Abstract
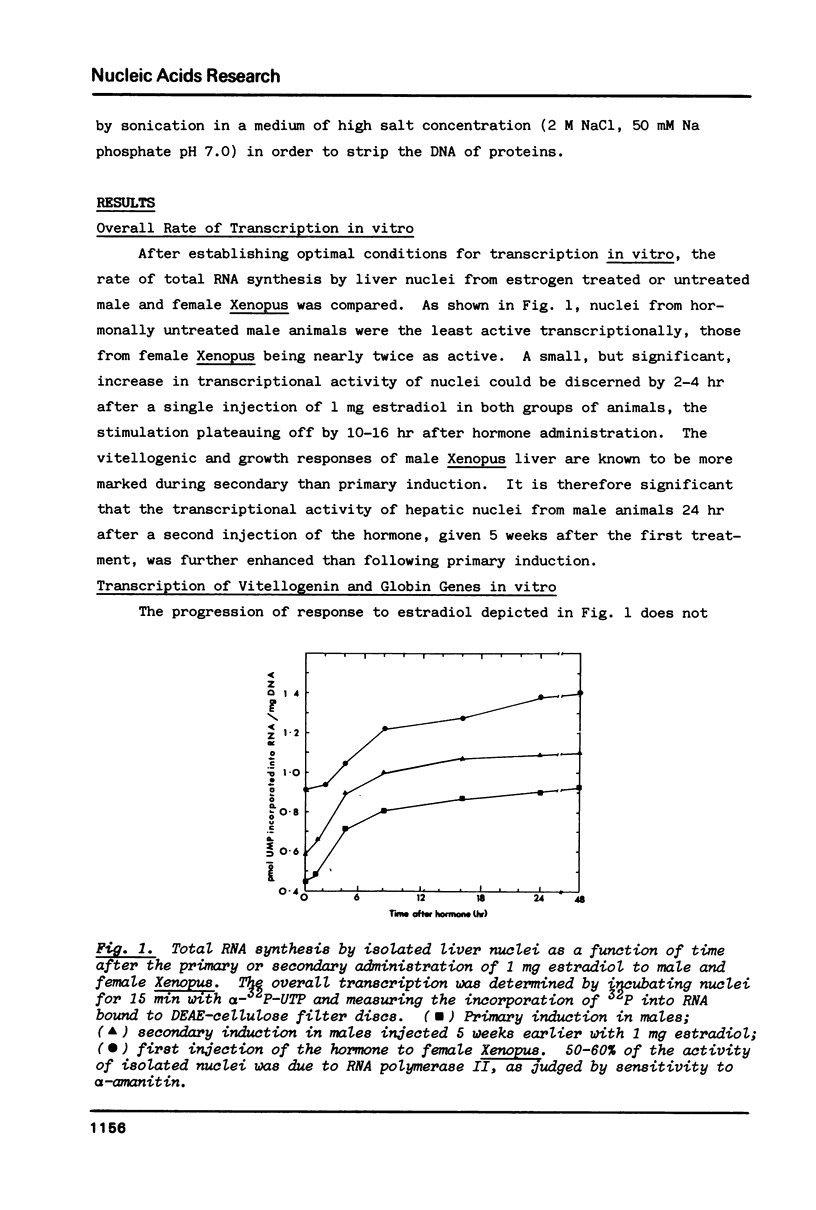
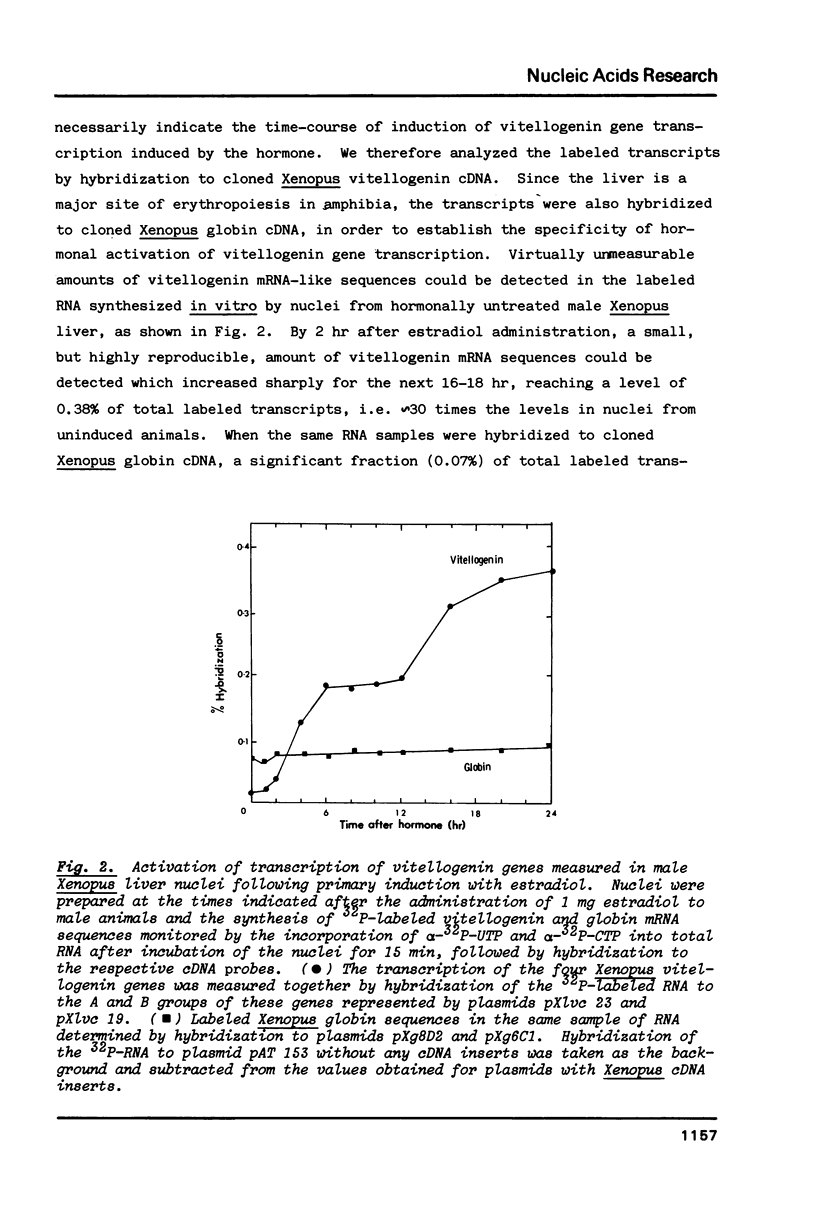
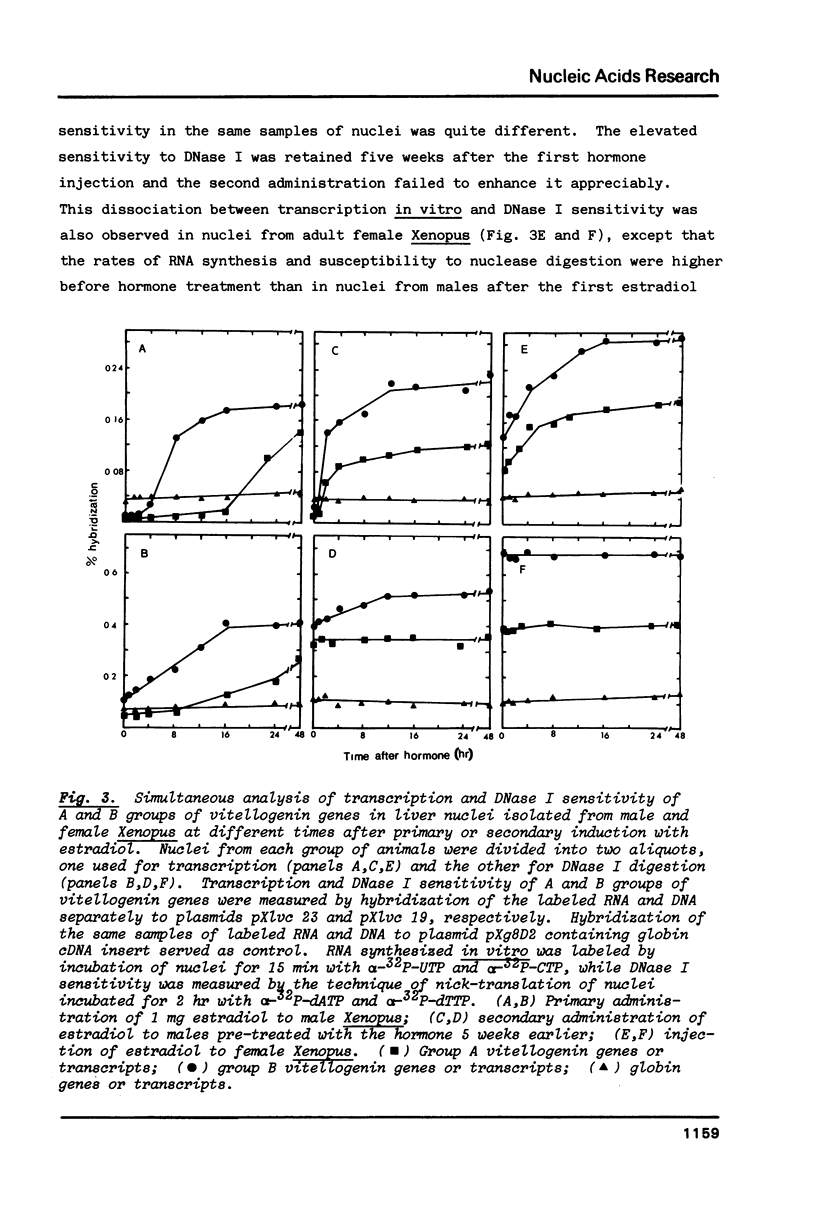
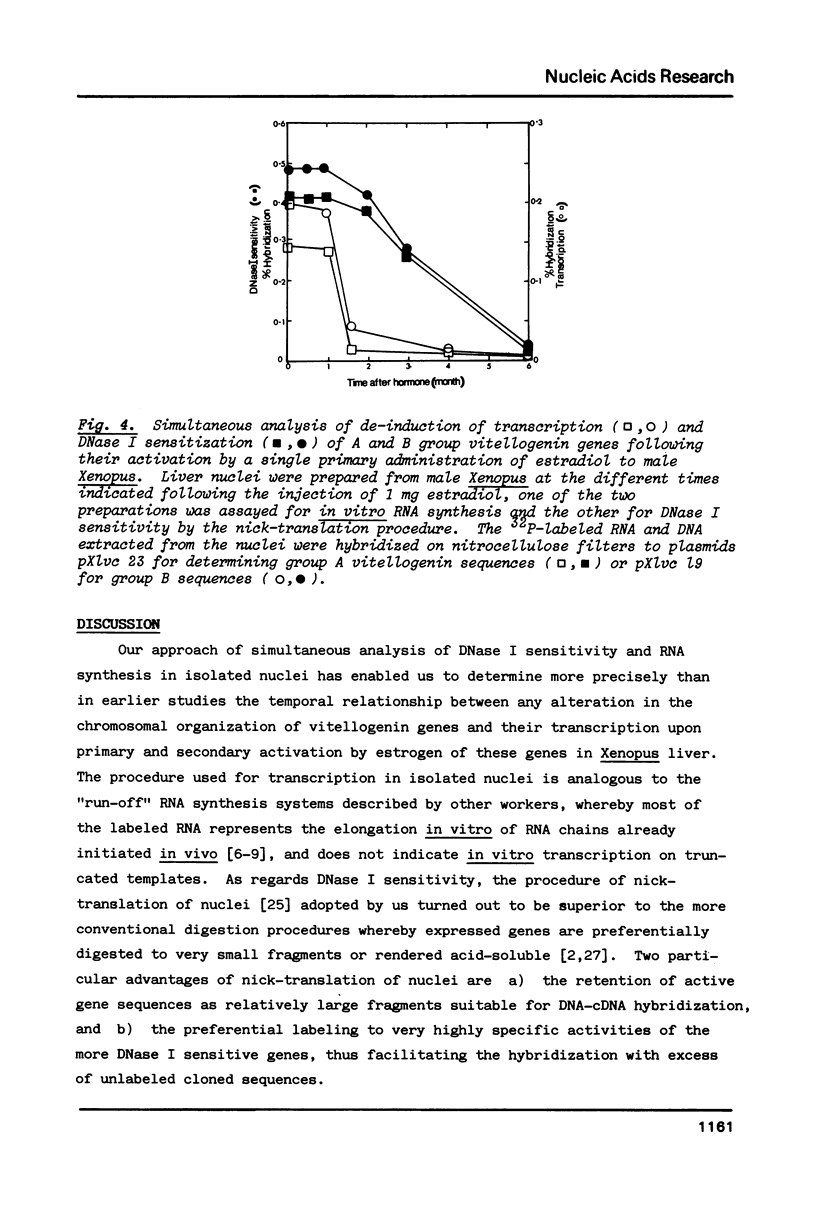
In male Xenopus, primary estradiol administration results in noncoordinate activation in the liver of the A and B groups of vitellogenin genes, both as judged by transcription and DNase I sensitivity in isolated nuclei, B group genes being activated preferentially in the first 20 hr. Secondary induction in males or "primary" induction in females results in a coordinate and equal transcription of these two groups of genes. The elevated transcriptional activity following primary estrogen stimulation returns to low levels rapidly but the high DNase I sensitivity of these genes persists for 2-3 months. A non-coordinate activation of the A and B groups of vitellogenin genes is however re-established in response to a second administration of estradiol 8 months after primary stimulation of male Xenopus.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baker H. J., Shapiro D. J. Kinetics of estrogen induction of Xenopus laevis vitellogenin messenger RNA as measured by hybridization to complementary DNA. J Biol Chem. 1977 Dec 10;252(23):8428–8434. [PubMed] [Google Scholar]
- Chambon P. Eukaryotic nuclear RNA polymerases. Annu Rev Biochem. 1975;44:613–638. doi: 10.1146/annurev.bi.44.070175.003145. