Abstract
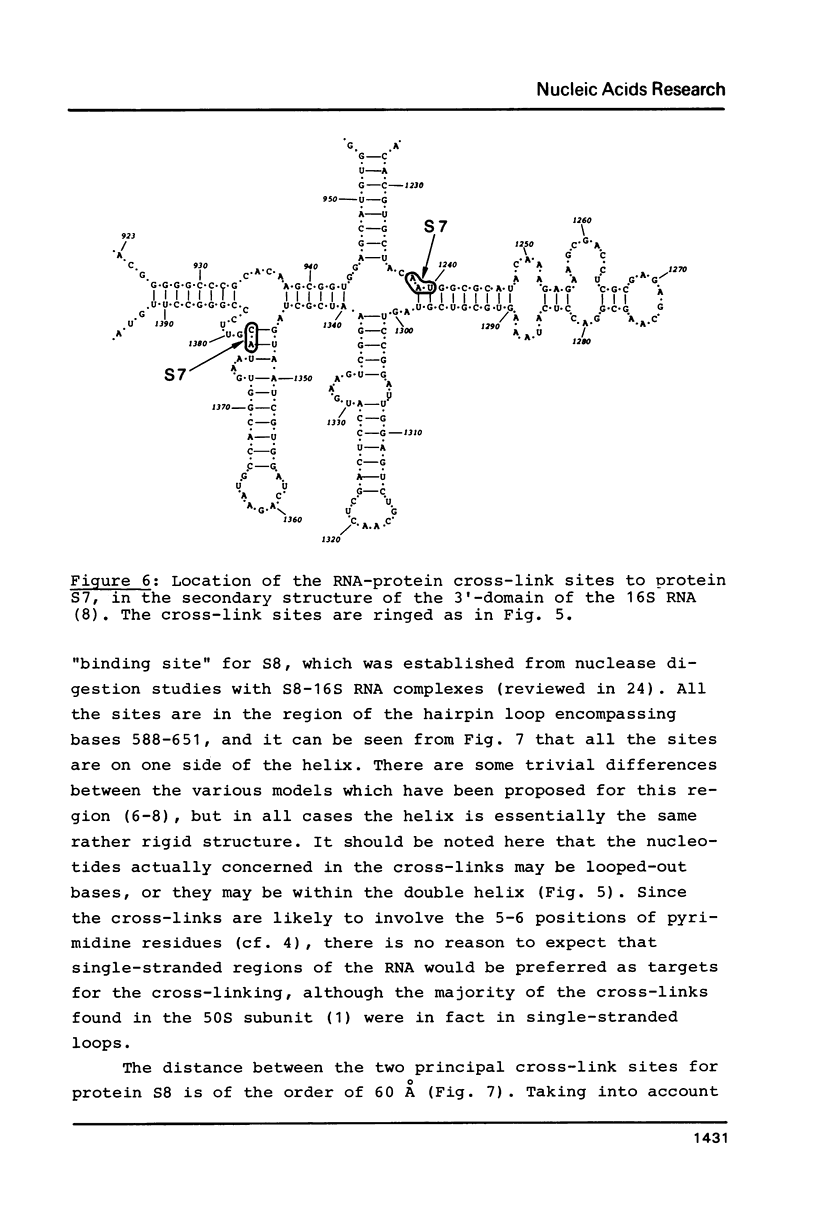
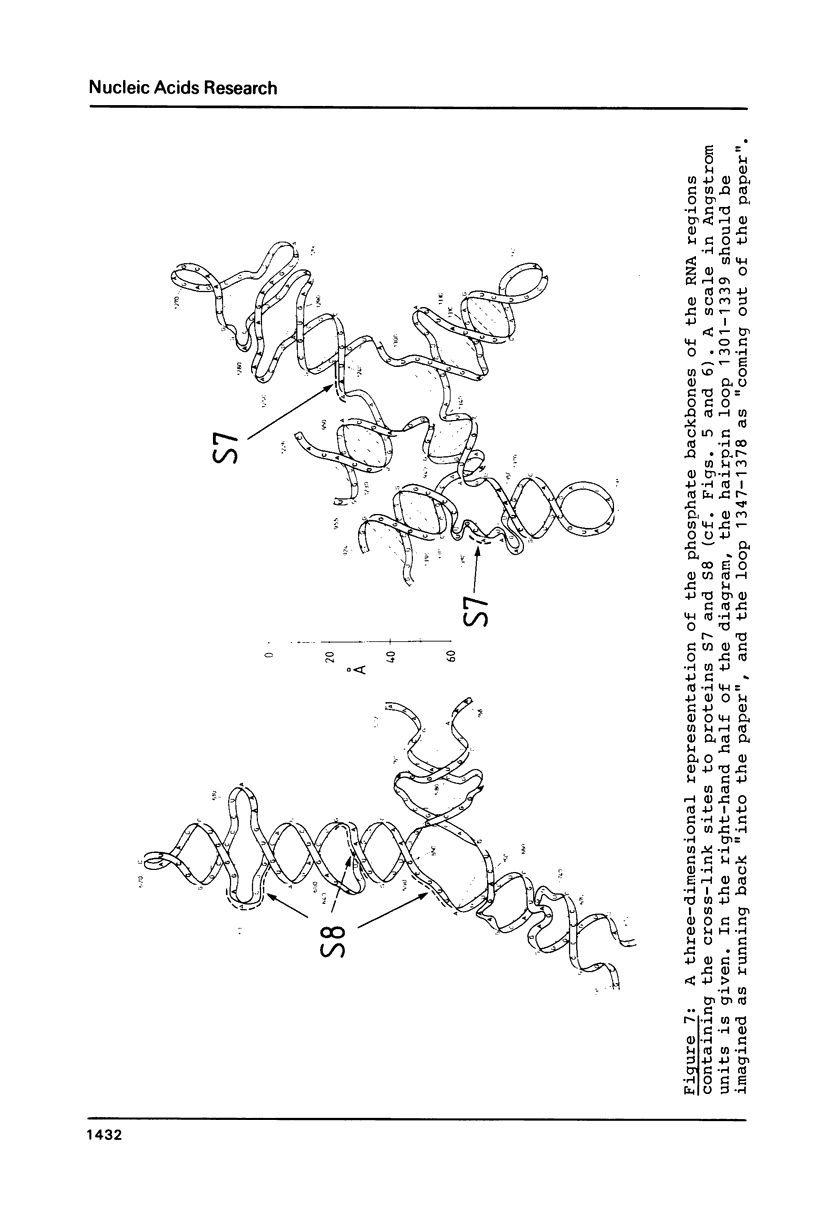
RNA-protein cross-links were introduced into E. coli 30S ribosomal subunits by reaction with 2-iminothiolane followed by a mild ultraviolet irradiation treatment. After removal of non-reacted protein and partial nuclease digestion of the cross-linked 16S RNA-protein moiety, a number of individual cross-linked complexes could be isolated and the sites of attachment of the proteins to the RNA determined. Protein S8 was cross-linked to the RNA at three different positions, within oligo-nucleotides encompassing positions 629-633, 651-654, and (tentatively) 593-597 in the 16S sequence. Protein S7 was cross-linked within two oligonucleotides encompassing positions 1238-1240, and 1377-1378. In addition, a site at position 723-724 was observed, cross-linked to protein S19, S20 or S21.
