Abstract
Outer membrane vesicles (OMVs) are produced by all Gram-negative microorganisms studied to date. The contributions of OMVs to biological processes are diverse and include mediation of bacterial stress responses, selective packaging and secretion of virulence determinants, modulation of the host immune response, and contributions to biofilm formation and stability. First characterized as transformasomes in Haemophilus, these membranous blebs facilitate transfer of DNA among bacteria. Nontypeable Haemophilus influenzae (NTHI), an opportunistic pathogen of the upper and lower respiratory tracts, produces OMVs in vivo, but there is a paucity of information regarding both the composition and role of OMVs during NTHI colonization and pathogenesis. We demonstrated that purified NTHI vesicles are 20 to 200 nm in diameter and contain DNA, adhesin P5, IgA endopeptidase, serine protease, and heme utilization protein, suggesting a multifaceted role in virulence. NTHI OMVs can bind to human pharyngeal epithelial cells, resulting in a time- and temperature-dependent aggregation on the host cell surface, with subsequent internalization. OMVs colocalize with the endocytosis protein caveolin, indicating that internalization is mediated by caveolae, which are cholesterol-rich lipid raft domains. Upon interaction with epithelial cells, NTHI OMVs stimulate significant release of the immunomodulatory cytokine interleukin-8 (IL-8) as well as the antimicrobial peptide LL-37. Thus, we demonstrated that NTHI OMVs contain virulence-associated proteins that dynamically interact with and invade host epithelial cells. Beyond their ability to mediate DNA transfer in Haemophilus, OMV stimulation of host immunomodulatory cytokine and antimicrobial peptide release supports a dynamic role for vesiculation in NTHI pathogenesis and clinically relevant disease progression.
INTRODUCTION
Nontypeable Haemophilus influenzae (NTHI) is a Gram-negative commensal inhabitant of the human nasopharynx that causes diseases of the mammalian upper and lower airways. NTHI predominates in otitis media (OM) and other localized respiratory diseases, such as acute sinusitis and community-acquired pneumonia, and has important consequences in patients with chronic obstructive pulmonary disease (COPD) or cystic fibrosis, with direct and indirect costs of diagnosing and managing OM exceeding $5 billion annually in the United States alone (21, 22, 32, 35, 40, 41, 48). To gain a more comprehensive understanding of the dynamic interplay between microbe-expressed virulence factors and the host immune response, particularly during the transition from commensal to pathogen, the secretion of virulence-associated bacterial components must be investigated further. The blebbing of outer membrane vesicles (OMVs) is one common and recently appreciated mechanism of release of virulence-associated proteins by Gram-negative bacteria such as NTHI.
OMVs are ubiquitously shed among pathogenic and nonpathogenic Gram-negative bacteria (7, 23, 27). Vesicles are composed of luminal periplasmic material bound by a layer of bacterial outer membrane components (e.g., lipopolysaccharide, phospholipids, and outer membrane proteins) and play an active role in pathogenesis, as they are proposed to be vehicles for virulence factor delivery to host cells (3, 4, 15, 17, 19, 20, 51). The functions of bacterial OMVs are versatile, such as regulation of the bacterial stress response, reduction of toxic compounds in the environment, quorum sensing, and coaggregation of bacteria, thus enabling colonization and biofilm formation (7, 11, 24, 29, 34, 43, 53, 55). In addition to these functions, OMVs mediate an immunomodulatory role in bacterial pathogenesis (4). Moraxella OMVs contain the immunostimulatory superantigen molecule MID, which induces a nonspecific immune response that fails to target Moraxella whole bacterial cells, thus contributing to pathogenesis (50). Furthermore, OMVs stimulate Toll-like receptor 2 (TLR-2), TLR-4, and TLR-9 host cell signaling molecules and the release of immunomodulatory cytokines such as interleukin-6 (IL-6), IL-8, and IL-12 (7, 23, 49). The ability of OMVs to modulate robust host immune responses presents OMVs as attractive vaccine candidates; both natural and engineered OMVs have been shown to confer immunity to challenges with pathogenic bacteria (49, 50).
NTHI, a versatile opportunistic pathogen, has been reported to shed outer membrane vesicles both in vitro (6) and in vivo (14). Thus, NTHI OMVs are likely to be immunomodulatory, contributing to NTHI pathogenesis. Outer membrane vesicles produced by Haemophilus were described to have a role in natural competence (6) and were characterized as transformasomes due to their ability to transport DNA, both internally and on the surface, among bacterial cells (18). Importantly, Haemophilus vesicles could induce blood-brain barrier permeability during experimental meningitis (54). The biological role of outer membrane vesicles produced by clinically relevant NTHI strains remains unknown, and there is a paucity of comprehensive information regarding the components of vesicles and their role in clinically relevant Haemophilus disease states. Thus, we investigated OMV production, identified vesicle protein cargo, and monitored OMV interaction with and stimulation of host respiratory and middle ear epithelial cells. We further monitored this interaction with host epithelial cells by fluorescence microscopy and elucidated the mechanism of OMV internalization. The ability of OMVs to modulate host cell responses suggests that outer membrane vesicles are a potent contributor to NTHI pathogenesis and play a role in the delicate balance between the commensal and pathogenic lifestyles of NTHI.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
NTHI strain 86-028NP is a minimally passaged clinical isolate obtained at Nationwide Children's Hospital in Columbus, OH. This strain has been sequenced and characterized extensively (12, 31). Strain 86-028NP was grown on chocolate II agar (Becton Dickinson, Sparks, MD) or in brain heart infusion broth supplemented with 2 μg heme/ml and 1 μg NAD/ml (sBHI). Bacteria were cultured from overnight growth on chocolate II agar, resuspended in sBHI to an optical density at 490 nm (OD490) of 0.65, diluted 1:100 in fresh sBHI medium, and grown overnight (16 h) at 37°C and 180 rpm.
Purification and imaging of OMVs.
