Abstract
One of the solutions for reducing the global mortality and morbidity due to malaria is multivalent vaccines comprising antigens of several life cycle stages of the malarial parasite. Hence, there is a need for supplementing the current set of malaria vaccine candidate antigens. Here, we aimed to characterize glycosylphosphatidylinositol (GPI)-anchored micronemal antigen (GAMA) encoded by the PF08_0008 gene in Plasmodium falciparum. Antibodies were raised against recombinant GAMA synthesized by using a wheat germ cell-free system. Immunoelectron microscopy demonstrated for the first time that GAMA is a microneme protein of the merozoite. Erythrocyte binding assays revealed that GAMA possesses an erythrocyte binding epitope in the C-terminal region and it binds a nonsialylated protein receptor on human erythrocytes. Growth inhibition assays revealed that anti-GAMA antibodies can inhibit P. falciparum invasion in a dose-dependent manner and GAMA plays a role in the sialic acid (SA)-independent invasion pathway. Anti-GAMA antibodies in combination with anti-erythrocyte binding antigen 175 exhibited a significantly higher level of invasion inhibition, supporting the rationale that targeting of both SA-dependent and SA-independent ligands/pathways is better than targeting either of them alone. Human sera collected from areas of malaria endemicity in Mali and Thailand recognized GAMA. Since GAMA in P. falciparum is refractory to gene knockout attempts, it is essential to parasite invasion. Overall, our study indicates that GAMA is a novel blood-stage vaccine candidate antigen.
INTRODUCTION
Plasmodium falciparum is the causative agent of the most burdensome form of human malaria, affecting about 225 million individuals and killing about 0.8 million individuals in 2009 worldwide (37). The reemergence of drug-resistant parasites and insecticide-resistant mosquitoes aggravates the spread of malaria (19). The complex biology, extensive antigenic diversity, and immune evasion strategies of P. falciparum enable it to cause repeated and chronic infections. However, naturally acquired immunity to malaria does develop after repeated exposure (27), and several lines of evidence support the feasibility of vaccines to protect against malaria (16). The scope and expectation for malaria vaccine development have expanded dramatically in recent years, in large part due to the renewed focus on control, local elimination, and eventual global eradication efforts (3). However, despite intensive efforts, no malaria vaccine has yet been licensed, and there is an urgency to rapidly enrich the pipeline of vaccine development with novel vaccine candidates. The availability of the P. falciparum genome sequence, along with its transcription and proteomic profiles and insights, has provided great opportunities to identify new candidates for development into vaccines (15).
Highly efficacious malaria vaccines will certainly need to be multicomponent vaccines that comprise several different alleles of an antigen and/or several different antigens and/or comprise antigens of several life cycle stages to overcome the antigenic diversity and immune evasion capacity of P. falciparum and, hence, provide broad and sustained protection. This provides a strong rationale for developing blood-stage vaccines as part of the strategy (27). Although an increasing number of merozoite antigens are being identified, few antigens have been evaluated as vaccine candidates or as targets of immunity (14, 27). Therefore, we were interested in identifying novel blood-stage vaccine candidate antigens.
In order to find novel blood-stage vaccine candidates, basic research on the molecular basis of invasion and subsequent modification of the host cell is indispensable. The invasion-related merozoite proteins are either located on the merozoite surface (mostly via glycosylphosphatidylinositol [GPI] anchors) or stored initially in apical organelles (i.e., micronemes, rhoptries, and dense granules) and later translocated onto the surface of the invading parasite. Since these proteins are eventually exposed to the human immune system, they are leading blood-stage vaccine candidate antigens (18, 20). For instance, merozoite surface proteins 1 and 2 (MSP1 and MSP2, respectively) and the micronemal protein apical membrane antigen 1 (AMA1) have been explored as blood-stage vaccine candidates (27) and as targets of acquired human immunity (14).
Therefore, this study was taken up with the objective of identifying previously uncharacterized P. falciparum proteins that are targeted to either apical organelles or the parasite surface and assess them as novel blood-stage vaccine candidates. For this purpose, we used P. falciparum genome (15), transcriptome (4), and proteome (13) data as a starting point and screened the proteins in this data set based on four features: (i) late-schizont stage transcription, (ii) smaller gene size (<2.5 kbp), (iii) presence of predicted signal peptide (SP), and (iv) putative GPI anchor attachment site. Our bioinformatics searches identified PF08_0008 as a novel putative surface and/or apical protein. Previous bioinformatics searches by Haase et al. (using transcriptional and structural features) (20) and Gilson et al. (using their GPI anchor site prediction software trained on P. falciparum sequences) (18) have also predicted that PF08_0008 may be an invasion-related, surface or apical organellar, merozoite antigen. Recently, Hinds et al. (21) have experimentally shown that PF08_0008 is a novel GPI-anchored erythrocyte binding protein that appears to be localized in the apical organelle of P. falciparum merozoites and, hence, designated the protein GPI-anchored micronemal antigen (GAMA). However, antibodies (Abs) raised against recombinant GAMA expressed in Escherichia coli were not inhibitory to invasion or growth of the parasite, and therefore, the role of GAMA as a vaccine candidate antigen is unclear (21). In our previous studies (32, 34, 35), we have demonstrated that the wheat germ cell-free system is an optimal system for the synthesis of correctly folded recombinant malaria proteins in sufficient quantities. Therefore, in this study, we attempted to test our hypothesis that GAMA may be a vaccine candidate by using recombinant GAMA expressed in the wheat germ cell-free system and further define its subcellular localization by immunoelectron microscopy (IEM) and characterize its erythrocyte binding region and its receptor on the erythrocyte membrane.
MATERIALS AND METHODS
Parasite culture and culture supernatant.
P. falciparum asexual stages (3D7 strain) were cultured in vitro in human erythrocytes (blood group O+ or A+) obtained from the Japanese Red Cross Society as previously described (6). To harvest parasite pellets, mature schizonts were purified by using Percoll (GE Healthcare, Camarillo, CA) density gradient centrifugation and further treated with tetanolysin, washed with phosphate-buffered saline (PBS) containing Complete protease inhibitor (Roche, Mannheim, Germany), and stored at −80°C until used. For culture supernatant preparation, tightly synchronized, purified schizonts were cultured for 20 h at 37°C in the absence of erythrocytes for rupture and merozoite release. The culture medium was centrifuged at 3,000 × g for 20 min at 4°C to remove cellular debris, and subsequently, the supernatant was concentrated 5-fold in a centrifugal filter (Amicon Ultra 10K device; Millipore, Billerica, MA) and stored in aliquots at −80°C until used.
RNA isolation and cDNA synthesis.
Total RNA was isolated from 3D7 parasite-infected erythrocytes rich in schizonts by using the RNeasy minikit (Qiagen, Hilden, Germany) and stored at −80°C. Following DNase treatment, cDNA was generated with random hexamers by using an Omniscript reverse transcription kit (Qiagen).
Production of recombinant PF08_0008 proteins and antisera.
