributes to ROS generation both via O2− generation during the catalytic conversion of prostaglandin (PG) G2 to PGH2, and through PG-facilitated oxidative alterations of the inflammatory process [58]. Nitric oxide synthase (NOS), which normally produces nitric oxide (NO), generates O2− when it becomes “uncoupled”, during a deficiency of the NOS substrate L-arginine or the NOS cofactor BH4, which occurs in the presence of increased oxidative stress [59, 60]. How these ROS-producing enzymes affect the redox status of cardiac proteins during hypertrophy and heart failure, their importance relative to Noxs, and the interaction between these mechanisms and Noxs remain to be elucidated. Noxs may promote ROS generation through activation of other ROS-producing enzymes and inactivation of anti-oxidants such as thioredoxin-1 (Trx1), thereby amplifying total levels of ROS [59, 61, 62].
Myocardial damage/death and NADPH oxidase
The loss of CMs through apoptosis/necrosis leads to decreases in cardiac function in the heart subjected to chronic myocardial infarction (MI) or PO [63]. Oxidative stress is involved in the pathogenesis of apoptosis through various pathways including: (1) activation of enzymes involved in pro-apoptotic signaling (JNK, p38, ASK-1, CaMKII, etc.) [64, 65]; (2) direct effects of ROS on mitochondria, leading to cytochrome-c release; (3) effects on the cellular anti-apoptotic signaling (Akt, heat shock proteins, ERK-1/2, etc.).
In cultured CMs, induction of apoptosis by Ang II was abolished in the presence of apocynin, which prevents the association of p47phox with Nox2, suggesting that Nox2 is involved in Ang II-induced CM apoptosis [66]. Ang II-induced increases in CM apoptosis in the heart were abolished in p47phox knockout mice, suggesting that Nox2 plays an important role in mediating Ang II-induced CM apoptosis in vivo [65]. Induction of apoptosis in the post-MI heart is also attenuated in both p47phox and Nox2 knockout mice [61, 67], supporting the involvement of Nox2 in CM apoptosis during cardiac remodeling. Interestingly, isoproterenol-induced increases in CM apoptosis were not significantly affected in p47phox knockout mice [65], suggesting that increases in oxidative stress due to stimulation of the β-adrenergic receptor are mediated by Nox2-independent mechanisms. These results correlate with the fact that O2− production by Nox2 is inducible in response to certain types of agonists, including Ang II.
Then what is the role of Nox4 in mediating CM apoptosis? Although O2− production from Nox4 is not generally inducible, increased expression of Nox4 in cultured CMs, as well as in the mouse heart, induces CM apoptosis [10]. Protein expression of Nox4 is upregulated in response to hypertrophic stimuli, such as Ang II and pressure overload, as well as aging [10]. Thus, chronic upregulation of Nox4 during cardiac hypertrophy could lead to increases in apoptosis, which in turn contribute to gradual decreases in cardiac function. Nox4-induced apoptosis in cultured CMs was accompanied by cytochrome-c release, and was prevented by the presence of Bcl-xL or MnSOD [10, 44], suggesting that Nox4 activates the mitochondrial apoptotic pathway in CMs. Since Nox4 is localized primarily in mitochondria, mitochondrial generation of ROS may initiate Nox4-induced apoptosis in CMs [10]. Importantly, increases in CM apoptosis in response to pressure overload were significantly attenuated in cardiac-specific Nox4 knockout mice [44], suggesting that upregulation of endogenous Nox4 during pressure overload plays an important role in mediating CM apoptosis.
Cardiac hypertrophy
Cardiac hypertrophy initially develops as an adaptation of the heart in response to increased mechanical stress. However, since prolonged hypertrophy leads to cardiac dysfunction, cardiac hypertrophy is considered an independent risk factor for the development of congestive heart failure [68]. Redox-sensitive mechanisms play an essential role in mediating the development of cardiac hypertrophy, in response to either chronic PO or neurohumoral stimuli. In cultured CMs, ROS mediate hypertrophy in response to Ang II, α-adrenergic agonists, TNF-α, endothelin-1 or mechanical stretch [14, 69, 70]. Activation of pro-hypertrophic transcription factors, including NFAT, GATA4 and MEF2, plays a critical role in mediating pathological cardiac hypertrophy [71–73]. The activity of these transcription factors is negatively regulated by class II histone deacetylases (HDACs). There are currently 11 known mammalian HDACs, which are categorized into 4 families (class I, IIa, IIb and IV) based on their structure and expression patterns [74]. In addition to the classical HDACs, mammalian genomes encode another group of deacetylases, the sirtuin family, which are referred to as class III HDACs.