OMVs were isolated from strain 86-028NP cultured overnight (16 h) at 37°C and 180 rpm in sBHI broth. Whole cells were removed by centrifugation (10,000 × g for 10 min at 4°C). Supernatants were concentrated through a 100-kDa filter by use of a tangential-flow filtration system (Millipore, Billerica, MA) to a final volume that was one-sixth or less of the starting volume (approximately 250 ml for a 2-liter culture). The concentrated supernatant was filter sterilized (0.22 μm; Millipore), and OMVs were subsequently isolated from concentrated supernatants according to published methods (2, 19). Briefly, the purified retentate was centrifuged at 38,000 × g for 1 h (4°C) to pellet crude vesicles. Crude OMVs were resuspended in sterile 50 mM HEPES–150 mM NaCl. Crude vesicles were further purified by density gradient centrifugation (overnight, 150,000 × g, 4°C, SW41T.i rotor) on a discontinuous OptiPrep-iodixanol (Grenier Bio-One, Monroe, NC) gradient (3 ml 50%, 2 ml 45%, 2 ml 40%, 2 ml 35%, 2 ml 30%, and 1 ml 25% OptiPrep in 50 mM HEPES–150 mM NaCl). Gradient fractions (780 μl) were collected from top to bottom and resolved in a 12% acrylamide gel, and vesicle proteins were identified by silver nitrate staining. Vesicle-containing fractions were pooled, diluted 10× in 50 mM HEPES–150 mM NaCl, and pelleted (150,000 × g, 1 h, 4°C, 70Ti rotor). Vesicles were resuspended in 250 μl 50 mM HEPES–150 mM NaCl, stored at 4°C, and utilized within 4 weeks. Vesicle sterility was confirmed by plating 10 μl of each preparation on a chocolate II agar plate. No colonies were detected in any of the purified vesicle preparations. Bacterium-free OMVs were quantified for protein content by Bradford assay. By protein amount, 1 liter of culture yielded approximately 80 μg of purified OMVs.
Purified OMV samples (in 50 mM HEPES–150 mM NaCl) were applied to carbon-coated copper grids (Electron Microscopy Sciences), stained with 2% uranyl acetate, and air dried. Samples were visualized at 80 kV on a Hitachi H-7650 transmission electron microscope with AMtv542 software.
Purified OMVs were fluorescently labeled as previously described (20). Briefly, purified vesicles were pelleted (150,000 × g for 30 min, MTX 150 tabletop ultracentrifuge, S55-A2 fixed-angle microtube rotor) and resuspended in binding buffer (0.1 M sodium bicarbonate). Alexa Fluor 488 (Invitrogen, Carlsbad, CA) was added to the binding buffer suspension and incubated for 1 h at room temperature on a rotator. Vesicles were then pelleted and washed three times with Dulbecco's phosphate-buffered saline (D-PBS) to remove unbound dye. A final suspension was made in 50 mM HEPES–150 mM NaCl. The labeled OMVs were tested for sterility prior to use, stored at 4°C, and utilized within 4 weeks of labeling. Labeled OMVs were quantified by Bradford assay and did not contain aggregates as observed by fluorescence microscopy.
Proteinase K treatment of OMVs.
Vesicles (50 μg) were incubated with 100 μg proteinase K/ml at 37°C on a rotator for 30 min. As a control, an equivalent amount of vesicles was incubated in the absence of proteinase K. Following incubation, vesicles were pelleted (150,000 × g for 30 min, MTX 150 tabletop ultracentrifuge, S55-A2 fixed-angle microtube rotor) and resuspended in D-PBS. This washing procedure was repeated 2 additional times to remove proteinase K. Washed vesicles were brought up in 50 mM HEPES–150 mM NaCl, stored at 4°C, and utilized within 1 week of proteinase K treatment.
DNA quantitation.
Quantitation of DNA content in OMVs was performed as previously described (39), with the following modifications. Briefly, surface-associated and luminal DNAs were quantified by PicoGreen assay (Invitrogen). Ten micrograms of OMV protein was pelleted (150,000 × g, 30 min) and resuspended in 50 mM HEPES–150 mM NaCl or GES lysis reagent (5 M guanidinium thiocyanate, 100 mM EDTA, 0.5% Sarkosyl) to release DNA from OMVs. OMVs were treated with Ambion DNase according to the manufacturer's instructions to digest DNA bound to the outer surfaces of OMVs. Vesicles were pelleted (150,000 × g, 30 min) and resuspended in HEPES buffer, and supernatants were saved for DNA quantitation.
SDS-PAGE analysis of NTHI protein fractions.
OMV samples were compared to NTHI 86-028NP cytoplasmic (CP), periplasmic (PP), and outer membrane (OMP) proteins prepared as previously described (9) and analyzed by SDS-PAGE. Enriched NTHI periplasmic and cytoplasmic protein fractions were obtained through whole-cell fractionation. Briefly, NTHI was grown to mid-log phase in sBHI. Cells were pelleted by centrifugation and resuspended in 2 mg polymyxin B sulfate/ml (Sigma) to permeabilize the outer membrane and release periplasmic proteins. Spheroplasts were pelleted by centrifugation. Periplasmic proteins were removed in the supernatant. The spheroplast pellet was lysed by freeze-thawing, membranes were pelleted by ultracentrifugation, and the supernatant (containing cytoplasm) was removed. All protein concentrations were determined by Bradford assay and normalized to 1 μg total protein. The proteins were resolved in a 12% acrylamide gel at 100 V for 120 min in 1× TGS buffer (Bio-Rad, Hercules, CA). Protein bands were visualized by silver nitrate staining.
Proteome analysis.
Proteomic analysis was performed at the Institute for Genome Sciences and Policy Service Core at Duke University, Durham, NC. Briefly, purified OMVs were probe sonicated and resuspended in 50 μl of 50 mM Ambic buffer to a protein concentration of approximately 1.0 mg/ml (total of 50 μg of protein). Vesicles were resuspended in a volume of 500 μl for a protein concentration of 0.01 μg/μl. One hundred microliters of the vesicle suspension was digested, lyophilized, and resuspended in 20 μl of liquid chromatography (LC) buffer. A volume of 8 μl was used for mass spectrometry (MS) and analyzed using LC-MS/MS. Peptide masses were matched with the NCBI species-specific database for NTHI 86-028NP. There were approximately 1,830 entries in the database, which was downloaded from the NCBI website on 20 April 2011. There were approximately 100 proteins identified with 2 or more peptides and another approximately 50 proteins identified with reasonable confidence but only 1 peptide. The overall false discovery rate for the data set was ∼4%; however, it was virtually 0% for the proteins with 2 or more peptides.
IL-8 and LL-37 detection.
Primary chinchilla middle ear epithelial (CMEE) cells were isolated from adult chinchilla middle ear mucosa and cultured in CMEE growth medium at 37°C with 90% humidity and 5% CO2 (33). Human pharyngeal (Detroit 562; CCL-138) epithelial cells were obtained from the ATCC (Manassas, VA) and cultured in Eagle's minimal essential medium (EMEM; ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) at 37°C and incubated at a 90% humidity and 5% CO2. The cells were passaged and grown in 96-well cell culture plates or an 8-well chamber slide system (Lab-Tek II; Thermo Fisher Scientific) to 80% confluence prior to utilization for IL-8 and LL-37 release and fluorescence microscopy.
CMEE and Detroit 562 cells were exposed to 1 μg of purified vesicles in a 120-μl total volume and were cultured at 37°C. Supernatants were collected at 24 and 48 h. IL-8 levels in the supernatants of both experimental and control (medium alone) wells were determined by enzyme-linked immunosorbent assay (ELISA), and absorbance values were compared to a standard curve prepared following the manufacturer's instructions (BD OptEIA human IL-8 ELISA kit). P values were determined by paired Student's t test.