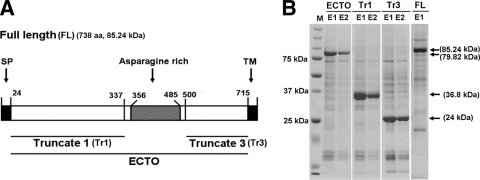
The nucleotide sequence of the PF08_0008 gene was obtained from the P. falciparum 3D7 genome database (http://plasmodb.org). Full-length and different truncated versions of PF08_0008 proteins were synthesized and used for raising antibodies (see Fig. 1). Briefly, the PF08_0008 fragments encoding constructs designated FL (full-length GAMA, comprising amino acid [aa] 1 to aa 738), ECTO (full-length GAMA without signal peptide and transmembrane [TM] regions and comprising aa 25 to aa 714 and a hexahistidine [His] tag at the C terminus), Tr1 (truncated protein 1; comprising aa 25 to aa 337 and a His tag at the C terminus), and Tr3 (truncated protein 3; comprising aa 500 to aa 714 and a His tag at the C terminus) were amplified by using sense primers with XhoI sites and antisense primers with NotI restriction sites (shown in lowercase letters in the primer sequences below), by PCR from P. falciparum 3D7 cDNA. The primer pairs FLf (5′-ctcgagATGAAATATTATACATCTTTGTACGTTGC-3′) and FLr (5′-gcggccgcCTAATTTAACAAGTTAATTAAAATGAACGAAAAAAAAAGG-3′), ECTOf (5′-ctcgagaTGAATTCGAACACTCCTCAGGCCTTC-3′) and ECTOr (5′-gcggccgcCTAATGATGATGATGATGGTGTGCCTTTGCATTTGGTCCTTGAAAAG-3′), Tr1f (5′-ctcgagATGAATTCGAACACTCCTCAGGCCTTC-3′) and Tr1r (5′-gcggccgcCTAATGATGATGATGATGGTGATGCTTATATGCATTTAGTTTATTAAGTATATC-3′), and Tr3f (5′-ctcgagATGAAGGATATTATAAAATTATTAAAAGATTTAATAAAATATTTAC-3′) and Tr3r (5′-gcggccgcCTAATGATGATGATGATGGTGTGCCTTTGCATTTGGTCCTTGAAAAG-3′) were used to generate the DNA fragments encoding FL, ECTO, Tr1, and Tr3 proteins, respectively. The underlined sequences in the primers above indicate the regions that encode His tags.
Fig. 1.
Structure and recombinant proteins of GAMA. (A) Schematic of the primary structure of GAMA. The GAMA protein consists of 738 amino acids, with a calculated molecular mass of 85.24 kDa. Indicated are the predicted signal peptide (SP; residues 1 to 24), asparagine-rich region (residues 356 to 485), and C-terminal transmembrane domain (TM; residues 715 to 738). FL GAMA (residues 1 to 738) and three regions of GAMA (Tr1 [residues 25 to 337], Tr3 [residues 500 to 714], and ECTO [residues 25 to 714]) were expressed in recombinant form and used to raise specific antisera. (B) SDS-PAGE of recombinant proteins of GAMA. All the recombinant proteins were synthesized using a wheat germ cell-free protein expression system. The fractions of purified ECTO (79.82 kDa), Tr1 (36.8 kDa), Tr3 (24 kDa), and FL (85.24 kDa) proteins resolved in an SDS-PAGE gel and stained with Coomassie brilliant blue R-250 are shown. M represents a molecular weight marker. E1 and E2 represent the first and the second fractions of purified proteins eluted from affinity purification columns, respectively. Arrows indicate specific bands.
The amplified fragments were then restricted and ligated into the wheat germ cell-free expression vectors (CellFree Sciences, Matsuyama, Japan). Fragments of ECTO, Tr1, and Tr3 were cloned into the pEU-E01-MCS vector. Fragments of FL were cloned into the pEU-E01-GST-TEV-N2 vector. The cloned inserts were sequenced by using an ABI PRISM 3100-Avant genetic analyzer (Applied Biosystems, Foster City, CA). The recombinant proteins with either glutathione S-transferase (GST) or His tags were expressed using a wheat germ cell-free system (CellFree Sciences) and purified using either a glutathione-Sepharose 4B column (GE Healthcare) or a nickel-Sepharose column (GE Healthcare) as described previously (36). Only in the case of the full-length protein, the N-terminal GST tag was removed by eluting GST-tagged FL bound to a glutathione-Sepharose 4B column by using tobacco etch virus (TEV) protease which cleaves the TEV recognition site between the GST tag and full-length GAMA. To generate antisera of these proteins (FL without GST tag, ECTO, Tr1, and Tr3), two BALB/c mice were immunized subcutaneously with 20 μg of purified recombinant proteins emulsified with Freund's complete adjuvant, followed by 20 μg of the proteins with Freund's incomplete adjuvant thereafter. Japanese white rabbits were immunized subcutaneously with 250 μg of purified proteins with Freund's complete adjuvant, followed by 250 μg of purified proteins with Freund's incomplete adjuvant thereafter. All immunizations were done 3 times at 3-week intervals. The antisera were collected 14 days after the last immunization. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Ehime University, and the experiments were conducted according to the Ethical Guidelines for Animal Experiments of Ehime University. The rabbit anti-erythrocyte binding antigen 175 (regions 3 to 5) (EBA175) serum was prepared as previously described (22).
Western blot analysis.
For the analysis of total schizont material, purified parasite pellets were directly lysed in an appropriate amount of 2× reducing or nonreducing SDS-PAGE sample buffer. The lysate was centrifuged at 10,000 × g for 10 min at room temperature (RT), and supernatants were collected, boiled at 95°C for 10 min, and resolved by electrophoresis in a 12.5% polyacrylamide gel (ATTO, Tokyo, Japan). Proteins were then transferred onto a 0.2-μm polyvinylidene difluoride (PVDF) membrane (Hybond LFP; GE Healthcare). The membranes were blocked with PBSTM buffer (PBS containing 0.1% [vol/vol] Tween 20 and 5% [wt/vol] nonfat milk) and then probed with appropriate primary antibodies diluted in PBSTM buffer. Bound primary antibodies were detected by incubation with an appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (GE Healthcare) diluted in PBSTM buffer, followed by visualization reaction with Immobilon Western chemiluminescent HRP substrate (Millipore). The relative molecular sizes of the proteins were calculated with reference to a molecular weight size marker (MagicMark XP; Invitrogen, Carlsbad, CA).
Indirect immunofluorescence assay (IFA).
P. falciparum (3D7) blood-stage parasites were cultured to approximately 8% parasitemia as previously described (6). Blood smears were prepared on glass slides when the majority of the parasites were at late trophozoite and schizont stages. Then, slides were fixed with ice-cold acetone for 3 min, dried, and stored at −80°C. Before use, the slides were thawed and blocked with PBS containing 5% nonfat milk at 37°C for 30 min. After the blocking, the slides were incubated with primary antibodies (both rabbit anti-FL and mouse anti-P. falciparum apical membrane antigen 1 [PfAMA1]) at 37°C for 1 h, followed by Alexa 488-conjugated goat anti-rabbit IgG secondary Ab (Invitrogen), Alexa 546-conjugated goat anti-mouse IgG secondary Ab (Invitrogen), and nuclear stain with DAPI (4′,6-diamidino-2-phenylindole) at 37°C for 30 min. The slides were mounted in ProLong Gold antifade reagent (Invitrogen) and visualized under oil immersion in a confocal scanning laser microscope (LSM5 PASCAL; Carl Zeiss MicroImaging, Thornwood, NY) using a Plan-Apochromat 63×/1.4 oil differential interference contrast (DIC) objective lens. Images were captured with LSM5 PASCAL software and prepared for publication with Adobe Photoshop (Adobe Systems, San Jose, CA).
IEM.
