Abstract
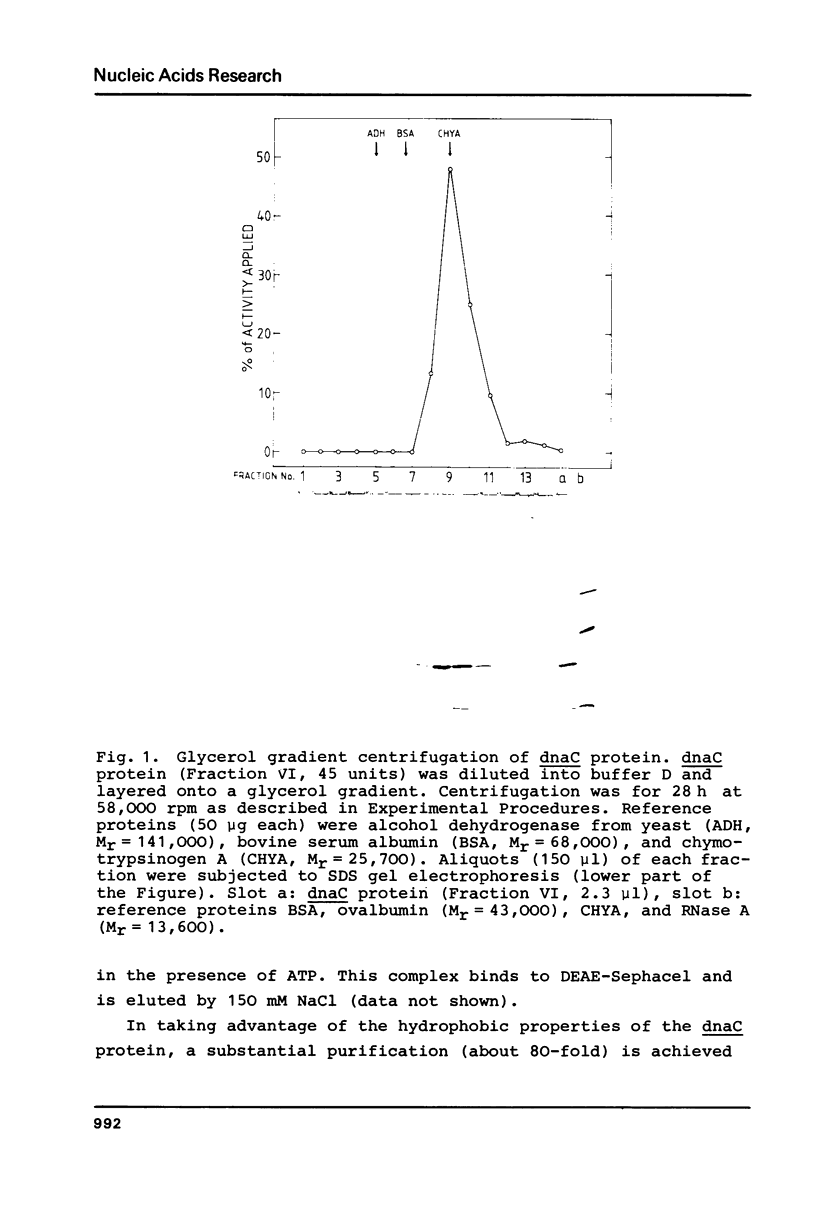
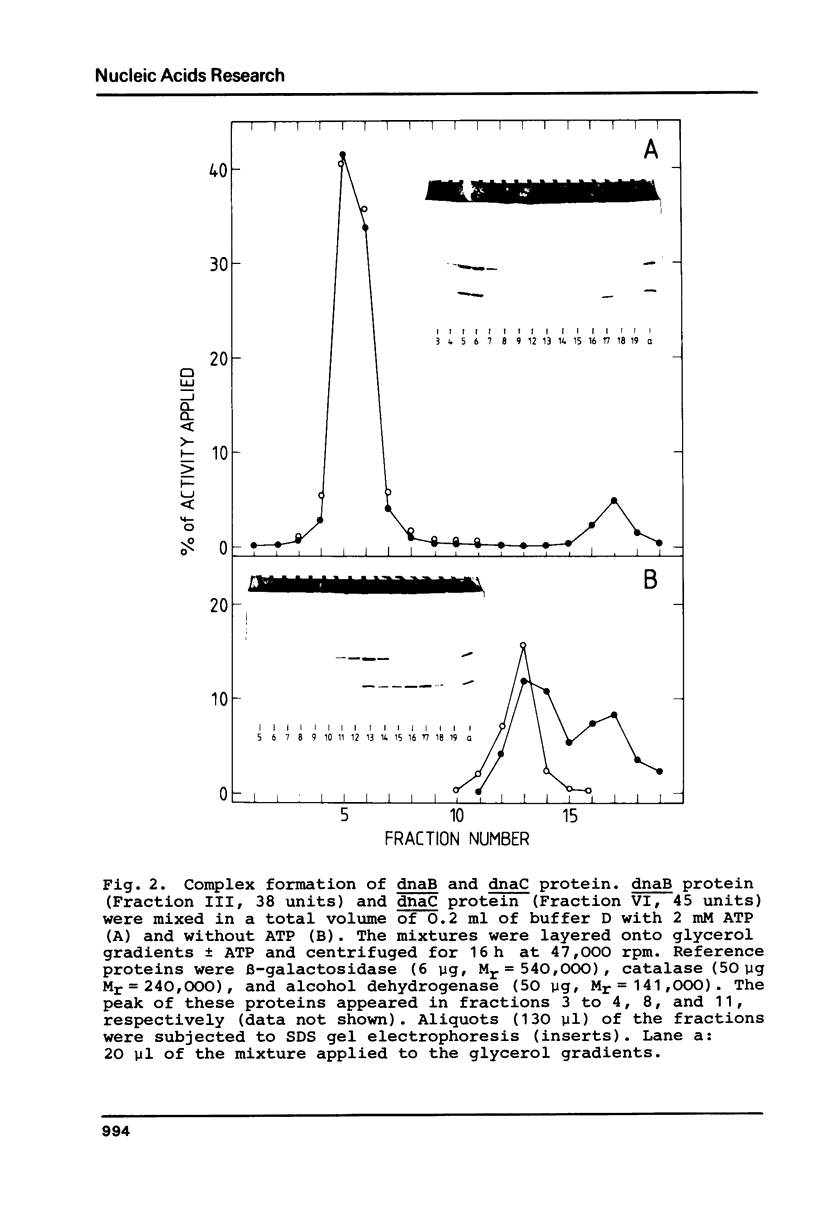
E.coli dnaC protein was purified to near-homogeneity in using a dnaC complementation assay [S.Wickner, I.Berkower, M.Wright, and J.Hurwitz (1973) Proc. Natl. Acad. Sci. USA 70, 2369-2373]. Purification was achieved by taking advantage of the hydrophobic interaction of dnaC protein with aliphatic and aromatic matrixes and with Brij58 as stabilizing agent. A sedimentation coefficient for the dnaC protein of 2.6 S corresponding to a molecular weight of approximately 26,000 was estimated from glycerol gradient centrifugation. A polypeptide molecular weight of 28,000 was determined by densitometry on a denaturing gel. In the presence of ATP the dnaC protein forms a complex with dnaB protein [S.Wickner and J.Hurwitz (1975) Proc.Natl.Acad.Sci. USA 72, 921-925]. For the dnaB . dnaC complex a sedimentation coefficient of 14.5 S was measured by glycerol gradient centrifugation, indicating a molecular weight of about 400,000. The ratio of the dnaC and dnaB polypeptides in the complex is approximately 1, as determined on a denaturing gel. It is suggested that the complex consists of the dnaB protein hexamer and six dnaC polypeptides amounting to a calculated molecular weight of about 450,000.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kornberg A. A general priming system employing only dnaB protein and primase for DNA replication. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4308–4312. doi: 10.1073/pnas.76.9.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Yasuda S., Kornberg A. Mechanism of dnaB protein action. I. Crystallization and properties of dnaB protein, an essential replication protein in Escherichia coli. J Biol Chem. 1981 May 25;256(10):5247–5252. [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Collins J., Williams P., Helinski D. R. Plasmid ColE1 DNA replication in Escherichia coli strains temperature-sensitive for DNA replication. Mol Gen Genet. 1975;136(4):273–289. doi: 10.1007/BF00341713. [DOI] [PubMed] [Google Scholar]