Detroit 562 cells in a 96-well plate were exposed to 5 μl of proteinase K-treated vesicles, nontreated vesicles, or proteinase K alone. Supernatants were collected after 24 h, and IL-8 release was determined by comparison to a standard curve prepared according to the manufacturer's instructions (BD OptEIA human IL-8 ELISA kit).
Additionally, to determine whether OMVs stimulate components of host innate immunity, the 24-h supernatants were resolved by SDS-PAGE (Ready Gel Tris-HCl precast gels; Bio-Rad, Hercules, CA), transferred to polyvinylidene difluoride (PVDF; Bio-Rad, Hercules, CA) membranes, and blocked in 3% skim milk. Membranes were incubated with rabbit anti-LL-37 (Phoenix Pharmaceuticals, Burlingame, CA) overnight at 4°C, washed, and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG(H+L) (Invitrogen, Carlsbad, CA). Membranes were washed, and peroxidase activity was detected using SuperSignal West Femto chemiluminescent substrate (Thermo Scientific, Rockford, IL).
Fluorescence microscopy.
Detroit 562 human pharynx epithelial cells were grown in an eight-well chamber slide system (Lab-Tek II) and exposed to fluorescently labeled vesicles. Cells were incubated at 37°C and 4°C (in parallel) for 30 min and then incubated, at their respective temperatures, for 2 h with 1 μg total Alexa Fluor-labeled OMVs suspended in EMEM. Alternately, cells were treated with 1 μg filipin/ml for 30 min prior to exposure to 1 μg Alexa Fluor-conjugated OMVs for 4 h. Following incubation with OMVs, cells were washed 3 times with PBS and stained for 10 min; plasma membranes were stained with 1 mg/ml of wheat germ agglutinin-Alexa Fluor 594 in PBS (Invitrogen, Carlsbad, CA), and DNA was stained with 0.5 mg/ml Hoechst 34580 (Invitrogen, Carlsbad, CA). Cells were fixed with 4% paraformaldehyde overnight at 4°C, and a coverslip was attached using ProLong Gold (Invitrogen). Slides were imaged with an Axiovert 200 M inverted epifluorescence microscope equipped with an Axiocam MRM charge-coupled device (CCD) camera (Carl Zeiss Inc., Thornwood, NY). Three-dimensional reconstructions of planar image stacks were processed using ImageJ computer software. Images shown are representative of three independent experiments.
Quantitative analysis of vesicle aggregation.
Representative image data sets from three independent experiments were rendered into a 3-dimensional image. The image was flattened in the z dimension such that the vesicles in all planes remained in focus in a single image. The image of the green channel only (representing the Alexa 488-labeled vesicles) was inverted in color tone in black and white such that the vesicles appear black in Adobe Photoshop. The “threshold” algorithm was applied in NIH ImageJ (http://rsbweb.nih.gov/ij/) across the entire image until all of the smallest vesicles were observed. The “analyze particles” function of NIH ImageJ was applied, using the parameters of a particle size of 0 to 1,000 and a circularity of 0 to 1. Each image set analyzed contained 160 to 414 vesicles. There were a total of 677 vesicles in the no-filipin group and 934 vesicles in the filipin-treated group. There was a statistically significant difference between the sizes of the vesicles in the treatment groups (P < 0.0258) by unpaired Student's t test (Graphpad Prism).
Subcellular fractionation of OMV-treated epithelial cells.
To determine whether NTHI OMVs are internalized by host epithelial cells via a caveola-dependent mechanism, we isolated detergent-insoluble epithelial lipid raft domains according to a protocol modified from a previously described method (47). Briefly, human pharyngeal Detroit 562 cells were grown to 85% confluence in a 25-cm2 flask and were incubated with or without 50 μg NTHI OMVs for 24 h. Following incubation, the cells were washed three times with ice-cold D-PBS and solubilized in 1 ml 1% Triton X-100 and MBS (0.25 M NaCl and 25 mM morpholineethanesulfonic acid [MES], pH 6.8) supplemented with protease inhibitor cocktail (CalBioChem, La Jolla, CA) on ice for 1 h without agitation. Cells were removed with a cell scraper, and 1 ml cell lysate suspension was adjusted to 40% OptiPrep in MBS, overlaid with 2 ml each of 35, 25, 15, and 5% OptiPrep in MBS, and centrifuged overnight at 100,000 × g. Twelve equal fractions (1 ml) were collected from the top of the gradient and were assayed for NTHI outer membrane proteins, caveolin, and clathrin heavy chain by immunoblot analysis. Subcellular fractions were separated by SDS-PAGE, transferred to nitrocellulose (Bio-Rad, Hercules, CA), and blocked in 3% skim milk. Membranes were incubated with mouse anti-caveolin (BD Biosciences Bioimaging, Rockville, MD), mouse anti-clathrin heavy chain (BD Biosciences Bioimaging, Rockville, MD), or chinchilla anti-NTHI OMP serum overnight at 4°C, washed, and incubated with goat anti-mouse IgG(H+L)–HRP (Invitrogen, Carlsbad, CA) or anti-protein A–HRP (Invitrogen, Carlsbad, CA). Membranes were washed, and peroxidase activity was detected using ECL Plus Western blotting detection reagent (GE Healthcare Life Sciences, Piscataway, NJ). OMVs alone were subjected to OptiPrep gradient centrifugation and assayed for endocytosis markers. OMVs did not cross-react with either clathrin or caveolin antibodies. Additionally, cells alone were probed for NTHI OMPs and did not display a protein signal by immunoblotting, and endocytosis markers were found in high-density fractions.
RESULTS
NTHI produces outer membrane vesicles that contain lipooligosaccharide (LOS), DNA, and virulence-associated proteins.
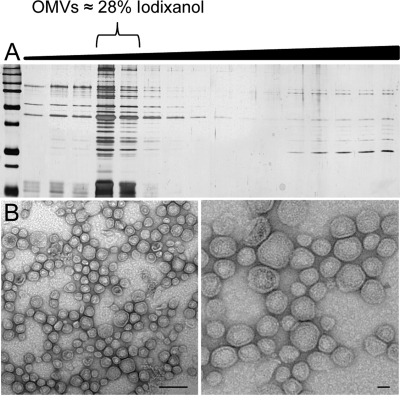
There has been little information regarding Haemophilus OMVs since their initial characterization as transformasomes 30 years ago. Given that OMV production by other Gram-negative bacteria is biologically relevant (7), we purified and characterized the production of NTHI OMVs. We determined that a clinical NTHI isolate, the prototypic strain 86-028NP, produced protein-rich OMVs from cells grown to stationary phase in rich medium. NTHI vesicle preparations were purified by density gradient purification as previously described (15). We observed that NTHI vesicles equilibrated to a density of 28% in OptiPrep solution, as determined by refractometry of gradient fractions separated by SDS-PAGE (Fig. 1A). Peak fractions containing OMVs were pooled and visualized by transmission electron microscopy. We observed spherical blebs of 20 to 200 nm in diameter which contained electron-dense luminal components and were encased in a spherical membrane (Fig. 1B).