The purification of Tr3-specific IgGs from protein G-purified total rabbit IgGs raised against Tr3 was done using an antigen affinity chromatography method. One milligram of purified recombinant Tr3 was buffer exchanged into coupling buffer (0.2 M NaHCO3, 0.5 M NaCl) by using PD-10 desalting columns (GE Healthcare) and covalently coupled to a HiTrap N-hydroxysuccinimide (NHS)-activated HP column (GE Healthcare) according to the manufacturer's protocol. By use of this antigen column, Tr3-specific IgG was purified from total rabbit IgG raised against Tr3 protein. For immunoelectron microscopy (IEM), schizont stages of parasites were fixed for 15 min on ice in a mixture of 1% paraformaldehyde–0.1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). Fixed specimens were washed, dehydrated, and embedded in LR White resin (Polysciences, Warrington, PA) as previously described (6). Ultrathin sections were blocked at 37°C for 30 min in PBS containing 5% nonfat milk and 0.01% Tween 20 (PBS-MT). The grids were then incubated at 4°C overnight with rabbit Tr3-specific IgG or control sera in PBS-MT. After washing with PBS containing 10% Block Ace (Yukijirushi, Sapporo, Japan) and 0.01% Tween 20 (PBS-BT), the grids were incubated at 37°C for 1 h with goat anti-rabbit IgG conjugated to 10-nm gold particles (Amersham Life Science, Arlington, IL) diluted 1:20 in PBS-MT, rinsed with PBS-BT, and fixed on ice for 10 min in 2.5% glutaraldehyde to stabilize the gold. The grids were then rinsed with distilled water, dried, and stained with uranyl acetate and lead citrate. Samples were examined with a transmission electron microscope (JEM-1230; JEOL, Tokyo, Japan).
Erythrocyte binding assay.
Pure human erythrocytes were obtained from the Japanese Red Cross Society. It was stored at 4°C for up to 4 weeks and washed three times in incomplete RPMI medium (iRPMI; RPMI 1640 medium with l-glutamine, 25 mM HEPES buffer, and 50 mg/liter of hypoxanthine without sodium bicarbonate [Invitrogen]) before use. Enzyme treatments of erythrocytes were done as previously described (9). Briefly, sialic acid (SA) residues were removed by incubating 100 μl of packed human erythrocytes with neuraminidase (final concentration [fc] of 66.7 mU/ml in iRPMI), on a rotating wheel, for 1 h at 37°C. For trypsin or chymotrypsin treatment, 100 μl of packed human erythrocytes was incubated with trypsin or chymotrypsin (final concentration of 1 mg/ml in iRPMI), on a rotating wheel, for 1 h at 37°C and subsequently incubated with soybean trypsin inhibitor (final concentration of 0.5 mg/ml in iRPMI) for 10 min at 37°C to inhibit the trypsin or chymotrypsin. After the enzyme treatments, the erythrocytes were washed twice with 10 ml of iRPMI, then resuspended in iRPMI at a 50% hematocrit, stored at 4°C, and used within a week.
For erythrocyte binding assays with recombinant GAMA, 5 to 10 μg of recombinant protein (either ECTO, Tr1, or Tr3) was incubated with 100 μl of untreated, neuraminidase-treated, trypsin-treated, or chymotrypsin-treated human erythrocytes on a rotating wheel for 30 min at RT. After the incubation, the reaction mixture was layered over silicone oil (HIVAC F4; Shin-Etsu Silicones, Tokyo, Japan) and centrifuged in order to remove unbound proteins in the supernatant and collect pelleted erythrocytes. Proteins bound to erythrocytes were either eluted directly from the pelleted erythrocytes or eluted after the pelleted erythrocytes were washed once with iRPMI. Elution was done by incubating erythrocytes with 20 μl of 0.5 M NaCl in PBS, pH 7.4, for 15 min at RT. An amount of 2× SDS reducing sample buffer equal to the 20μl NaCl in PBS was added to the eluted proteins and incubated at 37°C for 20 min. The samples were separated by SDS-PAGE and detected by Western blotting using mouse monoclonal anti-penta-His antibodies (Qiagen).
For the erythrocyte binding assay with native GAMA and EBA175 shed in the culture supernatant, 100 μl of five-times-concentrated culture supernatant was incubated with 100 μl of untreated and enzyme-treated human erythrocytes, on a rotating wheel, for 30 min at RT. The reaction mixture was then layered over silicone oil and centrifuged to collect erythrocytes. Bound protein was eluted from the pelleted erythrocytes by incubating erythrocytes with 20 μl of 0.5 M NaCl in PBS, pH 7.4, for 15 min at RT. An amount of 2× SDS nonreducing sample buffer equal to the 20μl NaCl in PBS was added to the eluted proteins and incubated at 37°C for 20 min. The samples were separated by SDS-PAGE and detected by Western blotting using the respective rabbit antibodies.
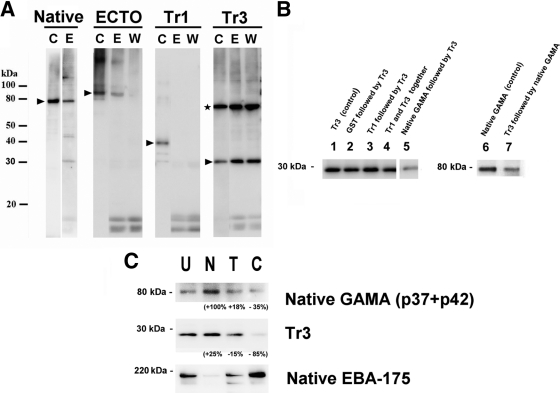
To check whether Tr3 binds to the receptor of native GAMA (see Fig. 5B), the following seven erythrocyte binding reactions were done using the procedure described above: (i) erythrocytes were incubated with 10 μg of Tr3, (ii) erythrocytes were incubated initially with 10 μg of GST followed by centrifugation, removal of supernatant containing the unbound GST fraction, and subsequent incubation with 10 μg of Tr3, (iii) erythrocytes were incubated initially with 10 μg of Tr1 followed by centrifugation, removal of supernatant containing the unbound Tr1 fraction, and subsequent incubation with 10 μg of Tr3, (iv) erythrocytes were incubated with a mixture containing 10 μg each of Tr1 and Tr3, (v) erythrocytes were incubated initially with 100 μl of 5-times-concentrated culture supernatant followed by centrifugation, removal of supernatant, and subsequent incubation with 10 μg of Tr3, (vi) erythrocytes were incubated with 100 μl of 5-times-concentrated culture supernatant, and (vii) erythrocytes were incubated initially with 10 μg of Tr3, followed by centrifugation, removal of supernatant containing the unbound Tr3 fraction, and subsequent incubation with 100 μl of 5-times-concentrated culture supernatant. After the final incubations, bound proteins were eluted as described above, and the quantities of Tr3 and native GAMA proteins in eluted proteins were detected by Western blotting with anti-penta-His and anti-FL antibodies, respectively.
Fig. 5.
Erythrocyte binding assay with native and recombinant GAMA. (A) Erythrocyte binding activities of native and recombinant GAMA proteins. The native GAMA protein in the culture supernatant or recombinant proteins (ECTO, Tr1, and Tr3) were incubated with human erythrocytes. The bound proteins were eluted with 0.5 M NaCl in PBS, pH 7.4, either directly from the incubated erythrocytes (E) or from the erythrocytes washed once with iRPMI (W). The eluted protein was detected by Western blotting either with rabbit anti-FL antibody (for GAMA) or with anti-penta-His antibodies (for ECTO, Tr1, and Tr3). For each experiment, the intact protein (without incubation or elution) was also detected by Western blotting as a control (C) (arrowheads). (Given that Tr3 has a cysteine residue, Tr3 forms an artificial homodimer [marked by an asterisk] with erythrocyte binding capacity.) (B) Tr3 competes for binding to a receptor(s) against native GAMA. The bands in lanes 1 through 5 and bands in lanes 6 and 7 refer to Tr3 and native GAMA, respectively, present in the blot of erythrocyte-bound proteins eluted from either controls or different treatments used in the binding assay (described above the lanes; also refer to Materials and Methods). Tr3 and native GAMA in the blot were detected with anti-penta-His and anti-FL antibodies, respectively. (C) GAMA binds erythrocytes in a receptor-specific manner. The erythrocyte binding abilities of native GAMA (present in the culture supernatant) and recombinant Tr3 were tested by incubation with untreated (U), neuraminidase-treated (N), trypsin-treated (T), and chymotrypsin-treated (C) erythrocytes, then elution with 0.5 M NaCl in PBS, pH 7.4, and detection by Western blotting with anti-penta-His (for Tr3) or anti-FL (for GAMA) antibodies. As a control for erythrocyte treatment, native EBA175 in the identical culture supernatant was also examined and detected by anti-EBA175 (regions 3 to 5) antibody. The values indicate percent changes in signal intensity of the relevant band relative to the band in lane U, calculated using Image J.