Class I HDACs (HDAC1, 2, 3, and 8) are expressed primarily in neuronal, renal, epidermal, prostate, and pancreatic cells [75–78]. Class I HDACs inhibit expression of anti-hypertrophic genes, thereby stimulating cardiac hypertrophy [79–81]. HDAC2 also induces cardiac hypertrophy by suppressing inositol polyphosphate-5-phosphatase f (Inpp5f) expression. As Inpp5f removes a 5'-phosphate from PIP2 and PIP3, HDAC2 causes accumulation of PIP3, which in turn enhances Akt signaling, inhibits GSK-3β, a negative regulator of cardiac hypertrophy, and induces cardiac hypertrophy [81]. HDAC2 is regulated by S-nitrosylation in neuronal cells [82]. NO generated in response to neurotrophin enters the nucleus and nitrosylates HDAC2 at cysteine residues 262 and 274, which releases HDAC2 from chromatin and facilitates gene expression. Whether the status of S-nitrosylation of HDAC2 in the heart is regulated by hypertrophic stimuli, and, if so, whether or not cardiac hypertrophy is regulated by S-nitrosylation of HDAC2 are currently unknown.
Class IIa HDACs (HDAC4, 5, 7, and 9) are expressed primarily in differentiated cell types, including neurons and CMs. Class IIa HDACs repress transcription through pro-hypertrophic transcription factors, including NFAT, GATA4, and MEF2 [83, 84]. Subcellular localization of class II HDACs is primarily regulated by phosphorylation induced by hypertrophic stimuli, which in turn controls the activity of these transcription factors [85]. Phosphorylation of class IIa HDACs by HDAC kinases, including CaMK, PKD and GRK5, in response to hypertrophic stimuli induces interaction of HDACs with 14-3-3, which leads to unmasking of the nuclear export signal (NES) to Crm1 (exportin) and suppression of the nuclear localization signal (NLS) [86]. Class IIa HDACs are then exported to the cytosol, where they no longer suppress the pro-hypertrophic transcription factors [87]. Importantly, recent evidence suggests that the nucleo-cytoplasmic shuttling of HDAC4 is also regulated by oxidation of evolutionarily conserved cysteine residues [88, 89]. Treatment of cultured CMs with phenylephrine (PE) induces formation of a disulfide bond between cysteines 667 and 669 in HDAC4, which in turn induces cytosolic translocation of HDAC4 via a Crm1-dependent nuclear export mechanism. Since oxidation of HDAC4 by PE takes place earlier than phosphorylation (within 5 min vs 30 min), we speculate that PE-induced cytoplasmic translocation of HDAC4 takes place initially through cysteine oxidation, whereas subsequent phosphorylation allows HDAC4 to stay in the cytoplasm. The cysteine resides subjected to disulfide bond formation in response to PE are readily reduced by Trx1, and reduction of the cysteine residues induces nuclear translocation of HDAC4 even in the presence of serine phosphorylation. These results suggest that the redox-dependent mechanism overrides the phosphorylation-dependent one to control the nucleo-cytoplasmic shuttling of class II HDACs [90]. As PE induces cysteine oxidation of HDAC4 within 5 minutes, ROS responsible for oxidation of HDAC4 appear to be generated by an enzyme whose activity is rapidly stimulated by PE. Although Nox4 is partially localized in the nucleus, at present we do not yet know whether Noxs are involved in oxidation of HDAC4 by PE.