Full text
PDF




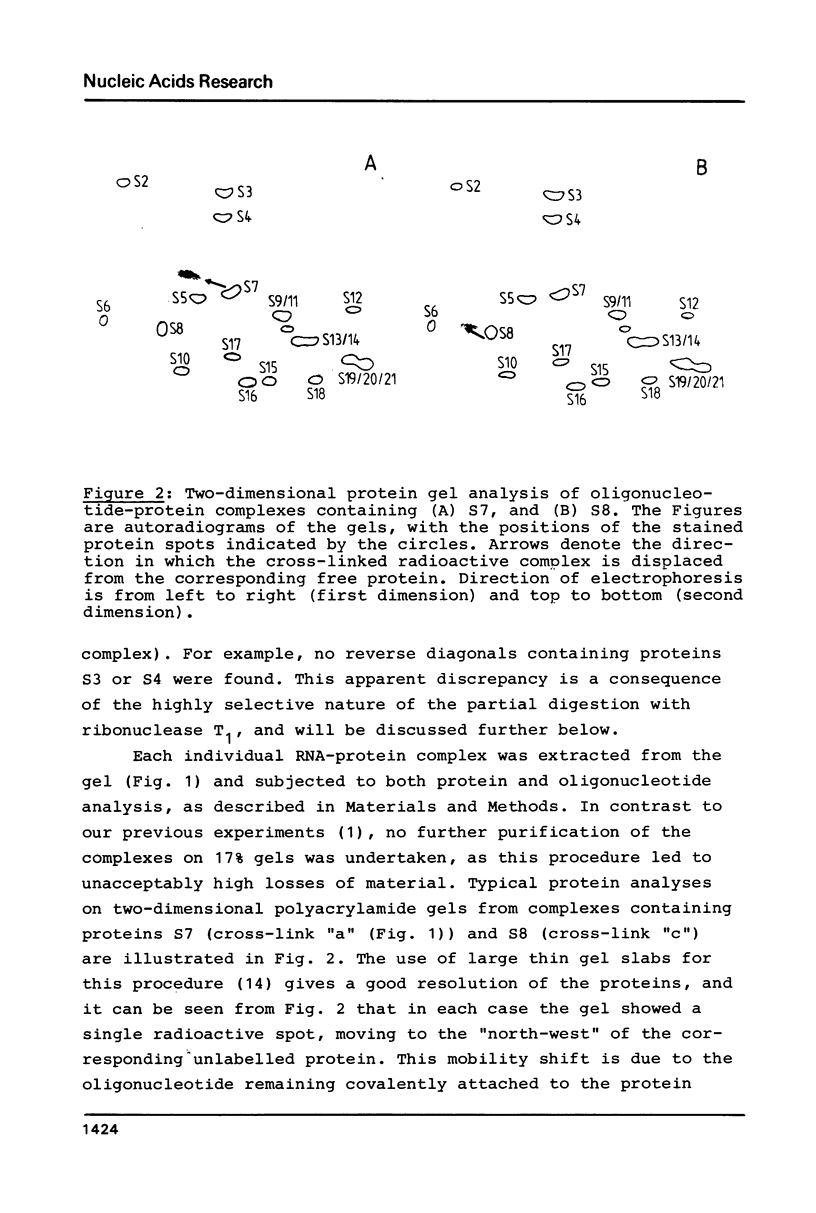


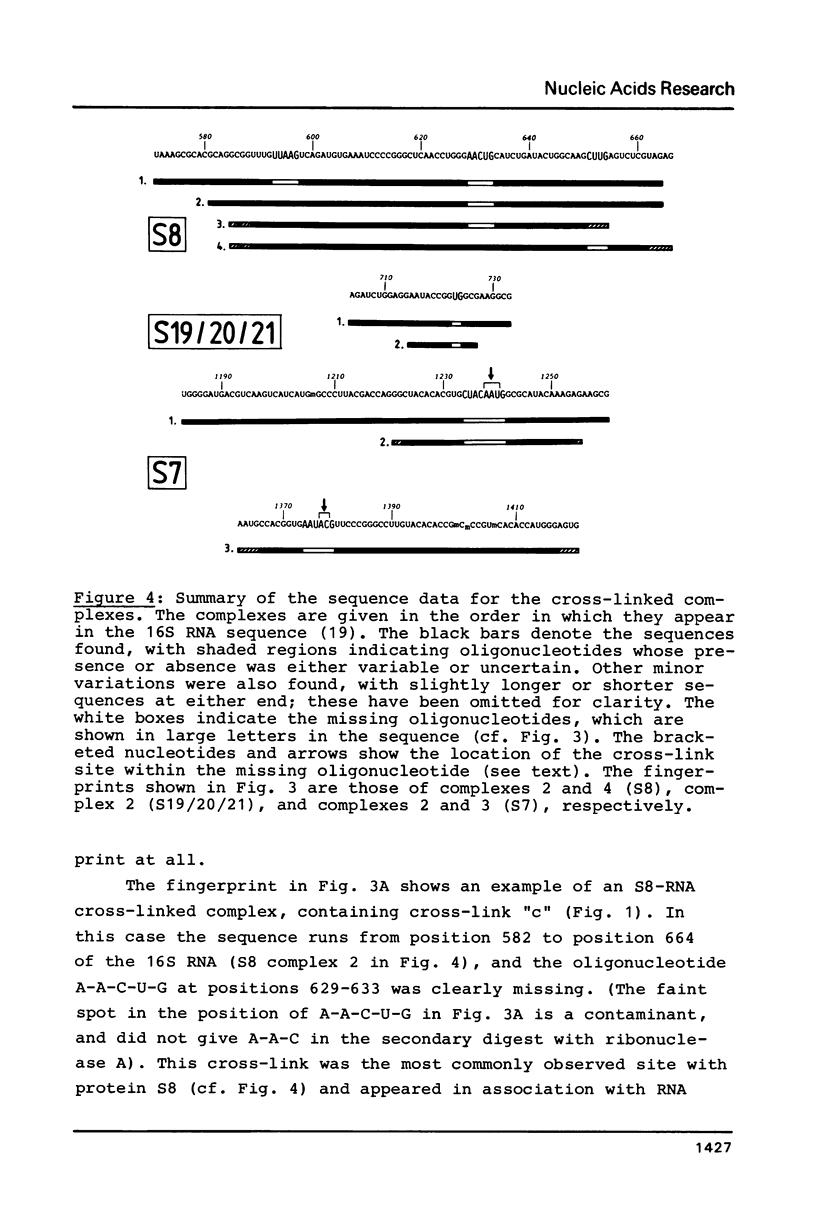


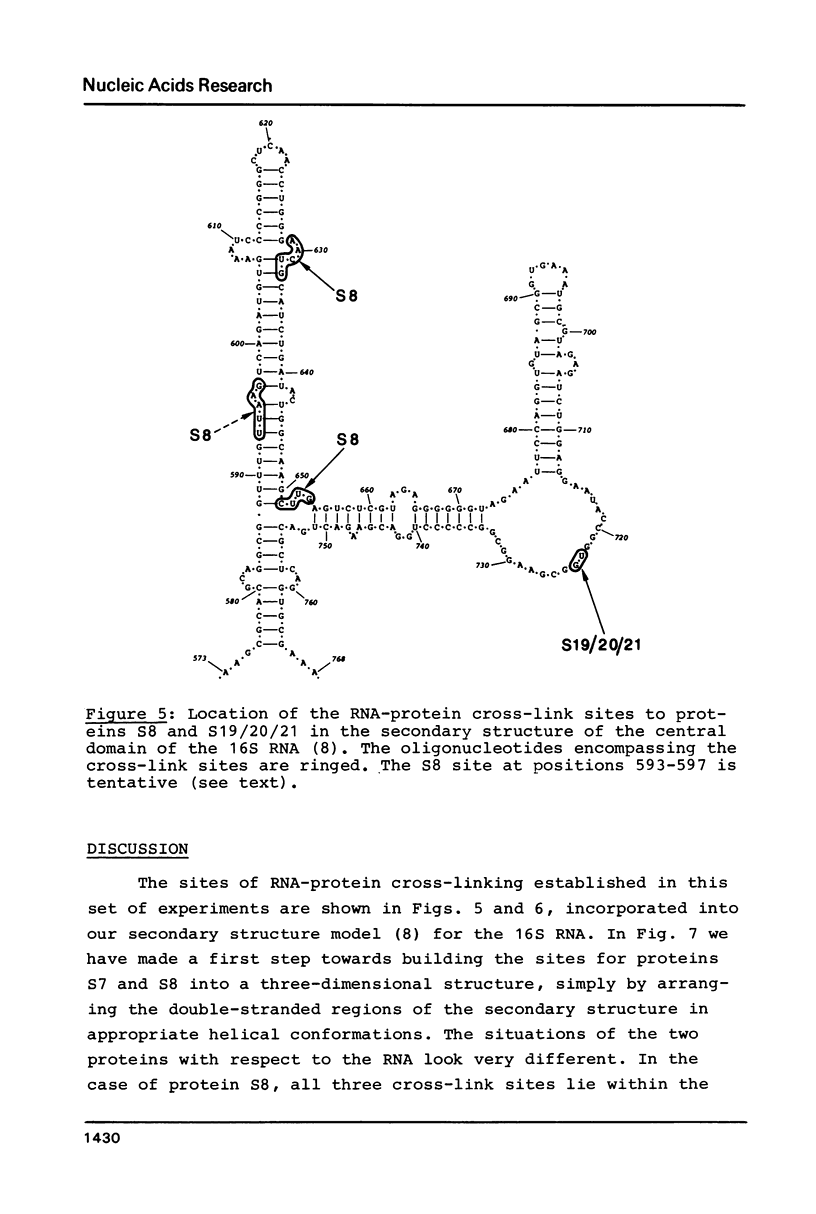





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Wittmann-Liebold B. The amino acid sequence of the ribosomal protein S8 of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1509–1525. doi: 10.1515/bchm2.1978.359.2.1509. [DOI] [PubMed] [Google Scholar]
- Backendorf C., Ravensbergen C. J., Van der Plas J., van Boom J. H., Veeneman G., Van Duin J. Basepairing potential of the 3' terminus of 16S RNA: dependence on the functional state of the 30S subunit and the presence of protein S21. Nucleic Acids Res. 1981 Mar 25;9(6):1425–1444. doi: 10.1093/nar/9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Kurland C. G., Stöffler G. 30S ribosomal proteins associated with the 3'-terminus of 16S RNA. FEBS Lett. 1975 Oct 15;58(1):281–284. doi: 10.1016/0014-5793(75)80279-1. [DOI] [PubMed] [Google Scholar]
- Ehresmann B., Backendorf C., Ehresmann C., Millon R., Ebel J. P. Effect of ultraviolet irradiation on 30-S ribosomal subunits. Identification of the RNA region crosslinked to protein S7. Eur J Biochem. 1980 Feb;104(1):255–262. doi: 10.1111/j.1432-1033.1980.tb04423.x. [DOI] [PubMed] [Google Scholar]
- Giri L., Littlechild J., Dijk J. Hydrodynamic studies on the Escherichia coli robosomal proteins S8 and L6, prepared by two different methods. FEBS Lett. 1977 Jul 15;79(2):238–244. doi: 10.1016/0014-5793(77)80795-3. [DOI] [PubMed] [Google Scholar]
- Glotz C., Brimacombe R. An experimentally-derived model for the secondary structure of the 16S ribosomal RNA from Escherichia coli. Nucleic Acids Res. 1980 Jun 11;8(11):2377–2395. doi: 10.1093/nar/8.11.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotz C., Zwieb C., Brimacombe R., Edwards K., Kössel H. Secondary structure of the large subunit ribosomal RNA from Escherichia coli, Zea mays chloroplast, and human and mouse mitochondrial ribosomes. Nucleic Acids Res. 1981 Jul 24;9(14):3287–3306. doi: 10.1093/nar/9.14.