GIA.
Total IgGs to be tested in a growth inhibition assay (GIA) were purified from rabbit antisera with HiTrap protein G Sepharose columns (GE Healthcare) according to the manufacturer's protocol. Purified antibodies were further buffer exchanged into iRPMI, concentrated using Amicon Ultra-15 centrifugal devices (Millipore), filter sterilized using Ultrafree-MC GV 0.22-μm tubes (Millipore), and preabsorbed using 25 μl of packed human O+ erythrocytes per purified IgG from 1 ml of antiserum for 1 h at RT on a shaker. Finally, the concentrations of all antibodies were adjusted to 40 mg/ml in iRPMI. The inhibitory activity of antibodies was tested over one cycle of parasite replication, and parasitemia was measured by flow cytometry as described previously (2, 25). Briefly, the parasite cultures were synchronized the day before the start of the GIA. At the commencement of the GIA, the majority of parasites were at the late trophozoite to schizont stage. Twenty microliters of parasite suspension (0.3% parasitemia and 2% hematocrit) and 20 μl of antibodies were added per well of half-area flat-bottom 96-well cell culture microplates (Corning, Corning, NY) and gently mixed. For a control, 20 μl of iRPMI was added to the parasite suspension. Cultures were incubated at 37°C in a humidified, gassed (90% N2, 5% O2, and 5% CO2) box. After 25 h of incubation, the cultures were pelleted via centrifugation (1,300 × g for 5 min) and washed in 100 μl PBS. The cells were then incubated with 50 μl of diluted (1:1,000 in PBS) SYBR green I nucleic acid gel stain (Invitrogen) for 10 min at RT. Cells were washed once in PBS and resuspended in PBS. Parasitemia was measured by flow cytometry using a FACSCantII (BD Biosciences, San Jose, CA) with an acquisition of 50,000 events per sample. Data were analyzed with FlowJo 9.1 software (Tree Star, Ashley, OR). Samples were tested in triplicate in each experiment, and three independent experiments were performed. The GIA based on the parasite lactate dehydrogenase (pLDH) assay was done as previously described (8). Antibodies were also tested for inhibitory activity over two cycles of parasite replication, as described previously, with parasitemia measured by flow cytometry (24, 25); antibodies were tested at a 1/10 dilution (final concentration of 2 or 4 mg/ml). In these assays, inhibition of growth by antibodies greater than 10% compared to the control values is considered significant (25, 38). For GIAs using neuraminidase-treated erythrocytes, erythrocytes were treated with neuraminidase (final concentration of 66.7 mU/ml in iRPMI), on a rotating wheel, for 1 h at 37°C and washed twice with 10 ml of iRPMI, then resuspended in iRPMI at a 50% hematocrit, stored at 4°C, and used within a week.
ELISA.
Human plasma samples were collected from adults living in three areas of malaria endemicity in Mali (29). The study was approved by the ethical review committees of the Faculty of Medicine, Pharmacy, and Dentistry at the University of Bamako (Mali) and the NIAID, National Institutes of Health (Bethesda, MD). Individual written informed consent was obtained from all participants. Human serum samples from Thailand were collected from asymptomatic parasite carriers infected with P. falciparum alone with written informed consent as previously described (7). The study was approved by the Ethics Committee of the Thai Ministry of Public Health and the Institutional Review Board of the Walter Reed Army Institute of Research (7). Measurement of antibodies against P. falciparum GAMA in the 1:1,000-diluted Mali or Thai sera was performed as previously described (29). Briefly, 96-well enzyme-linked immunosorbent assay (ELISA) plates were coated with 50 ng/well of purified FL in coating buffer (20 mM boric acid, pH 8.9) and incubated at 4°C overnight. The plates were blocked with 2 mg/ml of gelatin in coating buffer. The sera were diluted (1:1,000) in phosphate-buffered saline with 0.1% Tween 20 (PBS-T), added to antigen-coated wells in duplicate, and incubated for 1 h at 37°C. After the plates were washed, they were incubated with 1:3,000-diluted HRP-conjugated rabbit anti-human IgG (DakoCytomation, Glostrup, Denmark) in PBS-T for 1 h at 37°C. After the plates were washed, they were incubated with 0.5 mg/ml azino-bis-3-ethylbenthiazoline-6-sulfonic acid (Wako, Osaka, Japan) diluted in citrate buffer (0.1 M citric acid, pH 4.1) for 20 min at RT. The reaction was stopped with 0.1 M citric acid, and optical densities (ODs) were measured at 415 nm by using a precision microplate reader (Molecular Devices, Sunnyvale, CA). The ELISA experiments were replicated twice independently.
Statistical analysis.
For the ELISA, the Mann-Whitney U test was performed. For the GIA, a one-way analysis of variance (ANOVA) was performed. If the overall test was significant, Bonferroni's pairwise multiple-comparison tests were used to compare each experimental group to the control. All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
RESULTS
Recombinant GAMA proteins and antibodies.
Sequence information of GAMA encoded by PF08_0008 retrieved from PlasmoDB (http://www.plasmodb.org) revealed that GAMA in P. falciparum is a 738-amino-acid protein (with a predicted molecular mass of 85.2 kDa) (Fig. 1 A). GAMA has a signal peptide (residues 1 to 24), a long asparagine-rich region (residues 356 to 485), and a transmembrane domain (residues 715 to 738) (Fig. 1A). We used the wheat germ cell-free system to synthesize the recombinant full-length GAMA (FL) and truncated versions of GAMA proteins, namely, ECTO (GAMA without the SP and TM regions), Tr1 (region upstream of the asparagine-rich region), and Tr3 (region downstream of the asparagine-rich region), without codon optimization (Fig. 1A and B). Figure 1B shows the different truncated GAMA proteins resolved in a 12.5% SDS-polyacrylamide gel. Almost all of the GAMA proteins were recovered in the supernatant fraction and easily purified as a single dominant band (Fig. 1B, arrows) by affinity chromatography. These results demonstrate that the wheat germ cell-free system is able to translate the native GAMA gene sequences and produce soluble proteins. These proteins were used to immunize rabbits and mice to produce antibodies.
Proteolytic processing and shedding of GAMA into culture supernatant.
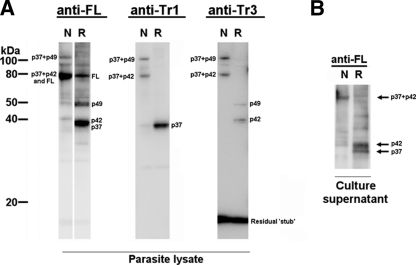
The Western blotting of schizont material and culture supernatant with anti-GAMA antisera reconfirmed the previous findings (21) that primary (FL to p37-p49 dimer) and secondary (p49 to p42 and residual stub) processing events occur in GAMA (Fig. 2 A), and GAMA is shed into culture supernatant as a dimer (p37-p42) following erythrocyte invasion (Fig. 2B). Moreover, p37-p42 and the residual stub were clearly detected in our blot of parasite lysates. These results also confirm the quality and specificity of the different antibodies raised against GAMA synthesized in a wheat germ cell-free system.