Recent evidence suggests that CaMKII, an HDAC kinase, is activated not only through Ca2+-calmodulin-dependent mechanisms, but also by methionine oxidation in a Ca2+-independent manner [65]. Ang II-induced oxidation of the methionine residues in CaMKII was abolished in p47phox knockout CMs, suggesting that Nox2 plays an important role in mediating Ang II-induced activation of CaMKII. The methionine oxidation in CaMKII can be reversed in the presence of methionine sulfoxide oxidase, whose activity is regulated by Trx1. Thus, the redox status of cardiomyocytes has a significant influence on pathological hypertrophy through modulation of key signaling molecules.
Noxs play an important role in mediating cardiac hypertrophy in response to PO and α-adrenergic receptor stimulation [14]. Experimental cardiac hypertrophy induced by short-term infusion of Ang II is inhibited in Nox2 knockout mice, indicating that endogenous Nox2 mediates Ang II-induced hypertrophy [9, 31]. Genetic deletion of Rac1, an essential cytosolic component of the Nox2 NADPH oxidase complex, abolishes Ang II-induced increases in O2− production, activation of hypertrophy signaling mechanisms, including Ask1 and NF-κB, and cardiac hypertrophy, again suggesting the critical involvement of Nox2 in Ang II-induced cardiac hypertrophy [15]. Cardiac hypertrophy after chronic MI is significantly attenuated in p47phox knockout mice [61]. Since p47phox is an essential cytosolic cofactor for Nox2, this observation suggests that Nox2 plays a critical role in mediating cardiac remodeling after MI. Increases in cardiac fibrosis and contractile dysfunction in response to PO were also attenuated in systemic Nox2 knockout mice. Interestingly, however, cardiac hypertrophy was not affected in this animal model [91], suggesting that Nox2 does not mediate PO-induced hypertrophy. Recently, we have shown that increases in oxidative stress in response to PO are significantly reduced in cardiac specific Nox4 knockout mice [44]. Cardiac specific Nox4 knockout mice showed less hypertrophy and better left ventricular systolic function in response to PO than wild type mice. These results suggest that ROS produced by Nox4 contribute to the formation of cardiac hypertrophy and cardiac dysfunction in response to PO. It should be noted that transgenic mice with cardiac-specific overexpression of Nox4 display cardiac dysfunction without remarkable cardiac hypertrophy at the organ level, raising the possibility that the contribution of Nox4 to cardiac hypertrophy may be secondary to cardiac dysfunction. Indeed, in cultured CMs, overexpression of Nox4 induces apoptotic cell death but not hypertrophy, suggesting that the primary effect of Nox4 is cell death rather than cell growth in CMs. Expression of Nox4 is increased by hypertrophic stimuli, including Ang II, PE and PO. Thus, PO induces upregulation of Nox4 in mitochondria, which in turn induces ROS, cell death and cardiac dysfunction, leading to pathological hypertrophy. Downregulation of Nox4 inhibits PO-induced cardiac hypertrophy by disrupting the feed-forward mechanism of oxidative stress, cell death and consequent increases in mechanical loading. It has been shown recently, however, that cardiac hypertrophy induced by suprarenal banding is enhanced by systemic Nox4 knock-out, whereas it is attenuated in mice with cardiac-specific Nox4 overexpression [92]. Although cardiac specific versus systemic knock-out of Nox4 and transverse versus suprarenal aortic constriction could induce distinct functional consequences, Nox4 could mediate both detrimental and protective functions in hearts under stress, depending upon the conditions. For example, Nox4 expression under PO not only stimulates cell death but also induces angiogenesis. It is possible that the overall consequence of Nox4 regulation could be determined by the balance between the dichotomous functions (See below).
Thus, Nox2 and Nox4 appear to have distinct roles in mediating hypertrophy. A possible explanation for the distinct functions of Nox2 and Nox4 is their distinct subcellular localizations. Identifying downstream molecules whose redox status is regulated by each Nox isoform at distinct subcellular locations may allow us to elucidate the molecular mechanisms of cardiac hypertrophy initiated by activation/upregulation of distinct Nox isoforms.