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golinska B., Millon R., Backendorf C., Olomucki M., Ebel J. P., Ehresmann B. Identification of a 16-S RNA fragment crosslinked to protein S1 within Escherichia coli ribosomal 30-S subunits by the use of a crosslinking reagent: ethyl 4-azidobenzoylaminoacetimidate. Eur J Biochem. 1981 Apr;115(3):479–484. doi: 10.1111/j.1432-1033.1981.tb06227.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Maly P., Rinke J., Ulmer E., Zwieb C., Brimacombe R. Precise localization of the site of cross-linking between protein L4 and 23S ribonucleic acid induced by mild ultraviolet irradiation of Escherichia coli 50S ribosomal subunits. Biochemistry. 1980 Sep 2;19(18):4179–4188. doi: 10.1021/bi00559a007. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Two-dimensional polyacrylamide gel electrophoresis: an improved method for ribosomal proteins. Anal Biochem. 1974 Jan;57(1):200–210. doi: 10.1016/0003-2697(74)90065-7. [DOI] [PubMed] [Google Scholar]
- Möller K., Brimacombe R. Specific cross-linking of proteins S7 and L4 to ribosomal RNA, by UV irradiation of Escherichia coli ribosomal subunits. Mol Gen Genet. 1975 Dec 9;141(4):343–355. doi: 10.1007/BF00331455. [DOI] [PubMed] [Google Scholar]
- Möller K., Zwieb C., Brimacombe R. Identification of the oligonucleotide and oligopeptide involved in an RNA--protein crosslink induced by ultraviolet irradiation of Escherichia coli 30 S ribosomal subunits. J Mol Biol. 1978 Dec 15;126(3):489–506. doi: 10.1016/0022-2836(78)90055-4. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Kop J., Wheaton V., Brosius J., Gutell R. R., Kopylov A. M., Dohme F., Herr W., Stahl D. A., Gupta R. Secondary structure model for 23S ribosomal RNA. Nucleic Acids Res. 1981 Nov 25;9(22):6167–6189. doi: 10.1093/nar/9.22.