Fig. 2.
Processing and shedding of GAMA. (A) Detection of GAMA in schizont lysate. Total schizont material was examined by Western blotting under reducing (R) and nonreducing (N) conditions, using the rabbit anti-FL, mouse anti-Tr1, and rabbit anti-Tr3 antisera. (B) Detection of GAMA in culture supernatant. Culture supernatant was analyzed by Western blotting under reducing and nonreducing conditions using the rabbit anti-FL.
Subcellular location of GAMA.
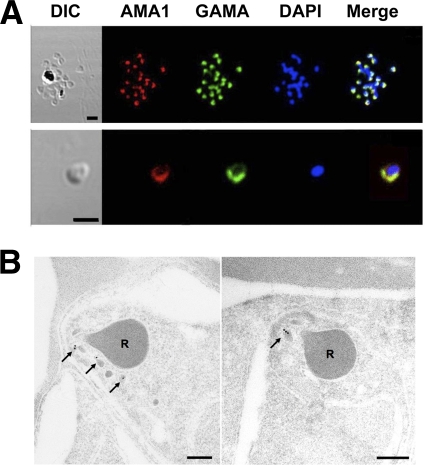
To confirm the localization of GAMA, an IFA was performed (Fig. 3 A). When the acetone-fixed smears of parasites in the late schizont stage were stained with rabbit anti-FL antiserum, green fluorescence was seen in the apical region (Fig. 3A, top panel), suggesting that GAMA resides in an apical organelle. The colocalization of GAMA with AMA1 indicates that GAMA may be localized in micronemes (Fig. 3A, top panel). These results in Fig. 3A were in good agreement with the previous findings (21). In order to validate the IFA data by electron microscopy, parasites in late schizont stages were stained with Tr3-specific IgGs and subsequently with secondary antibody labeled with gold particles. The signals of gold particles were found in micronemes (Fig. 3B, arrows), indicating that GAMA is indeed a micronemal protein. When free merozoites were stained with anti-FL antiserum, a circumferential green fluorescence was detected on the parasite surface (Fig. 3A, bottom panel), suggesting that GAMA resides on the surface of free merozoites.
Fig. 3.
Localization of GAMA in asexual blood-stage parasites. (A) GAMA localization using an immunofluorescence assay. Acetone-fixed P. falciparum 3D7 mature schizonts (top panel) and free merozoites (bottom panel) were probed with rabbit anti-FL (green) and mouse anti-PfAMA1 (microneme marker) (red). Parasite nuclei were stained with DAPI (blue). Scale bars represent 2 μm. (B) GAMA localization using immunoelectron microscopy. The two sections of merozoites in schizont-infected erythrocytes were probed with purified rabbit anti-Tr3 antibody and subsequently by secondary antibody conjugated with gold particles. The arrows indicate the micronemal localization of signals from gold particles. Bars represent 200 nm. Arrows mark micronemes. R's mark rhoptries.
GIA.
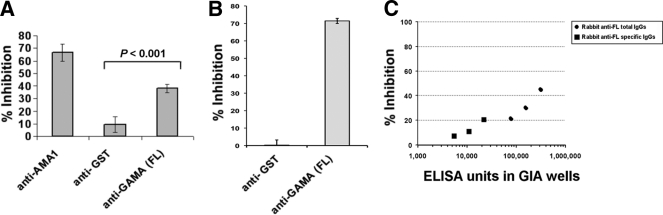
In order to test whether antibodies to GAMA could block parasite invasion, rabbit polyclonal IgG to FL GAMA was tested initially for inhibition of parasite growth over one cycle of replication with measurement of parasite growth by using flow cytometry. When anti-FL, anti-AMA1 (positive control), and anti-GST (negative control) were tested at final concentrations of 20 mg/ml (total IgG concentration), they inhibited the invasion by (mean) 38%, 66%, and 9%, respectively (Fig. 4 A). Using a GIA based on parasite detection using the pLDH assay, both total and antigen-specific anti-FL IgGs inhibited invasion and/or growth (up to about 43% and 21%, respectively) in a dose-dependent manner (Fig. 4C). IgGs to FL, Tr1, and Tr3 were also tested for their growth-inhibitory activities over two cycles of replication at final concentrations of 2.3 mg/ml, 4 mg/ml, and 4 mg/ml, respectively. The levels of inhibition were (mean ± SEM) 20% ± 2.6%, 32% ± 0.5%, and 20% ± 3.1%, respectively. The normal rabbit IgG at the same concentration gave no significant inhibition (mean ± SEM, 0.3% ± 3.3%).
Fig. 4.
Anti-FL antibody inhibits parasite invasion in vitro. (A) Anti-FL antibodies have invasion-inhibitory activity in vitro. The ability of the anti-FL antibodies to inhibit the parasite invasion into erythrocytes was tested in a one-cycle growth inhibition assay. Anti-AMA1 and anti-GST antibodies were used as positive and negative controls, respectively. The error bars represent the standard deviations of the means of the three independent experiments. One-way ANOVA was performed (P < 0.001) and followed by Bonferroni's pairwise multiple-comparison tests to compare anti-GST and anti-FL. (B) GAMA plays a role in the SA-independent invasion pathway. The ability of the anti-FL antibody to inhibit parasite invasion into neuraminidase-treated erythrocytes was tested in a one-cycle growth inhibition assay. Anti-GST antibody was used as a negative control. The bars represent the standard deviations of the means of the three independent experiments. (C) Anti-FL antibodies inhibit parasite invasion in a dose-dependent manner in vitro. The graph shows that the anti-FL antibodies, both total and antigen-specific IgGs, inhibited the invasion and/or growth in a dose-dependent manner in a one-cycle growth inhibition assay, as determined by measuring parasite LDH. The ELISA unit value was assigned as the reciprocal of the dilution giving an optical density (OD) at 415 nm equal to 1 in a standardized assay.
The erythrocyte binding region of GAMA.
Hinds et al. have previously reported that native GAMA (p37-p42 heterodimer) in the culture supernatant can bind to erythrocyte membranes and the bound GAMA can be eluted (using 0.5 M NaCl in PBS, pH 7.4) from erythrocyte membranes after extensive washing with PBS (21). However, the location of the erythrocyte binding region of GAMA has not yet been defined. Characterization of the erythrocyte binding region will be helpful for understanding the molecular basis of invasion inhibition by anti-GAMA antibodies that we have observed in our GIA. Therefore, we tested the erythrocyte binding abilities of recombinant GAMA ECTO, Tr1, and Tr3 proteins.
First, we reconfirmed that native GAMA (the p37-p42 dimer), shed by extracellular merozoites into the culture supernatant, has the ability to bind erythrocytes (Fig. 5 A), as previously reported (21). Second, we tested the erythrocyte binding abilities of the recombinant proteins. After incubation of recombinant proteins with erythrocytes, bound proteins were eluted from erythrocytes, with or without a wash with iRPMI, and detected in a blot by using anti-penta-His antibodies (Qiagen). ECTO was detected in an immunoblot of proteins eluted from unwashed erythrocytes, but not from washed erythrocytes, suggesting that unprocessed ECTO has a weak erythrocyte binding ability, and hence, the binding did not withstand a wash with iRPMI (Fig. 5A). When Tr1 and Tr3 were tested, only Tr3 and not Tr1 was detected in an immunoblot of proteins eluted both from unwashed and washed erythrocytes, suggesting that Tr3, not Tr1, has the erythrocyte binding ability and, hence, an erythrocyte binding epitope. The persistence of Tr3 binding even after erythrocytes were washed with iRPMI suggests that Tr3 has a stronger erythrocyte binding capacity than ECTO (Fig. 5A).