Fig. 1.
Purification of NTHI OMVs. NTHI produces OMVs that are 20 to 200 nm in diameter and contain bacterial proteins. (A) Differential density centrifugation of NTHI OMVs. Crude vesicle preparations were layered on the bottom of an iodixanol density gradient solution and centrifuged, and equal-volume fractions were obtained from the top, low-density portion of the gradient (increases in fraction density are indicated by a black wedge). Fractions were resolved by SDS-PAGE and visualized by silver staining for OMV-associated proteins. OMVs floated to the density of a 28% iodixanol solution. LOS was observed in fractions containing OMVs. (B) Purified NTHI vesicles were placed on copper grids and stained with uranyl acetate for visualization by electron microscopy at increasing magnifications. Bar, 100 nm.
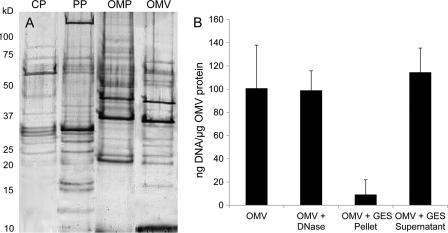
In order to characterize their composition and identify protein cargo of NTHI OMVs, we compared OMV proteins to enriched cytoplasmic, periplasmic, and outer membrane protein fractions from whole NTHI bacteria. Proteins were resolved by SDS-PAGE and visualized by silver staining (Fig. 2). OMVs contained proteins of similar size and mass to those found in the periplasm and outer membrane of NTHI, consistent with the current model of OMV biogenesis (5, 7, 26, 27, 52). NTHI OMVs contained proteins with masses of 32 or 37 kDa (P5 fimbrin) and 39 kDa (P2) that were identified as outer membrane proteins, as they were not detected in periplasmic or cytoplasmic fractions (data not shown). The outer membrane protein P5 has been shown previously to mediate NTHI pathogenicity and is currently targeted as an otitis media vaccine candidate (36). In addition to outer membrane proteins, NTHI OMVs contain lipooligosaccharide and DNA. Based on the previous results demonstrating that NTHI blebs mediate transformation, we also determined the DNA content of the purified OMVs and found that they contained approximately 100.8 ng DNA/1 μg OMV protein. DNA was packaged primarily within the vesicle lumen (94%) and was released upon vesicle lysis (Fig. 2B). Approximately 6% of the DNA, likely contained on the external surface, was susceptible to DNase. Surface-associated DNA may contribute to biofilm nucleation (43), as OMVs have been identified as a component of the biofilm matrix (13, 44, 55). Our initial characterization revealed that NTHI OMVs contain virulence-associated OMPs and DNA, yet we observed additional proteins compared to NTHI outer membrane components (Fig. 2). Protein selectivity or exclusion from NTHI OMVs may dictate their functional role and better define the mechanism of OMV formation.
Fig. 2.
OMVs package outer membrane and periplasmic proteins and contain surface and luminal DNAs. (A) The OMV protein profile was compared to NTHI strain 86-028NP cytoplasmic (CP), periplasmic (PP), and outer membrane (OMP) proteins isolated from whole cells. Protein preparations were separated by SDS-PAGE and visualized by silver staining. (B) Surface-associated and luminal DNAs were quantified for OMVs, OMVs treated with DNase, and the OMV pellet and supernatant following incubation with GES lysis reagent. Error bars represent the standard errors of the means for three independent assays repeated in triplicate (n = 3).
To further define the proteomic profile of NTHI OMVs, vesicles were subjected to tryptic digestion and mass spectrometry. Proteomic analysis identified 142 Haemophilus proteins that were packaged in NTHI OMVs, including mainly periplasmic and outer membrane proteins (Table 1; see Table S1 in the supplemental material). Proteins found in vesicles from other bacterial species have been discovered to exhibit a wide range of functions. Concordant with these observations, the vast array of proteins found in NTHI OMVs includes ABC transporters, adhesins, virulence factors, and proteins known to contribute to bacterial survival. Therefore, NTHI OMVs contain not only DNA and LOS but also a vast array of proteins that could contribute to their physiological role in the host.
Table 1.
NTHI 86-028NP OMV proteins identified by LC-MS/MS
| Identified protein (over 95% confidence) | Gene no. | GenBank accession no. | Molecular mass (kDa) | No. of protein hitsa |
|---|---|---|---|---|
| Outer membrane protein P2 precursor | NTHI0225 | 68056946 | 40 | 135 |
| Outer membrane protein P5 | NTHI1332 | 68057911 | 38 | 46 |
| Probable amino acid ABC transporter binding protein | NTHI1243 | 68057836 | 28 | 34 |
| HMW1B (OMP-85-like protein required for secretion of HMW1A and HMW2A) | NTHI1984 | 68058015 | 61 | 34 |
| HMW2B (OMP-85-like protein required for HMW1A and HMW2A secretion) | NTHI1449 | 68058015 | 61 | 34 |
| IgA-specific serine endopeptidase | NTHI1164 | 68057774 | 197 | 32 |
| Outer membrane protein P1 precursor | NTHI0522 | 68057203 | 50 | 30 |
| Probable d-methionine-binding lipoprotein MetQ | NTHI0877 | 68057518 | 30 | 27 |
| NAD nucleotidase | NTHI0303 | 68057010 | 66 | 26 |
| Heme-binding protein A | NTHI1021 | 68057646 | 61 | 26 |
| Spermidine/putrescine-binding periplasmic protein 1 precursor | NTHI1823 | 68058340 | 43 | 26 |
| Probable periplasmic serine protease do/HhoA-like precursor | NTHI1905 | 68058410 | 49 | 25 |
| Heme utilization protein | NTHI1390 | 68057962 | 103 | 25 |
| l-Lactate dehydrogenase | NTHI2049 | 68058536 | 42 | 25 |
| Hemoglobin-haptoglobin binding protein B | NTHI0782 | 68057439 | 114 | 24 |
| Putative periplasmic chelated iron binding protein | NTHI0481 | 68057164 | 32 | 23 |
| 2′,3′-Cyclic-nucleotide 2′-phosphodiesterase | NTHI0741 | 68057402 | 73 | 22 |
| Periplasmic oligopeptide-binding protein | NTHI1292 | 68057874 | 61 | 22 |
| Phosphate-binding periplasmic protein precursor PstS | NTHI1774 | 68058299 | 37 | 18 |
| Elongation factor Tu | NTHI0712 | 68057378 | 43 | 17 |
| Putative lipoprotein | NTHI1957 | 68058456 | 63 | 16 |
| Protective surface antigen D15 | NTHI1084 | 68057702 | 88 | 16 |
| Iron utilization periplasmic protein hFbpA | NTHI0177 | 68056907 | 36 | 15 |
| Glycerol-3-phosphate transporter | NTHI0809 | 68057464 | 53 | 15 |
| TRAP-type C4-dicarboxylate transport system, periplasmic component | NTHI0232 | 68056953 | 36 | 14 |
| HMW1A (high-molecular-weight adhesin 1) | NTHI1983 | 68058480 | 154 | 14 |
| d-Galactose-binding periplasmic protein precursor | NTHI0987 | 68057616 | 38 | 10 |
| Conserved hypothetical protein | NTHI1208 | 68057809 | 41 | 10 |
| TolB | NTHI0502 | 68057184 | 45 | 10 |
| Conserved hypothetical lipoprotein | NTHI0266 | 68056981 | 29 | 10 |
| Glycerophosphoryl diester phosphodiesterase precursor | NTHI0811 | 68057465 | 42 | 10 |
Purified OMVs isolated from 86-028NP were analyzed using LC-MS/MS and were matched with the NCBI H. influenzae 86-028NP database. Listed proteins include those with 10 or more protein hits, all of which have over 95% confidence. A full list of identified proteins (142) can be found in Table S1 in the supplemental material.