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Woese C. R. Secondary structure of 16S ribosomal RNA. Science. 1981 Apr 24;212(4493):403–411. doi: 10.1126/science.6163215. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Sjöberg B. Small-angle X-ray scattering study of the proteins S1, S8, S15, S16, S20 from Escherichia coli ribosomes. FEBS Lett. 1978 Sep 1;93(1):115–119. doi: 10.1016/0014-5793(78)80817-5. [DOI] [PubMed] [Google Scholar]
- Reinbolt J., Tritsch D. The primary structure of ribosomal protein S7 from E. coli strains K and B. FEBS Lett. 1978 Jul 15;91(2):297–301. doi: 10.1016/0014-5793(78)81196-x. [DOI] [PubMed] [Google Scholar]
- Rinke J., Meinke M., Brimacombe R., Fink G., Rommel W., Fasold H. The use of azidoarylimidoesters in RNA-protein cross-linking studies with Escherichia coli ribosomes. J Mol Biol. 1980 Mar 5;137(3):301–304. doi: 10.1016/0022-2836(80)90318-6. [DOI] [PubMed] [Google Scholar]
- Serdyuk I. N., Zaccai G., Spirin A. S. Globular conformation of some ribosomal proteins in solution. FEBS Lett. 1978 Oct 15;94(2):349–352. doi: 10.1016/0014-5793(78)80974-0. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Aplin R. T. A mixed photoproduct of uracil and cysteine (5-S-cysteine-6-hydrouracil). A possible model for the in vivo cross-linking of deoxyribonucleic acid and protein by ultraviolet light. Biochemistry. 1966 Jun;5(6):2125–2130. doi: 10.1021/bi00870a046. [DOI] [PubMed] [Google Scholar]
- Sommer A., Traut R. R. Identification of neighboring protein pairs in the Escherichia coli 30 S ribosomal subunit by crosslinking with methyl-4-mercaptobutyrimidate. J Mol Biol. 1976 Oct 5;106(4):995–1015. doi: 10.1016/0022-2836(76)90348-x. [DOI] [PubMed] [Google Scholar]