- Edelbluth C., Lanka E., von der Hude W., Mikolajczyk M., Schuster H. Association of the prophage P1ban protein with the dnaB protein of Escherichia coli. Overproduction of ban protein by a P1bac crr mutant. Eur J Biochem. 1979 Mar;94(2):427–435. doi: 10.1111/j.1432-1033.1979.tb12910.x. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther E., Mikolajczyk M., Schuster H. Stabilization by ATP and ADP of Escherichia coli dnaB protein activity. J Biol Chem. 1981 Dec 10;256(23):11970–11973. [PubMed] [Google Scholar]
- Hasunuma K., Sekiguchi M. Effect of dna mutations on the replication of plasmid pSC101 in Escherichia coli K-12. J Bacteriol. 1979 Mar;137(3):1095–1099. doi: 10.1128/jb.137.3.1095-1099.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranias E. G., Dumas L. B. Replication of bacteriophage phiX174 DNA in a temperature-sensitive dnaC mutant of escherichia coli C. J Virol. 1974 Jan;13(1):146–154. doi: 10.1128/jvi.13.1.146-154.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanka E., Edelbluth C., Schlicht M., Schuster H. Escherichia coli dnaB protein. Affinity chromatography on immobilized nucleotides. J Biol Chem. 1978 Aug 25;253(16):5847–5851. [PubMed] [Google Scholar]
- Lanka E., Mikolajczyk M., Schlicht M., Schuster H. Association of the prophage P1ban protein with the dnaB protein of Escherichia coli. J Biol Chem. 1978 Jul 10;253(13):4746–4753. [PubMed] [Google Scholar]
- McMacken R., Kornberg A. A multienzyme system for priming the replication of phiX174 viral DNA. J Biol Chem. 1978 May 10;253(9):3313–3319. [PubMed] [Google Scholar]
- Reha-Krantz L. J., Hurwitz J. The dnaB gene product of Escherichia coli. I. Purification, homogeneity, and physical properties. J Biol Chem. 1978 Jun 10;253(11):4043–4050. [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Schuster H., Mikolajczyk M., Rohrschneider J., Geschke B. phiX174 DNA-dependent DNA synthesis in vitro: requirement for P1 ban protein in dnaB mutant extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3907–3911. doi: 10.1073/pnas.72.10.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster H., Schlicht M., Lanka E., Mikolajczyk M., Edelbluth C. DNA synthesis in an Escherichia coli dna B dnaC mutant. Mol Gen Genet. 1977 Feb 28;151(1):11–16. doi: 10.1007/BF00446907. [DOI] [PubMed] [Google Scholar]