Outer membrane vesicles stimulate production of the immunomodulatory cytokine IL-8 and the antimicrobial propeptide hCAP18/LL-37.
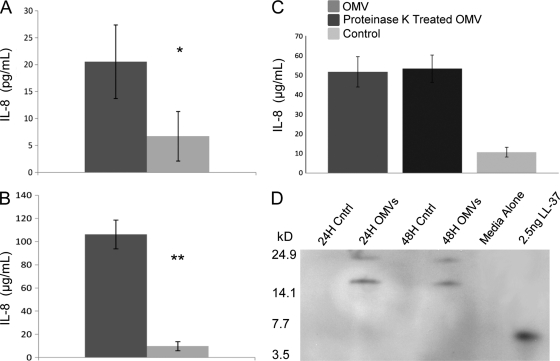
Since NTHI OMVs contain LOS and package proteins previously shown to contribute to NTHI pathogenesis, we exposed host epithelial cells to purified NTHI OMVs and assessed secretion of IL-8, an immunomodulatory cytokine. Human pharynx cells and primary chinchilla middle ear epithelial cells exposed to NTHI OMVs secreted significantly more IL-8 than cells exposed to medium alone (Fig. 3). In order to determine the contribution of OMV surface proteins to IL-8 stimulation and release, epithelial cells were exposed to OMVs pretreated with proteinase K to degrade surface-exposed proteins (see Fig. S1 in the supplemental material). We determined that proteinase K-treated OMVs stimulated the release of similar amounts of IL-8 to those for nontreated vesicles (Fig. 3C). These data suggest that OMV LOS and luminal proteins, and possibly other vesicle-associated molecules, such as DNA, contribute to IL-8 release from host cells (42). We hypothesize that LOS is the key modulator of cytokine stimulation in host cells, however, since proteinase K treatment did not alter the ability of vesicles to interact with and stimulate host epithelial cells. Furthermore, the majority of the DNA was contained within the vesicle lumen, not on the vesicle surface, and OMV failed to stimulate increased expression of TLR-9 (data not shown), suggesting that DNA is not a significant contributor to modulation of host cell responses. These data are consistent with the recent observation that DNA does not contribute significantly to the inflammatory response to vesicles (8).
Fig. 3.
NTHI OMVs stimulate IL-8 production and hCAP18/LL-37 release. Primary CMEE cells (A) and Detroit 562 human pharyngeal cells (B) release significant amounts of IL-8 upon OMV exposure compared to cells incubated in the absence of OMVs (medium alone). Data represent three independent experiments (n = 3) performed in triplicate. P values were determined by paired Student's t test. *, P ≤ 0.0153; **, P ≤ 0.0001. (C) Proteinase K treatment does not diminish OMV-induced IL-8 release. Detroit 562 cells release IL-8 in response to both OMVs and proteinase K-treated OMVs. Data represent one experiment performed in triplicate. (D) hCAP18/LL-37 propeptide (18 kDa) is released from epithelial cells in response to OMV exposure for 24 and 48 h. The cleaved product peptide, LL-37, is approximately 4.7 kDa.
Our observation of IL-8 elicitation led us to investigate the role of OMVs in stimulation of other host innate immune responses, such as antimicrobial peptides (AMPs). AMPs are small, cationic molecules that target and disrupt bacterial membranes. NTHI OMVs were found to induce the production of the antimicrobial propeptide hCAP18/LL-37 by host epithelial cells. We exposed human pharyngeal cells to purified NTHI OMVs and assessed LL-37 secretion by immunoblotting (Fig. 3D). We observed the presence of a protein with an apparent molecular mass of 18 kDa by immunoblotting after both 24 and 48 h of OMV exposure. This protein is consistent with the propeptide form of LL-37 that is stored in epithelial cells, released, and activated upon cleavage by an extracellular protease (46, 56). These data suggest that OMVs are capable of stimulating host cells to release host defense peptides and, taken together, demonstrate that NTHI OMVs elicit a multifaceted immunomodulatory response from host epithelial cells.
NTHI vesicles bind to and are internalized by epithelial host cells.
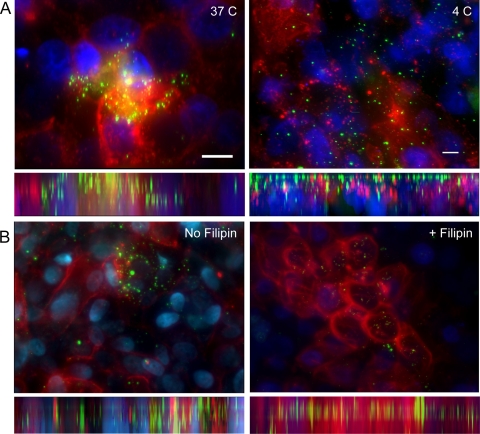
The ability of NTHI OMVs to stimulate production and release of the immunomodulatory cytokine IL-8 suggests direct interaction with the host cell. In order to further elucidate the mechanism of OMV-host cell interaction, we exposed human pharyngeal cells to Alexa Fluor-labeled vesicles and visualized the OMV-host cell interaction by fluorescence microscopy (Fig. 4). Rendered three-dimensional reconstructions of images revealed that OMVs bind to and interact with the host cell surface (Fig. 4, left panels). OMVs were observed to bind in a time-dependent manner, with OMV aggregates forming after as little as 30 min of exposure. OMVs remained surface associated with the epithelial cells after a series of washes, indicating an intimate interaction between host cells and NTHI OMVs. Additionally, host cells appeared to internalize OMVs, and OMV-associated fluorescence was observed throughout the cell layer (Fig. 4, left panels, side view).