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G. J., Tata J. R. Differential sensitization to deoxyribonuclease I of Xenopus vitellogenin and albumin genes during primary and secondary induction of vitellogenesis by oestradiol. Biochem J. 1982 Feb 15;202(2):491–497. doi: 10.1042/bj2020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Henshaw E. C., Berridge M. V., Tata J. R. Translation of Xenopus vitellogenin mRNA during primary and secondary induction. Nature. 1978 Jun 1;273(5661):401–403. doi: 10.1038/273401a0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Gerber-Huber S., Meier C., May F. E., Westley B., Weber R., Ryffel G. U. Quantitation of DNase I sensitivity in Xenopus chromatin containing active and inactive globin, albumin and vitellogenin genes. Nucleic Acids Res. 1981 Jun 11;9(11):2455–2474. doi: 10.1093/nar/9.11.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Maurhofer S., Jaggi R. B., Wyler T., Wahli W., Ryffel G. U., Weber R. Isolation and translation in vitro of four related vitellogenin mRNAs of estrogen-stimulated Xenopus laevis. Eur J Biochem. 1980 Mar;105(1):17–24. doi: 10.1111/j.1432-1033.1980.tb04469.x. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Multigene families encoding actin and tubulin. Cell. 1981 Apr;24(1):6–7. doi: 10.1016/0092-8674(81)90494-3. [DOI] [PubMed] [Google Scholar]
- Hayward M. A., Mitchell T. A., Shapiro D. J. Induction of estrogen receptor and reversal of the nuclear/cytoplasmic receptor ratio during vitellogenin synthesis and withdrawal in Xenopus laevis. J Biol Chem. 1980 Dec 10;255(23):11308–11312. [PubMed] [Google Scholar]