Cell proliferation and fibrosis
Nox-derived ROS are involved in cell proliferation in endothelial cells, vascular smooth muscle cells and fibroblasts [93–95]. These cell types express Nox1, Nox2 and Nox4 [96, 97]. Both Nox2 and Nox4 are abundantly expressed in endothelial cells and promote proliferation [98–100]. Nox1 plays a critical role in mediating regulation of cell proliferation in vascular smooth muscle cells, but has little influence on endothelial proliferation, as Nox1 is rarely expressed in endothelial cells [99, 100]. In addition, Nox-mediated cell proliferation is observed in non-vascular cells as well.
Interstitial fibrosis is an important feature of pathological cardiac hypertrophy and heart failure [101]. Several lines of evidence suggest that Noxs are associated with increases in fibrosis in many organs, including the heart [102–106]. Interstitial fibrosis of the heart induced by Ang II infusion is significantly attenuated in systemic Nox2 knock-out mice as compared to wild-type mice [9, 31]. Interstitial cardiac fibrosis was also inhibited in Nox2 knock-out mice subjected to PO [91]. Multiple mechanisms are involved in the profibrotic effect of Nox2, including activation of NF-κB and upregulation of CTGF and MMP-2, and infiltration of inflammatory cells [106]. Nox4 also mediates TGF-β-induced differentiation of cardiac fibroblasts into myofibroblasts [34]. Cardiac-specific overexpression of Nox4 in mice induces fibrosis in an age-dependent manner, whereas that of dominant negative Nox4 leads to significantly less fibrosis [10], suggesting that Nox4 expressed in CMs affects interstitial fibrosis. Interestingly, Nox4 primarily induces apoptosis in neonatal rat CMs, whereas it induces proliferation in cultured cardiac fibroblasts isolated from the same heart [10]. The molecular mechanism through which Nox4 promotes proliferation of cardiac fibroblasts remains to be elucidated. Recent evidence suggests that regulation of protein tyrosine phosphatases and Ca2+ signaling by Nox1 and Nox5 may be involved in interleukin (IL)-4 signaling, which executes pleiotropic functions, including induction of Th (helper T-lymphocyte) 2-differentiation and B cell proliferation, suppression of Th1-differentiation, and macrophage activation in immune cells [107].
Cell differentiation
Redox signaling is also involved in the differentiation of CMs. The differentiation of ES cells into the cardiomyogenic cell lineage is dependent on Nox-mediated ROS [108]. Low concentrations of ROS derived from either endogenous Nox [109] or exogenous H2O2 induce cell-cycle reentry in ES-cell-derived and neonatal CMs, and significantly upregulates expression of MLC2a, MLC2v, α-actin, ANP and β-MHC, as well as cardiac-specific transcription factors, such as MEF2C, DTEF, Nkx-2.5, and GATA-4, and BMP-10, a growth factor involved in embryonic heart formation. Nox1 expression peaks around embryonic day 6–8, when cardiomyogenesis occurs. Nox2 expression peaks on day 12, whereas Nox4 expression peaks around day 14. These observations suggest that ROS derived from Nox1 may play a critical role in the initial stage of cardiomyogenesis. Since Noxs regulate the redox state of the heart under stress and heart failure [10, 61, 110], ROS derived from Noxs may regulate proliferation and differentiation of cardiac progenitor cells during cardiac repair and regeneration [32].
Physiological functions of NADPH oxidases
Although excessive production of ROS by Noxs is detrimental, local and modest production of O2− and H2O2 by Noxs allows them to act as signaling molecules, thereby mediating physiological responses. For example, since Noxs are functional at low pO2, Noxs may act as a sensor, and ROS generated by Noxs as a transducer, for hypoxia [29, 111]. Erythropoietin (EPO) synthesis occurs in the renal tubular cells, where Nox4 is abundantly expressed [112, 113]. Since DPI not only blocks oxygen sensing but also inhibits Nox4 in renal tubular cells, it has been proposed that Nox4 is an O2 sensor in the kidney and may regulate EPO production [114]. The causative role of Nox4 in mediating EPO synthesis through its function as an O2 sensor remains to be shown. Recently, a role of Nox4 in mediating angiogenesis during cardiac hypertrophy was reported [92]. Pathological hypertrophy induces upregulation/activation of Nox4, which in turn causes stabilization of HIF-1α, upregulation of VEGF, and increases in angiogenesis [92]. It appears that the protective effect of Nox4 prevails under the authors' experimental conditions. It remains unknown, however, whether such a mechanism is sufficient to overcome increases in cell death and mitochondrial dysfunction directly caused by upregulation of Nox4 in response to hypertrophic stimuli [10, 44].