Fig. 4.
NTHI vesicles interact with host cells and are internalized. Alexa Fluor 488-labeled OMVs (1 μg; green) were incubated with Detroit 562 cells for 2 h at 37°C, washed, and monitored for interaction with host epithelial cells. Cell membranes were labeled with wheat germ agglutinin (red; Molecular Probes), and cell nuclei (blue) were stained with Hoechst dye (Molecular Probes). All images are 3-dimensional renderings of planar optical sections with top-down views (above) and orthogonal side views (below). (A) Temperature-dependent uptake of OMVs by epithelial host cells. Detroit 562 human pharyngeal cells were exposed to labeled vesicles and incubated at 37°C and 4°C. Bar, 10 μm. (B) Detroit 562 cells were pretreated with filipin, an inhibitor of cholesterol recycling, and then exposed to labeled OMVs in the presence of filipin for 4 h. In parallel, cells were exposed to OMVs in the absence of filipin treatment and incubated for 4 h at 37°C. Images are representative of three independent experiments. There was a statistically significant difference (P < 0.0258) between the sizes of the vesicles in the filipin-treated group and the no-filipin group.
To confirm this observation, epithelial cells were incubated at 37°C or 4°C prior to and during exposure to labeled OMVs. Three-dimensional renderings revealed internalization in cells maintained at 37°C, represented by a robust OMV-associated signal throughout the epithelial cells (Fig. 4A, 37°C panel). In contrast, when cells were incubated at 4°C, OMVs were associated primarily with the cell surface and OMV-associated fluorescence did not appear throughout the cell layer, indicating a decreased ability of host cells to internalize vesicles (Fig. 4A, 4°C panel). These data suggest that NTHI outer membrane vesicles directly bind to, interact with, and are internalized by host epithelial cells in a temperature-dependent manner.
NTHI OMVs are internalized into epithelial cells via a caveola-dependent mechanism.
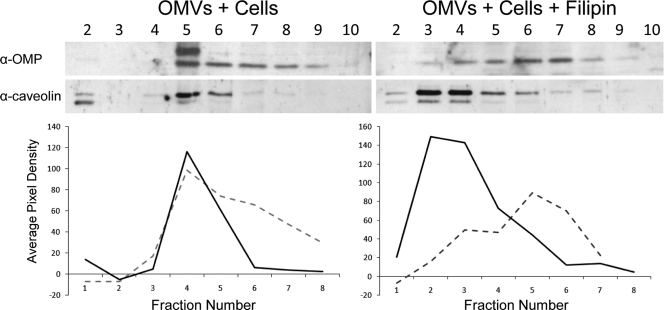
Host cells have been shown to internalize bacterial OMVs through cholesterol-enriched lipid rafts or clathrin-mediated endocytosis (10, 20, 38). To assess the mechanism of NTHI OMV internalization, human pharyngeal cells were incubated with NTHI OMVs for 24 h, lysed, and separated into subcellular fractions by density gradient centrifugation as previously described (47). In this manner, we determined whether vesicle-associated proteins would cofractionate with either caveolin or clathrin, protein components of caveola- and clathrin-dependent endocytosis mechanisms, respectively. We demonstrated that OMVs cofractionated with caveolin protein and not with clathrin, as determined by immunoblot (Fig. 5). In parallel gradients, vesicles alone did not equilibrate to the same density fraction as either endocytosis marker, nor did OMVs cofractionate with clathrin (see Fig. S2 in the supplemental material). Collectively, these data indicate that cell-associated OMVs colocalize with caveolin protein in the host cell, suggesting internalization via a caveola-dependent mechanism. In order to confirm this observation, we exposed host epithelial cells to OMVs in the presence or absence of filipin, an inhibitor of cholesterol biosynthesis which diminishes recycling of caveolae and cholesterol- and caveolin-enriched lipid rafts. We observed that filipin disrupted OMV colocalization with caveolin protein, as indicated by a shift in the density fraction localization of both OMPs and caveolin (Fig. 5). The shift in colocalization is represented graphically by measurement of the average pixel density of the immunoblots in relation to the background signal (graphs in Fig. 5). Additionally, internalization was not observed by fluorescence microscopy of filipin-treated cells exposed to OMVs (Fig. 4B, + Filipin panel). We monitored differences in vesicle size between the two groups as a measure of aggregation and internalization and calculated whether inhibition of caveola formation resulted in the diminished OMV aggregation. We demonstrated that there was a statistically significant reduction in overall vesicle size in filipin-treated cells compared to cells not exposed to the inhibitor (P < 0.0258). Collectively, these data support the hypothesis that host cells internalize NTHI OMVs by using a caveola-dependent mechanism.
Fig. 5.
NTHI OMVs are internalized via caveolae. Disruption of caveolae alters OMV association and uptake by epithelial cells. OMVs cofractionated with caveolin following exposure to Detroit 562 pharyngeal cells for 24 h at 37°C. The addition of filipin inhibited colocalization of OMV proteins and caveolin. Signal intensities of caveolin and OMV fractions were determined by densitometry (average pixel density relative to background signal). Dashed lines, OMV signals; solid lines, caveolin signals.
DISCUSSION
Recent reports that describe the production, characterization, and functional roles of OMVs in bacterial virulence and their immunomodulatory effects on host cells have propelled an interest in further defining the contributions of OMVs to commensal and pathogenic bacterial behaviors (7). Although all Gram-negative organisms studied to date produce OMVs, there have been no structural or functional studies of OMVs released from clinically relevant NTHI strains. Thus, we investigated NTHI OMV release, protein composition, and modulation of host cell activity. NTHI OMVs contain proteins known to mediate NTHI virulence, elicit a potent response from epithelial cells, and are poised to play a significant role in NTHI infection and pathogenesis.
We identified 142 Haemophilus proteins packaged within these membranous structures, including virulence-associated outer membrane proteins, adhesins, endopeptidases, and nutrient utilization proteins (Table 1; see Table S1 in the supplemental material). This proteomic profile characterizes the distribution of these proteins outside the bacterial cell by vesicle secretion. Gradient-purified NTHI OMVs contained primarily outer membrane and periplasmic components and were mostly devoid of cytoplasm-associated proteins, in support of current models of OMV biogenesis (5, 7, 27).