- Stiege W., Zwieb C., Brimacombe R. Precise localisation of three intra-RNA cross-links in 23S RNA and one in 5S RNA, induced by treatment of Escherichia coli 50S ribosomal subunits with bis-(2-chloroethyl)-methylamine. Nucleic Acids Res. 1982 Nov 25;10(22):7211–7229. doi: 10.1093/nar/10.22.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Zuker M., Ebel J. P., Ehresmann C. Structural organization of the 16S ribosomal RNA from E. coli. Topography and secondary structure. Nucleic Acids Res. 1981 May 11;9(9):2153–2172. doi: 10.1093/nar/9.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut R. R., Bollen A., Sun T. T., Hershey J. W., Sundberg J., Pierce L. R. Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973 Aug 14;12(17):3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. Micro thin-layer techniques for rapid sequence analysis of 32P-labeled RNA: double digestion and pancreatic ribonuclease analyses. Anal Biochem. 1977 Nov;83(1):228–239. doi: 10.1016/0003-2697(77)90531-0. [DOI] [PubMed] [Google Scholar]
- Wower I., Wower J., Meinke M., Brimacombe R. The use of 2-iminothiolane as an RNA-protein cross-linking agent in Escherichia coli ribosomes, and the localisation on 23S RNA of sites cross-linked to proteins L4, L6, L21, L23, L27 and L29. Nucleic Acids Res. 1981 Sep 11;9(17):4285–4302. doi: 10.1093/nar/9.17.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Brimacombe R. Localisation of a series of intra-RNA cross-links in 16S RNA, induced by ultraviolet irradiation of Escherichia coli 30S ribosomal subunits. Nucleic Acids Res. 1980 Jun 11;8(11):2397–2411. doi: 10.1093/nar/8.11.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Brimacombe R. Max-Planck-Institut für Molekulare Genetik, Abteilung Wittmann, Berlin-Dahlem, GFR. Nucleic Acids Res. 1979;6(5):1775–1790. doi: 10.1093/nar/6.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Glotz C., Brimacombe R. Secondary structure comparisons between small subunit ribosomal RNA molecules from six different species. Nucleic Acids Res. 1981 Aug 11;9(15):3621–3640. doi: 10.1093/nar/9.15.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]