- Hentschel C. C., Tata J. R. Differential activation of free and template-engaged RNA polymerase I and II during the resumption of development of dormant Artemia gastrulae. Dev Biol. 1977 Jun;57(2):293–304. doi: 10.1016/0012-1606(77)90216-0. [DOI] [PubMed] [Google Scholar]
- Iatrou K., Tsitilou S. G., Kafatos F. C. Developmental classes and homologous families of chorion genes in Bombyx mori. J Mol Biol. 1982 May 25;157(3):417–434. doi: 10.1016/0022-2836(82)90469-7. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. Developmental regulation of Drosophila alpha-tubulin genes. Cell. 1982 May;29(1):91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Levitt A., Axel R., Cedar H. Nick translation of active genes in intact nuclei. Dev Biol. 1979 Apr;69(2):496–505. doi: 10.1016/0012-1606(79)90307-5. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: in vitro initiation of adenovirus 2 major late mRNA precursor. Proc Natl Acad Sci U S A. 1979 Jan;76(1):160–164. doi: 10.1073/pnas.76.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Ohta T. Amino acid diversity of immunoglobulins as a product of molecular evolution. J Mol Evol. 1980 Mar;15(1):29–35. doi: 10.1007/BF01732581. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., McKnight G. S., Senear A. W. Regulation of gene expression in the chick oviduct by steroid hormones. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):639–647. doi: 10.1101/sqb.1978.042.01.066. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Jaehning J. A., Roeder R. G. Faithful gene transcription by eukaryotic RNA polymerases in reconstructed systems. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):577–587. doi: 10.1101/sqb.1978.042.01.060. [DOI] [PubMed] [Google Scholar]
- Royal A., Garapin A., Cami B., Perrin F., Mandel J. L., LeMeur M., Brégégègre F., Gannon F., LePennec J. P., Chambon P. The ovalbumin gene region: common features in the organisation of three genes expressed in chicken oviduct under hormonal control. Nature. 1979 May 10;279(5709):125–132. doi: 10.1038/279125a0. [DOI] [PubMed] [Google Scholar]
- Schibler U., Weber R. A new method for the isolation of undegraded nuclear and cytoplasmic RNA from liver of Xenopus larvae. Anal Biochem. 1974 Mar;58(1):225–230. doi: 10.1016/0003-2697(74)90461-8. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Tata J. R. Vitellogenin gene expression in male Xenopus hepatocytes during primary and secondary stimulation with estrogen in cell cultures. Cell. 1981 Mar;23(3):741–746. doi: 10.1016/0092-8674(81)90437-2. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Melchior W., Jr, Felsenfeld G. DNAase I, DNAase II and staphylococcal nuclease cut at different, yet symmetrically located, sites in the nucleosome core. Cell. 1978 Jul;14(3):611–627. doi: 10.1016/0092-8674(78)90246-5. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Baker B. S., Deeley J. V. Vitellogenin as a multigene family. Not all Xenopus vitellogenin genes may be in an "expressible" configuration. J Biol Chem. 1980 Jul 25;255(14):6721–6726. [PubMed] [Google Scholar]