Conclusions
In summary, Noxs play a crucial role in mediating pathological effects, including apoptosis, hypertrophy, fibrosis and mitochondrial dysfunction, in the heart. However, Noxs also act as an oxygen sensor and produce ROS as signaling molecules, thereby mediating cell protective functions, such as hypoxic adaptation, erythropoiesis and angiogenesis. We speculate that the excessive production of ROS caused by upregulation of Noxs is detrimental, but that downregulation of Noxs below certain levels could also be harmful since it could impair adaptive mechanisms essential for cell survival.
Many questions remain. First, Nox2 and Nox4 appear to have distinct functions in CMs. The isoform-specific functions of Noxs at baseline and in response to stress remain to be elucidated. Second, thus far, the molecular mechanism through which expression of Nox4 is upregulated by hypertrophic stimuli is unknown. Since upregulation of Nox4 in response to hypertrophic stimuli leads to increases in oxidative stress, mitochondrial dysfunction and cell death, elucidating the underlying mechanism is highly significant. Third, although the total activity of Nox4 in CMs is regulated primarily at the level of protein expression of Nox4, whether or not the activity of Nox4 is regulated through either posttranscriptional or posttranslational mechanisms is unknown. Fourth, it is unclear why CMs express Nox4 in mitochondria despite the fact that upregulation of Nox4 could lead to oxidation of mitochondria and cell death. Nox4 may exert physiological (protective) functions in mitochondria when its expression is not elevated. Finally, the identity of ROS directly produced by Nox4 and that of molecules whose redox status is directly regulated by either Nox2 or Nox4 in the heart remain to be elucidated. Further investigation is required in order to elucidate the specificity of Nox isoforms and their involvement in redox signaling pathways mediating both detrimental and protective mechanisms under various stress conditions in the heart.
Acknowledgments
The authors thank Daniela Zablocki for critical reading of the manuscript.
Sources of Funding This work was supported in part by U.S. Public Health Service Grants HL59139, HL67724, HL69020, HL91469, HL102738, and AG27211, and the Foundation of Leducq Transatlantic Network of Excellence.
Footnotes
Disclosures None
Reference
- [1].McCord JM, Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978;89:122–7. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- [2].Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–54. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- [4].Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- [5].Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- [6].Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. The Journal of clinical investigation. 1973;52:741–4. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–77. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- [8].Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–31. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–6. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- [10].Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–64. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cave A. Selective targeting of NADPH oxidase for cardiovascular protection. Current opinion in pharmacology. 2009;9:208–13. doi: 10.1016/j.coph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- [12].Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, et al. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–91. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- [13].Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, Shah AM, et al. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113:1235–43. doi: 10.1161/CIRCULATIONAHA.105.581397. [DOI] [PubMed] [Google Scholar]
- [14].Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002;282:C926–34. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- [15].Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Sci U S A. 2006;103:7432–7. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003;278:12094–100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- [17].Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90:1012–9. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- [18].Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- [19].Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1903–11. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- [20].Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94:14483–8. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–7. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- [22].Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, et al. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–50. doi: 10.1161/hh0202.103615. [DOI] [PubMed] [Google Scholar]
- [23].Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–70. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- [24].Uhlinger DJ, Taylor KL, Lambeth JD. p67-phox enhances the binding of p47-phox to the human neutrophil respiratory burst oxidase complex. J Biol Chem. 1994;269:22095–8. [PubMed] [Google Scholar]
- [25].Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, et al. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4474–9. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- [27].Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. The Journal of biological chemistry. 1993;268:20983–7. [PubMed] [Google Scholar]