- Sclafani R. A., Wechsler J. A. Deoxyribonucleic acid initiation mutation dnaB252 is suppressed by elevated dnaC+ gene dosage. J Bacteriol. 1981 Apr;146(1):418–421. doi: 10.1128/jb.146.1.418-421.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani R. A., Wechsler J. A. Suppression of dnaC alleles by the dnaB analog (ban protein) of bacteriophage P1. J Bacteriol. 1981 Apr;146(1):321–324. doi: 10.1128/jb.146.1.321-324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Lanka E., Schuster H. Replication of small plasmids in extracts of Escherichia coli: involvement of the dnaB and dnaC protein in the replication of early replicative intermediates. Mol Gen Genet. 1978 Jul 4;162(3):243–249. doi: 10.1007/BF00268849. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A. Genetic and phenotypic characterization of dnaC mutations. J Bacteriol. 1975 Feb;121(2):594–599. doi: 10.1128/jb.121.2.594-599.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli and phage lambda. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):303–310. doi: 10.1101/sqb.1979.043.01.037. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli. Annu Rev Biochem. 1978;47:1163–1191. doi: 10.1146/annurev.bi.47.070178.005503. [DOI] [PubMed] [Google Scholar]
- Wickner S., Berkower I., Wright M., Hurwitz J. Studies on in vitro DNA synthesis: purification of dna C gene product containing dna D activity from Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2369–2373. doi: 10.1073/pnas.70.8.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Interaction of Escherichia coli dnaB and dnaC(D) gene products in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):921–925. doi: 10.1073/pnas.72.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]