The contribution of NTHI OMV pathogenesis remains undefined, yet the presence of NTHI adhesins and proteins essential for NTHI survival may mediate biologically important functional roles. In fact, it has been well characterized that the outer membrane protein P5 (OMP P5), contained in OMVs, contributes to NTHI pathogenesis, and it is currently designated an attractive vaccine candidate for NTHI-mediated diseases (36). Interestingly, OMP P5 contains a “decoy” epitope that elicits a robust immune response which targets a surface-exposed loop of the P5 protein, yet this response does not protect from NTHI-mediated disease (37). The contribution of vesicle-associated P5 to this decoy response is currently unknown. The lack of other periplasmic binding proteins, such as SapA (28) and HitA (1), and of the outer membrane protein OMP P6 (32) may indicate selectivity of protein incorporation in vesicle biogenesis or an impact of environmental stressors on selective protein packaging.
The diverse array of protein cargo led us to investigate how NTHI OMVs interact with host cells. Using fluorescence microscopy, we observed vesicle interaction and internalization following exposure to host epithelial cells. We noted a decrease in surface-associated vesicles over time, presumably due to OMV internalization. This intimate vesicle-host cell interaction, characterized as vesicle internalization, was disrupted when cells were cultured at 4°C and also when cells were treated with filipin, suggesting that OMV internalization was mediated via caveolin-enriched endocytic uptake. Our observations are consistent with studies of OMV internalization in enterotoxigenic Escherichia coli (ETEC), Pseudomonas, and Moraxella species (8, 20, 42), all of which produce OMVs that are internalized via a caveola-dependent mechanism.
Vesicles interact dynamically with host cells and elicit a robust cellular inflammatory response which includes IL-6, IL-8, IL-12, NF-κB, and gamma interferon (IFN-γ) (49). Specifically, OMVs produced by Pseudomonas and ETEC have been shown to induce the immunomodulatory cytokine IL-8 (2, 8). We demonstrated that NTHI OMVs elicit release of significant amounts of IL-8 by human pharynx and primary chinchilla middle ear epithelial cells. IL-8 release was not dependent on surface proteins, as proteinase K treatment, which removed over 30% of total OMV protein, failed to significantly decrease the amount of IL-8 released by host cells (Fig. 3; see Fig. S1 in the supplemental material). The induction of IL-8 release by OMVs may be in response to other OMV components, such as LOS, luminal proteins, or DNA, as previously shown with Moraxella catarrhalis (42).
Yet why secrete OMVs that elicit an immunomodulatory cytokine release by the host epithelium? It is possible that OMVs act as a decoy, directing the immune response, in a nonspecific manner, away from the whole bacterium, thus providing opportunity for the commensal bacterium to establish colonization without intense bombardment from the immune system. M. catarrhalis OMVs act as a decoy to the immune system to enable the whole bacterium to evade immune detection (50). A redirected immune response may benefit NTHI survival and growth in vivo, as host inflammation serves to increase the availability of essential nutrients and thus provides a selective advantage for NTHI colonization. For instance, the inflammatory release of the iron-containing compound heme into the local environment would benefit the growth of NTHI, a heme auxotroph (25).
The role of OMVs is not limited to the stimulation of inflammation. The LOS and DNA that are present in OMVs are major components of bacterial biofilms (13, 16, 55), suggesting that OMVs may play a role in biofilm nucleation and maintenance. NTHI produces OMVs in vivo which have been observed in biofilms (14). Furthermore, vesicles are able to coaggregate bacteria, enabling colonization and biofilm formation (11, 24, 29, 43, 53). Taking the data together, we hypothesize that NTHI OMVs play a similar role in the establishment, architecture, and structural stability of NTHI biofilms. We have shown here that NTHI OMVs contain both LOS and DNA, integral components of the biofilm matrix and thus biofilm formation, a critical factor of NTHI pathogenesis (30).
Furthermore, NTHI OMVs stimulate the release of the host innate immune component LL-37, an AMP that is secreted from host epithelium in response to bacterial colonization (Fig. 3D) (45). We propose that vesiculation may provide a mechanism to dampen the host innate immune response to thus benefit NTHI evasion of host immunity. We are currently investigating whether AMP exposure will alter release of NTHI OMVs, a likely consequence of AMP-mediated stress on bacterial membranes.
Future studies will consider the many potential biological consequences of Haemophilus outer membrane vesicle release. These membranous blebs are complex packages of virulence proteins, DNA, and LOS, leading to abundant and diverse functional possibilities in the host. While we now know that OMVs interact with and are internalized by host cells, the breadth of their impact on the life and disease states of NTHI in the host remains unknown. We hypothesize that NTHI vesiculation contributes to numerous critical functions of NTHI pathogenesis, such as host interaction, innate immune resistance, and biofilm maintenance. It will be important to further define the contribution of OMVs and their interactions with the host to unravel their role in pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sheryl Justice for expertise and assistance with microscopy and image analysis and for a critical review of the manuscript. We also thank Daniel Rodriguez for assistance with preparing the samples for proteomic analysis.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 29 August 2011.