- [28].Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–4. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–23. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- [30].Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–33. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- [31].Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–5. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- [32].Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, et al. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–88. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–8. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–7. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- [35].Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, et al. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–6. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- [36].Pawson T, Raina M, Nash P. Interaction domains: from simple binding events to complex cellular behavior. FEBS letters. 2002;513:2–10. doi: 10.1016/s0014-5793(01)03292-6. [DOI] [PubMed] [Google Scholar]
- [37].Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–14. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–41. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- [39].Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- [40].Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation research. 2009;105:249–59. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–54. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–51. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–11. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- [44].Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15565–70. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–97. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- [46].Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–25. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Anilkumar N, Sirker A, Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–87. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- [48].Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–28. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- [49].Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Science's STKE : signal transduction knowledge environment. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- [50].Tsutsui H, Kinugawa S, Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res. 2009;81:449–56. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- [51].Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–63. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- [52].Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovascular research. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- [53].Gupte SA. Glucose-6-phosphate dehydrogenase: a novel therapeutic target in cardiovascular diseases. Curr Opin Investig Drugs. 2008;9:993–1000. [PubMed] [Google Scholar]
- [54].Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–27. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- [55].George J, Struthers AD. The role of urate and xanthine oxidase inhibitors in cardiovascular disease. Cardiovasc Ther. 2008;26:59–64. doi: 10.1111/j.1527-3466.2007.00029.x. [DOI] [PubMed] [Google Scholar]
- [56].Bergamini C, Cicoira M, Rossi A, Vassanelli C. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail. 2009;11:444–52. doi: 10.1093/eurjhf/hfp042. [DOI] [PubMed] [Google Scholar]
- [57].Kang SM, Lim S, Song H, Chang W, Lee S, Bae SM, et al. Allopurinol modulates reactive oxygen species generation and Ca2+ overload in ischemia-reperfused heart and hypoxia-reoxygenated cardiomyocytes. European journal of pharmacology. 2006;535:212–9. doi: 10.1016/j.ejphar.2006.01.013. [DOI] [PubMed] [Google Scholar]
- [58].Kim JW, Baek BS, Kim YK, Herlihy JT, Ikeno Y, Yu BP, et al. Gene expression of cyclooxygenase in the aging heart. J Gerontol A Biol Sci Med Sci. 2001;56:B350–5. doi: 10.1093/gerona/56.8.b350. [DOI] [PubMed] [Google Scholar]
- [59].Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Otani H. The role of nitric oxide in myocardial repair and remodeling. Antioxid Redox Signal. 2009;11:1913–28. doi: 10.1089/ars.2009.2453. [DOI] [PubMed] [Google Scholar]
- [61].Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, et al. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- [62].McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler Thromb Vasc Biol. 2005;25:1623–8. doi: 10.1161/01.ATV.0000170827.16296.6e. [DOI] [PubMed] [Google Scholar]
- [63].Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal. 2005;7:472–81. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- [65].Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Qin F, Patel R, Yan C, Liu W. NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: effects of apocynin. Free Radic Biol Med. 2006;40:236–46. doi: 10.1016/j.freeradbiomed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [67].Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–25. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- [68].Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009;52:153–67. doi: 10.1016/j.pcad.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [69].Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, et al. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794–9. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- [70].Hirotani S, Otsu K, Nishida K, Higuchi Y, Morita T, Nakayama H, et al. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105:509–15. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- [71].Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Molkentin JD, Kalvakolanu DV, Markham BE. Transcription factor GATA-4 regulates cardiac muscle-specific expression of the alpha-myosin heavy-chain gene. Molecular and cellular biology. 1994;14:4947–57. doi: 10.1128/mcb.14.7.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, et al. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. The Journal of clinical investigation. 2000;105:1395–406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell death and differentiation. 2006;13:539–50. doi: 10.1038/sj.cdd.4401769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hu E, Chen Z, Fredrickson T, Zhu Y, Kirkpatrick R, Zhang GF, et al. Cloning and characterization of a novel human class I histone deacetylase that functions as a transcription repressor. The Journal of biological chemistry. 2000;275:15254–64. doi: 10.1074/jbc.M908988199. [DOI] [PubMed] [Google Scholar]