In order to verify whether Tr3 and native protein both bind a common receptor, the quantities of Tr3 in the proteins present in the blot of erythrocyte-bound proteins eluted from erythrocytes of either control (lane 1) and different treatments (lanes 2 through 5) were compared based on the measurement of band intensity by ImageJ analysis (Fig. 5B) (1). It showed that the quantity of Tr3 bound to erythrocytes that were preincubated with native GAMA was reduced by 50% (lane 5) relative to the control (lane 1), indicating that native GAMA preempts the receptors otherwise available for Tr3. However, there was no reduction in Tr3 quantity in other negative-control treatments (lane 2 to 4), indicating that neither GST nor Tr1 affects Tr3 binding. Similarly, in the reciprocal experiment, the quantity of native GAMA bound to erythrocytes that were preincubated with Tr3 was reduced by 50% (lane 7) relative to the control (lane 6), indicating that Tr3 preempts the receptors otherwise available for native GAMA (Fig. 5B). Taken together, these data show that native GAMA and Tr3 bind to the same receptor.
GAMA binds erythrocytes in a receptor-specific manner.
The erythrocyte binding specificity of GAMA Tr3 was studied by testing the binding to enzyme-treated erythrocytes (Fig. 5C). Neuraminidase treatment of erythrocytes removes sialic acid (SA) residues in SA-containing erythrocyte receptors, and trypsin or chymotrypsin treatments differentially cleave the peptide backbones of erythrocyte receptors (33). The quantity of Tr3 protein detected in the immunoblot of proteins eluted from the surface of neuraminidase-treated erythrocytes was comparable to that of untreated erythrocytes. While the quantity of Tr3 eluted from trypsin-treated erythrocytes appeared to be only slightly reduced (15% reduction in signal intensity based on densitometry using Image J) (1), the quantity of Tr3 eluted from chymotrypsin-treated erythrocytes was much lower than that of untreated erythrocytes (85% reduction in signal intensity). The binding of native GAMA was resistant to neuraminidase and trypsin treatment but appeared sensitive to chymotrypsin treatment (35% reduction in signal intensity). As a control for enzyme treatments, EBA175 was also examined. As expected, EBA175 bound to erythrocytes in a neuraminidase-sensitive, trypsin-sensitive, and chymotrypsin-resistant manner (9). Taken together, these results suggest that GAMA binds a nonsialylated protein receptor.
GIA with neuraminidase-treated erythrocytes.
The binding of GAMA to a nonsialylated protein receptor suggests that GAMA might be an invasion ligand that plays a role in the SA-independent invasion pathway. In order to verify this proposition, we tested parasite invasion into neuraminidase-treated erythrocytes in the presence of anti-GAMA antibodies and measured parasite growth over one cycle of replication by using flow cytometry. When anti-FL and anti-GST (negative control) IgGs were tested at final concentrations of 20 mg/ml, they inhibited invasion by 72% and 0.27%, respectively (Fig. 4B), suggesting that GAMA is a ligand that plays a role in the SA-independent invasion pathway.
Additive effects of antibodies in GIAs.
It has been suggested that the presence of antibodies that target a broad range of invasion ligands (SA dependent and SA independent) involved in alternate invasion pathways would have greater growth-inhibitory activity (22). Since GAMA is an SA-independent ligand (that binds to neuraminidase-resistant, trypsin-resistant, and chymotrypsin-sensitive erythrocyte receptors) (Fig. 5C) and EBA175 is an SA-dependent ligand (that binds to neuraminidase-sensitive, trypsin-sensitive, and chymotrypsin-resistant receptors) (Fig. 5C) (26), we were interested to test the first hypothesis, that a combination of anti-GAMA and anti-EBA175 antibodies may block both SA-dependent and SA-independent pathways and therefore exhibit a more potent invasion-inhibitory effect than either anti-GAMA or anti-EBA175 antibody alone.
Because of the colocalization of GAMA and AMA1 in free merozoites in our IFA, we were also interested to test the second hypothesis, that a combination of anti-GAMA and anti-AMA1 antibodies might exhibit a greater invasion-inhibitory effect than either anti-GAMA or anti-AMA1 antibody alone.
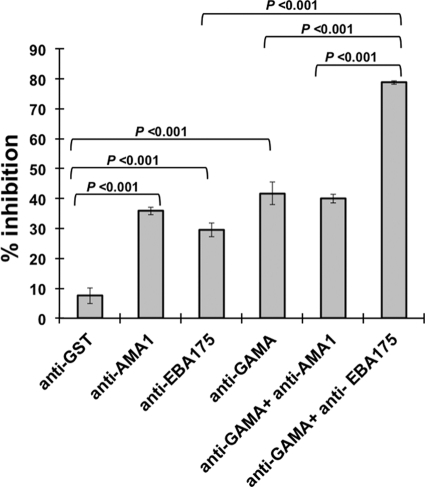
For testing of the two hypotheses described above, we did additive GIA experiments (Fig. 6). The following 6 antibody treatments were tried for inhibition of parasite growth over one cycle of replication with measurement of parasite growth by using flow cytometry: (i) anti-GST (negative control) (final concentration [fc], 20 mg/ml), (ii) anti-AMA1 (fc, 5 mg/ml), (iii) anti-EBA175 (regions 3 to 5) (fc, 1 mg/ml), (iv) anti-FL GAMA (fc, 15 mg/ml), (v) a mixture of anti-FL GAMA (fc, 15 mg/ml) and anti-AMA1 (fc, 5 mg/ml), and (vi) a mixture of anti-GAMA (fc, 15 mg/ml) and anti-EBA175 (regions 3 to 5) (fc, 1 mg/ml). The results (Fig. 6) showed that the invasion inhibition exhibited by the combination of anti-FL GAMA and anti-EBA175 antibodies was significantly greater (79%) than that by either anti-GAMA (41%) or anti-EBA175 (29%) alone. However, inhibition exhibited by the combination of anti-FL GAMA and anti-AMA1 antibodies (40%) was not significantly greater than that of either anti-GAMA (41%) or anti-AMA1 (35%) alone. The negative-control (anti-GST) antibodies inhibited invasion of 7%.
Fig. 6.
Additive blocking of invasion. Antibodies, either separately or in combinations, were tested for inhibition of parasite invasion into erythrocytes in a one-cycle growth inhibition assay. Anti-GST antibody was used as a negative control (for concentrations of IgGs, refer to Results). The error bars represent the standard deviations of the means of the three independent experiments. One-way ANOVA was performed (P < 0.001) and followed by Bonferroni's pairwise multiple-comparison tests to compare each experimental group. Statistical significance between other groups was not tested.
Reactivity of GAMA to human immune sera.