REFERENCES
- 1. Adhikari P., et al. 1995. Biochemical characterization of a Haemophilus influenzae periplasmic iron transport operon. J. Biol. Chem. 270:25142–25149 [DOI] [PubMed] [Google Scholar]
- 2. Bauman S. J., Kuehn M. J. 2006. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 8:2400–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beveridge T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bomberger J. M., et al. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deatherage B. L., et al. 2009. Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 72:1395–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deich R. A., Hoyer L. C. 1982. Generation and release of DNA-binding vesicles by Haemophilus influenzae during induction and loss of competence. J. Bacteriol. 152:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellis T. N., Kuehn M. J. 2010. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellis T. N., Leiman S. A., Kuehn M. J. 2010. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 78:3822–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filip C., Fletcher G., Wulff J. L., Earhart C. F. 1973. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 115:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furuta N., et al. 2009. Entry of Porphyromonas gingivalis outer membrane vesicles into human epithelial cells causes cellular functional impairment. Infect. Immun. 77:4761–4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grenier D., Mayrand D. 1987. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect. Immun. 55:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison A., et al. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 187:4627–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong W., et al. 2007. Phosphorylcholine decreases early inflammation and promotes the establishment of stable biofilm communities of nontypeable Haemophilus influenzae strain 86-028NP in a chinchilla model of otitis media. Infect. Immun. 75:958–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hong W., Pang B., West-Barnette S., Swords W. E. 2007. Phosphorylcholine expression by nontypeable Haemophilus influenzae correlates with maturation of biofilm communities in vitro and in vivo. J. Bacteriol. 189:8300–8307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horstman A. L., Kuehn M. J. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 275:12489–12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jurcisek J., et al. 2005. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect. Immun. 73:3210–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kadurugamuwa J. L., Beveridge T. J. 1997. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J. Antimicrob. Chemother. 40:615–621 [DOI] [PubMed] [Google Scholar]
- 18. Kahn M. E., Barany F., Smith H. O. 1983. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. Proc. Natl. Acad. Sci. U. S. A. 80:6927–6931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kesty N. C., Kuehn M. J. 2004. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 279:2069–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kesty N. C., Mason K. M., Reedy M., Miller S. E., Kuehn M. J. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23:4538–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kilpi T., Herva E., Kaijalainen T., Syrjanen R., Takala A. K. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654–662 [DOI] [PubMed] [Google Scholar]
- 22. Klein J. O. 1997. Role of nontypeable Haemophilus influenzae in pediatric respiratory tract infections. Pediatr. Infect. Dis. J. 16:S5–S8 [DOI] [PubMed] [Google Scholar]
- 23. Kulp A., Kuehn M. J. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64:163–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loeb M. R. 1974. Bacteriophage T4-mediated release of envelope components from Escherichia coli. J. Virol. 13:631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marx J. J. 2002. Iron and infection: competition between host and microbes for a precious element. Best Pract. Res. Clin. Haematol. 15:411–426 [PubMed] [Google Scholar]
- 26. Mashburn L. M., Whiteley M. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422–425 [DOI] [PubMed] [Google Scholar]
- 27. Mashburn-Warren L. M., Whiteley M. 2006. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61:839–846 [DOI] [PubMed] [Google Scholar]
- 28. Mason K. M., Bruggeman M. E., Munson R. S., Bakaletz L. O. 2006. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol. Microbiol. 62:1357–1372 [DOI] [PubMed] [Google Scholar]
- 29. McBroom A. J., Kuehn M. J. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63:545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moxon E. R., Sweetman W. A., Deadman M. E., Ferguson D. J., Hood D. W. 2008. Haemophilus influenzae biofilms: hypothesis or fact? Trends Microbiol. 16:95–100 [DOI] [PubMed] [Google Scholar]
- 31. Munson R. S., Jr., et al. 2004. Partial analysis of the genomes of two nontypeable Haemophilus influenzae otitis media isolates. Infect. Immun. 72:3002–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy T. F. 2003. Respiratory infections caused by non-typeable Haemophilus influenzae. Curr. Opin. Infect. Dis. 16:129–134 [DOI] [PubMed] [Google Scholar]
- 33. Nakamura A., DeMaria T. F., Lim D. J., van Blitterswijk C. A. 1991. Primary culture of chinchilla middle ear epithelium. Ann. Otol. Rhinol. Laryngol. 100:774–782 [DOI] [PubMed] [Google Scholar]
- 34. Nakamura S., et al. 2008. The roles of the quorum-sensing system in the release of extracellular DNA, lipopolysaccharide, and membrane vesicles from Pseudomonas aeruginosa. Jpn. J. Infect. Dis. 61:375–378 [PubMed] [Google Scholar]
- 35. Nizet V., Colina K. F., Almquist J. R., Rubens C. E., Smith A. L. 1996. A virulent nonencapsulated Haemophilus influenzae. J. Infect. Dis. 173:180–186 [DOI] [PubMed] [Google Scholar]
- 36. Novotny L. A., et al. 2009. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine 28:279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Novotny L. A., et al. 2002. Detection and characterization of pediatric serum antibody to the OMP P5-homologous adhesin of nontypeable Haemophilus influenzae during acute otitis media. Vaccine 20:3590–3597 [DOI] [PubMed] [Google Scholar]
- 38. Parker H., Chitcholtan K., Hampton M. B., Keenan J. I. 2010. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infect. Immun. 78:5054–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Renelli M., Matias V., Lo R. Y., Beveridge T. J. 2004. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology (Reading, England) 150:2161–2169 [DOI] [PubMed] [Google Scholar]
- 40. Resman F., et al. 2010. Invasive disease caused by Haemophilus influenzae in Sweden 1997–2009; evidence of increasing incidence and clinical burden of non-type b strains. Clin. Microbiol. Infect. doi:10.1111/j.1469-0691.2010.03417.x [DOI] [PubMed] [Google Scholar]
- 41. Roman F., Canton R., Perez-Vazquez M., Baquero F., Campos J. 2004. Dynamics of long-term colonization of respiratory tract by Haemophilus influenzae in cystic fibrosis patients shows a marked increase in hypermutable strains. J. Clin. Microbiol. 42:1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schaar V., et al. 2011. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell. Microbiol. 13:432–449 [DOI] [PubMed] [Google Scholar]
- 43. Schooling S. R., Beveridge T. J. 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188:5945–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schooling S. R., Hubley A., Beveridge T. J. 2009. Interactions of DNA with biofilm-derived membrane vesicles. J. Bacteriol. 191:4097–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schroder J. M. 1999. Epithelial antimicrobial peptides: innate local host response elements. Cell. Mol. Life Sci. 56:32–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi J., Ganz T. 1998. The role of protegrins and other elastase-activated polypeptides in the bactericidal properties of porcine inflammatory fluids. Infect. Immun. 66:3611–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shin J. S., Gao Z., Abraham S. N. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785–788 [DOI] [PubMed] [Google Scholar]
- 48. St. Geme J. W., III 2000. The pathogenesis of nontypable Haemophilus influenzae otitis media. Vaccine 19(Suppl. 1):S41–S50 [DOI] [PubMed] [Google Scholar]
- 49. Unal C. M., Schaar V., Riesbeck K. 2010. Bacterial outer membrane vesicles in disease and preventive medicine. Semin. Immunopathol. 33:395–408 [DOI] [PubMed] [Google Scholar]
- 50. Vidakovics M. L., et al. 2010. B cell activation by outer membrane vesicles—a novel virulence mechanism. PLoS Pathog. 6:e1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wai S. N., et al. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25–35 [DOI] [PubMed] [Google Scholar]
- 52. Wensink J., Witholt B. 1981. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur. J. Biochem. 116:331–335 [DOI] [PubMed] [Google Scholar]
- 53. Whitchurch C. B., Tolker-Nielsen T., Ragas P. C., Mattick J. S. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]
- 54. Wispelwey B., Hansen E. J., Scheld W. M. 1989. Haemophilus influenzae outer membrane vesicle-induced blood-brain barrier permeability during experimental meningitis. Infect. Immun. 57:2559–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yonezawa H., et al. 2009. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 9:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zanetti M., Litteri L., Gennaro R., Horstmann H., Romeo D. 1990. Bactenecins, defense polypeptides of bovine neutrophils, are generated from precursor molecules stored in the large granules. J. Cell Biol. 111:1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.