- [77].D'Souza SJ, Pajak A, Balazsi K, Dagnino L. Ca2+ and BMP-6 signaling regulate E2F during epidermal keratinocyte differentiation. The Journal of biological chemistry. 2001;276:23531–8. doi: 10.1074/jbc.M100780200. [DOI] [PubMed] [Google Scholar]
- [78].Patra SK, Patra A, Dahiya R. Histone deacetylase and DNA methyltransferase in human prostate cancer. Biochemical and biophysical research communications. 2001;287:705–13. doi: 10.1006/bbrc.2001.5639. [DOI] [PubMed] [Google Scholar]
- [79].Antos CL, McKinsey TA, Dreitz M, Hollingsworth LM, Zhang CL, Schreiber K, et al. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. The Journal of biological chemistry. 2003;278:28930–7. doi: 10.1074/jbc.M303113200. [DOI] [PubMed] [Google Scholar]
- [80].Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, et al. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. The Journal of clinical investigation. 2003;112:863–71. doi: 10.1172/JCI19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, et al. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3β activity. Nature Medicine. 2007;13:324–31. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- [82].Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–5. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- [83].McKinsey TA, Zhang CL, Olson EN. Signaling chromatin to make muscle. Current opinion in cell biology. 2002;14:763–72. doi: 10.1016/s0955-0674(02)00389-7. [DOI] [PubMed] [Google Scholar]
- [84].Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–88. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–11. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7835–40. doi: 10.1073/pnas.140199597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circulation research. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- [88].Kirsh O, Seeler JS, Pichler A, Gast A, Muller S, Miska E, et al. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. The EMBO journal. 2002;21:2682–91. doi: 10.1093/emboj/21.11.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, et al. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. The Journal of clinical investigation. 2007;117:2459–67. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–93. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- [91].Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, et al. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol. 2006;47:817–26. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- [92].Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18121–6. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Irani K. Oxidant signaling in vascular cell growth, death, and survival : a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circulation research. 2000;87:179–83. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- [94].Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Molecular and cellular biochemistry. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- [95].Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovascular research. 2007;75:679–89. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- [96].Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–35. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- [97].Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie. 2007;89:1107–12. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- [98].Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–60. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- [99].Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–17. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- [100].Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–84. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- [101].Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- [102].Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–22. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- [104].Ha H, Lee HB. Reactive oxygen species and matrix remodeling in diabetic kidney. J Am Soc Nephrol. 2003;14:S246–9. doi: 10.1097/01.asn.0000077411.98742.54. [DOI] [PubMed] [Google Scholar]
- [105].An SJ, Boyd R, Zhu M, Chapman A, Pimentel DR, Wang HD. NADPH oxidase mediates angiotensin II-induced endothelin-1 expression in vascular adventitial fibroblasts. Cardiovasc Res. 2007;75:702–9. doi: 10.1016/j.cardiores.2007.02.015. [DOI] [PubMed] [Google Scholar]
- [106].Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–8. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- [107].Sharma P, Chakraborty R, Wang L, Min B, Tremblay ML, Kawahara T, et al. Redox regulation of interleukin-4 signaling. Immunity. 2008;29:551–64. doi: 10.1016/j.immuni.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Buggisch M, Ateghang B, Ruhe C, Strobel C, Lange S, Wartenberg M, et al. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci. 2007;120:885–94. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- [109].Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1182–4. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- [110].Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–71. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- [111].Auzat I, Chapuy-Regaud S, Le Bras G, Dos Santos D, Ogunniyi AD, Le Thomas I, et al. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol Microbiol. 1999;34:1018–28. doi: 10.1046/j.1365-2958.1999.01663.x. [DOI] [PubMed] [Google Scholar]
- [112].Loya F, Yang Y, Lin H, Goldwasser E, Albitar M. Transgenic mice carrying the erythropoietin gene promoter linked to lacZ express the reporter in proximal convoluted tubule cells after hypoxia. Blood. 1994;84:1831–6. [PubMed] [Google Scholar]
- [113].Lacombe C, Da Silva JL, Bruneval P, Fournier JG, Wendling F, Casadevall N, et al. Peritubular cells are the site of erythropoietin synthesis in the murine hypoxic kidney. The Journal of clinical investigation. 1988;81:620–3. doi: 10.1172/JCI113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, et al. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal. 2006;18:499–507. doi: 10.1016/j.cellsig.2005.05.025. [DOI] [PubMed] [Google Scholar]