- Tata J. R., Baker B. Differential subnuclear distribution of polyadenylate-rich ribonuclei acid during induction of egg-yolk protein synthesis in male Xenopus liver by oestradiol-17 beta. Biochem J. 1975 Sep;150(3):345–355. doi: 10.1042/bj1500345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R. Selective steroid hormonal regulation of gene expression in multigene families. J Steroid Biochem. 1981 Dec;15:87–97. doi: 10.1016/0022-4731(81)90262-4. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Ryffel G. U., Weber R. Vitellogenesis and the vitellogenin gene family. Science. 1981 Apr 17;212(4492):298–304. doi: 10.1126/science.7209528. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Jaggi R. B., Weber R., Ryffel G. U. Vitellogenin in Xenopus laevis is encoded in a small family of genes. Cell. 1979 Mar;16(3):535–549. doi: 10.1016/0092-8674(79)90028-x. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Segall J., Harris B., Ng S. Y., Roeder R. G. Faithful transcription of eukaryotic genes by RNA polymerase III in systems reconstituted with purified DNA templates. J Biol Chem. 1979 Jul 10;254(13):6163–6173. [PubMed] [Google Scholar]
- Weisbrod S. T. Properties of active nucleosomes as revealed by HMG 14 and 17 chromatography. Nucleic Acids Res. 1982 Mar 25;10(6):2017–2042. doi: 10.1093/nar/10.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