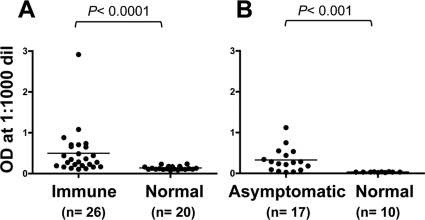
Since GAMA is mobilized from the microneme onto the surface of merozoites (Fig. 3A) and anti-GAMA antibodies inhibited invasion in vitro (Fig. 4), we were interested to investigate whether GAMA is exposed to the human immune system in P. falciparum-infected individuals and generates an immune response. In order to test for the presence of anti-GAMA antibody in the sera, we tested sera from immune adults of Mali and West Africa and naive, nonexposed U.S. adults for antibodies to GAMA FL recombinant protein by ELISA (Fig. 7 A) and sera from P. falciparum-infected asymptomatic adults of western Thailand and naive, nonexposed Thai adults for antibodies to GAMA FL recombinant protein (Fig. 7B). Sera of immune adults from Mali showed significantly higher reactivity to FL than those of malaria-naïve U.S. adults (P < 0.0001; Mann-Whitney U test) (Fig. 7A), and sera of asymptomatic Thai adults showed significantly higher reactivity to FL than those of malaria-naive Thai individuals (P < 0.001; Mann-Whitney U test) (Fig. 7B).
Fig. 7.
Human sera from areas of malaria endemicity in Mali and Thai recognize GAMA in an ELISA. Probing of FL with sera from immune adults of Mali (Immune) and naive, nonexposed U.S. adults (Normal) (A) and with sera from P. falciparum-infected asymptomatic adults of Thai (Asymptomatic) and naive, nonexposed Thai adults (Normal) (B). The P values were calculated by Mann-Whitney U test. n indicates the number of sera analyzed. OD, optical density; dil, dilution.
DISCUSSION
This study was performed with the objective of testing our hypothesis that GAMA may be a blood-stage vaccine candidate by using recombinant GAMA expressed in the wheat germ cell-free system.
Our Western blotting results (Fig. 2A) are in broad agreement with the previous findings (21) that primary and secondary processing events occur in GAMA. However, the detection of p37-p42 and the residual stub in our blot of parasite lysates, but not in that of the previous study (21), suggests that the secondary processing occurs prior to invasion and not at the time of invasion as described in the previous study. Our IEM results have confirmed for the first time that GAMA is indeed localized in micronemes of merozoites, and hence, GAMA represents a novel micronemal protein. It will be interesting to examine whether GAMA interacts with AMA1 or any other invasion-related merozoite proteins.
The GIA measures the capacity of antibodies to limit erythrocyte invasion and/or growth of P. falciparum in vitro (8). Our one-cycle GIA showed that anti-FL antibodies inhibited the merozoite invasion of erythrocytes. Similar assays using detection of parasitemia by parasite LDH assay also showed that anti-FL antibodies inhibited invasion and/or intraerythrocyte growth of the parasite in a dose-dependent manner. The two-cycle GIA showed that anti-FL, anti-Tr1, and anti-Tr3 antibodies inhibited invasion specifically.
Recently GAMA has been described as a novel erythrocyte binding protein (21). However, no recognizable protein motifs were identified in the primary structure of GAMA, and this raises the question of which regions of the protein are responsible for the demonstrated erythrocyte binding activity (21). In order to characterize the erythrocyte binding domain/epitope, truncated versions of GAMA were synthesized and tested for erythrocyte binding activity. Here, we have demonstrated that only Tr3, not Tr1, has the ability to bind erythrocytes, suggesting that the erythrocyte binding domain of GAMA resides in the C-terminal section of GAMA. Moreover, the binding of ECTO is weak, suggesting that while the unprocessed ECTO constrains the formation of proper erythrocyte binding domain in GAMA, processing of ECTO into the p37-p42 heterodimer is required for the facilitation of the formation and/or exposure of the erythrocyte binding domain. Our binding assays suggest that native GAMA and recombinant Tr3 bind to the same unknown receptor (Fig. 5B) and, importantly, revealed that the binding of native GAMA and Tr3 to human erythrocytes is neuraminidase resistant, SA independent, and chymotrypsin sensitive (Fig. 5C). However, it can be seen that the chymotrypsin treatment has a profound effect on binding of Tr3 rather than that of native GAMA. This may be due to the fact that Tr3 is a monomer and native GAMA is a heterodimer with p37 and p42 fragments, and additionally, p37 in native GAMA might per se mediate interaction with a different receptor that is neuraminidase and chymotrypsin resistant. Identification of the GAMA receptor(s) in future studies will be important to understanding the binding specificities of GAMA. Taken together, these results indicate that GAMA binds a nonsialylated protein receptor, and hence, GAMA represents a novel SA-independent invasion ligand that plays a role in the SA-independent invasion pathway. Our results indicating that the invasion-inhibitory effect of anti-FL is more profound with neuraminidase-treated erythrocytes (i.e., 72%; Fig. 4B) than with untreated erythrocytes (i.e., 38%; Fig. 4A) validate that GAMA indeed plays a role in the SA-independent invasion pathway.
Invasion pathways can be broadly classified into 2 main groups based on the use of SA on the erythrocyte surface by parasite ligands (22): (i) SA-independent (neuraminidase-resistant) invasion (11, 31) and (ii) SA-dependent (neuraminidase-sensitive) invasion (5, 17, 23, 26). Therefore, we were interested in finding combinations of antigens that induce more-potent synergistic antiparasite activity. Our results (Fig. 6) indicating that a combination of antibodies against GAMA (an SA-independent ligand) and EBA175 (an SA-dependent ligand) exhibited a significantly greater invasion-inhibitory effect than either anti-GAMA or anti-EBA175 antibody alone support the rationale that, for achieving greater invasion inhibition, targeting of both SA-dependent and SA-independent ligands/pathways is better than targeting either of them alone. It will be worthwhile to further test whether a more potent, or possibly synergistic, antiparasitic activity can be achieved with either a combination vaccine (mixture of GAMA and EBA175 antigens) or a fusion vaccine (chimeric GAMA-EBA175 fusion protein) in vivo.
Based on the colocalization of GAMA and AMA1 (Fig. 3A), we also hypothesized that the combination of anti-GAMA and anti-AMA1 antibodies might exhibit a greater invasion-inhibitory effect than either of them alone. However, our results (Fig. 6) showed that it was not the case. Given that GAMA interacts with an unknown erythrocyte receptor and AMA1 interacts with RON2 (30), one of the plausible explanations for our result is that GAMA-erythrocyte receptor interaction might be upstream of the AMA1-RON2 interaction and blocking of GAMA-receptor interaction by anti-GAMA antibodies makes the downstream AMA1-RON2 interaction impossible and, hence, renders the inhibitory effect of anti-AMA1 antibody superfluous.
Immunoreactive antigens involved in erythrocyte invasion represent potential candidates for malaria vaccine development (10), and designing a vaccine based on sequences of immunoreactive antigens with minimum polymorphisms is critical to preventing the parasite from evading the vaccine-induced immunity. Our results indicating that GAMA is localized on the surface of free merozoite (Fig. 3A) and antibodies are generated against GAMA during natural infection in humans (Fig. 7) suggest that GAMA is an immunogenic antigen. In order to study whether GAMA is exposed to the host immune pressure, we compared the single nucleotide polymorphisms (SNPs) in GAMA of 12 laboratory strains (see Table S1 in the supplemental material) deposited in PlasmoDB (http://plasmodb.org/plasmo/). GAMA contains a total of 7 nonsynonymous SNPs, and importantly, there are only 4 nonsynonymous SNPs outside the long asparagine-rich region (residues 356 to 485 in Fig. 1A); 3 of them (at positions 67, 229, and 258) are in Tr1, and only 1 (at position 632) is in the Tr3 region (containing the erythrocyte binding epitope) of GAMA. These preliminary SNP analyses suggest that GAMA is less polymorphic and, hence, may be a promising blood-stage vaccine candidate antigen. However, to validate this claim, we need further analysis of SNPs of GAMA in different field isolates worldwide.
In contrast to peripheral merozoite surface proteins and other apical proteins, most GPI-anchored proteins are refractory to genetic deletion (28). Knockouts of the GAMA gene in 3D7 and W2mef strains of P. falciparum were attempted, but no GAMA gene deletion mutant could be generated (see Fig. S1 in the supplemental material), and thus we believe that GAMA is essential to P. falciparum parasite invasion. However, it can be seen that the genetic disruption of the GAMA ortholog was successful in P. berghei (12). This discrepancy may be due to the differences in host erythrocyte receptors. However, additional research is required to address this discrepancy.
In summary, our data establish that GAMA is an important micronemal antigen. The data indicating that GAMA is exposed to the human immune system and anti-GAMA antibodies block merozoite invasion of erythrocytes in vitro validate GAMA as a novel blood-stage vaccine candidate antigen and suggest that it may be a target of antibodies that contribute to acquired immunity to malaria. Erythrocyte binding assays revealed that GAMA possesses an erythrocyte binding epitope in the C-terminal region and it binds a nonsialylated protein receptor. Growth inhibition assays with neuraminidase-treated erythrocytes reveal that GAMA represents a ligand that plays a role in the SA-independent invasion pathway. The significantly greater invasion-inhibitory effect exhibited by the combination of anti-GAMA or anti-EBA175 antibodies supports the rationale that targeting of both SA-dependent and SA-independent ligands/pathways is better than targeting either of them alone. This study also substantiates that the wheat germ cell-free system is a valuable tool for identification of novel malaria vaccine candidates.
Supplementary Material
ACKNOWLEDGMENTS
We thank Masachika Shudo, Integrated Center for Science, Ehime University, Japan, for technical assistance. We also thank the Japanese Red Cross Society for providing us the human erythrocytes and human plasma.
This research was supported in part by grants from The Bill and Melinda Gates Foundation, from the Ministry of Education, Culture, Sports, Science and Technology (21249028, 21022034, 23406007, and 23117008), and from the Ministry of Health, Labor, and Welfare, Japan (H21-Chikyukibo-ippan-005). This study was supported in part by the intramural program of the National Institute of Allergy and Infectious Diseases/NIH, and the GIA Reference Center is supported by the PATH/Malaria Vaccine Initiative.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 6 September 2011.
REFERENCES
- 1. Abramoff M. D., Magalhaes P. J., Ram S. J. 2004. Image processing with ImageJ. Biophotonics Int. 11:36–42 [Google Scholar]
- 2. Bei A. K., et al. 2010. A flow cytometry-based assay for measuring invasion of red blood cells by Plasmodium falciparum. Am. J. Hematol. 85:234–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Birkett A. J. 2010. PATH Malaria Vaccine Initiative (MVI): perspectives on the status of malaria vaccine development. Hum. Vaccin. 6:139–145 [DOI] [PubMed] [Google Scholar]
- 4. Bozdech Z., et al. 2003. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camus D., Hadley T. J. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230:553–556 [DOI] [PubMed] [Google Scholar]
- 6. Cao J., et al. 2009. Rhoptry neck protein RON2 forms a complex with microneme protein AMA1 in Plasmodium falciparum merozoites. Parasitol. Int. 58:29–35 [DOI] [PubMed] [Google Scholar]
- 7. Coleman R. E., et al. 2004. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J. Med. Entomol. 41:201–208 [DOI] [PubMed] [Google Scholar]
- 8. Crompton P. D., et al. 2010. In vitro growth-inhibitory activity and malaria risk in a cohort study in Mali. Infect. Immun. 78:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deans A. M., et al. 2007. Invasion pathways and malaria severity in Kenyan Plasmodium falciparum clinical isolates. Infect. Immun. 75:3014–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doolan D. L., et al. 2008. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 8:4680–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duraisingh M. T., et al. 2003. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 22:1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ecker A., Bushell E. S., Tewari R., Sinden R. E. 2008. Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Mol. Microbiol. 70:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Florens L., et al. 2002. A proteomic view of the Plasmodium falciparum life cycle. Nature 419:520–526 [DOI] [PubMed] [Google Scholar]
- 14. Fowkes F. J., Richards J. S., Simpson J. A., Beeson J. G. 2010. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 7:e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gardner M. J., et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Genton B. 2008. Malaria vaccines: a toy for travelers or a tool for eradication? Expert Rev. Vaccines 7:597–611 [DOI] [PubMed] [Google Scholar]
- 17. Gilberger T. W., et al. 2003. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J. Biol. Chem. 278:14480–14486 [DOI] [PubMed] [Google Scholar]
- 18. Gilson P. R., et al. 2006. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol. Cell Proteomics 5:1286–1299 [DOI] [PubMed] [Google Scholar]
- 19. Greenwood B. M., et al. 2008. Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118:1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haase S., et al. 2008. Characterization of a conserved rhoptry-associated leucine zipper-like protein in the malaria parasite Plasmodium falciparum. Infect. Immun. 76:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hinds L., Green J. L., Knuepfer E., Grainger M., Holder A. A. 2009. Novel putative glycosylphosphatidylinositol-anchored micronemal antigen of Plasmodium falciparum that binds to erythrocytes. Eukaryot. Cell 8:1869–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopaticki S., et al. 2011. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect. Immun. 79:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maier A. G., et al. 2003. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat. Med. 9:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCallum F. J., et al. 2008. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS One 3:e3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Persson K. E., Lee C. T., Marsh K., Beeson J. G. 2006. Development and optimization of high-throughput methods to measure Plasmodium falciparum-specific growth inhibitory antibodies. J. Clin. Microbiol. 44:1665–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Persson K. E., et al. 2008. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J. Clin. Invest. 118:342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richards J. S., Beeson J. G. 2009. The future for blood-stage vaccines against malaria. Immunol. Cell Biol. 87:377–390 [DOI] [PubMed] [Google Scholar]
- 28. Sanders P. R., et al. 2006. A set of glycosylphosphatidyl inositol-anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion. Infect. Immun. 74:4330–4338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh K., et al. 2010. Subdomain 3 of Plasmodium falciparum VAR2CSA DBL3x is identified as a minimal chondroitin sulfate A-binding region. J. Biol. Chem. 285:24855–24862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srinivasan P., et al. 2011. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc. Natl. Acad. Sci. U. S. A. 108:13275–13280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stubbs J., et al. 2005. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science 309:1384–1387 [DOI] [PubMed] [Google Scholar]
- 32. Takeo S., Arumugam T. U., Torii M., Tsuboi T. 2009. Wheat germ cell-free technology for accelerating the malaria vaccine research. Expert Opin. Drug Discov. 4:1191–1199 [DOI] [PubMed] [Google Scholar]
- 33. Tham W. H., et al. 2010. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc. Natl. Acad. Sci. U. S. A. 107:17327–17332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsuboi T., Takeo S., Arumugam T. U., Otsuki H., Torii M. 2010. The wheat germ cell-free protein synthesis system: a key tool for novel malaria vaccine candidate discovery. Acta Trop. 114:171–176 [DOI] [PubMed] [Google Scholar]
- 35. Tsuboi T., et al. 2008. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect. Immun. 76:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsuboi T., Takeo S., Sawasaki T., Torii M., Endo Y. 2010. An efficient approach to the production of vaccines against the malaria parasite. Methods Mol. Biol. 607:73–83 [DOI] [PubMed] [Google Scholar]
- 37. WHO 2010. World malaria report 2010. WHO Press, Geneva, Switzerland [Google Scholar]
- 38. Wilson D. W., Crabb B. S., Beeson J. G. 2010. Development of fluorescent Plasmodium falciparum for in vitro growth inhibition assays. Malar. J. 9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.