The Sec6 subunit of the multisubunit exocyst tethering complex interacts with the Sec1/Munc18 protein Sec1 and with the t-SNARE Sec9. Assembly of the exocyst upon vesicle arrival at sites of secretion is proposed to release Sec9 for SNARE complex assembly and to recruit Sec1 for interaction with SNARE complexes to facilitate fusion.
Abstract
Trafficking of protein and lipid cargo through the secretory pathway in eukaryotic cells is mediated by membrane-bound vesicles. Secretory vesicle targeting and fusion require a conserved multisubunit protein complex termed the exocyst, which has been implicated in specific tethering of vesicles to sites of polarized exocytosis. The exocyst is directly involved in regulating soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein receptor (SNARE) complexes and membrane fusion through interactions between the Sec6 subunit and the plasma membrane SNARE protein Sec9. Here we show another facet of Sec6 function—it directly binds Sec1, another SNARE regulator, but of the Sec1/Munc18 family. The Sec6–Sec1 interaction is exclusive of Sec6–Sec9 but compatible with Sec6–exocyst assembly. In contrast, the Sec6–exocyst interaction is incompatible with Sec6–Sec9. Therefore, upon vesicle arrival, Sec6 is proposed to release Sec9 in favor of Sec6–exocyst assembly and to simultaneously recruit Sec1 to sites of secretion for coordinated SNARE complex formation and membrane fusion.
INTRODUCTION
Exocytosis in eukaryotes requires the accurate trafficking of membrane-bound vesicles between functionally and chemically distinct organelles and to the plasma membrane for growth, secretion, and cell–cell communication. Trafficking is a conserved and highly regulated process, employing multiple classes of essential proteins to ensure proper spatial and temporal cargo delivery (Wickner and Schekman, 2008, and references therein). Soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein receptor (SNARE) proteins on the target membrane (t-SNAREs) and vesicle (v-SNAREs) form a four-helix bundle called the SNARE complex, which bridges the membranes for fusion. The formation of specific, fusion-competent SNARE complexes is regulated by multiple protein families, including tethering factors such as the exocyst (Munson and Novick, 2006; He and Guo, 2009) and the Sec1/Munc18 (SM) proteins (Sudhof and Rothman, 2009). The actions of tethers and SM proteins are likely to be coordinated at vesicle docking sites, but in many cases, direct evidence connecting them is lacking (Toonen and Verhage, 2003; Carr and Rizo, 2010).
The exocyst is a large conserved heterooligomeric complex that is essential for growth and secretion; it functions in exocytosis, endocytosis, cytokinesis, and autophagy, and has been implicated in ciliogenesis, cancer, and bacterial pathogenesis (TerBush et al., 1996; Guo et al., 1999; He and Guo, 2009; Munson and Novick, 2006; Wu et al., 2008; Nichols and Casanova, 2010). It is composed of eight subunits—Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84—which localize to sites of polarized growth and secretion through binding phosphoinositides (He et al., 2007; Baek et al., 2010; Yamashita et al., 2010) and small GTPases of the Ras superfamily (Wu et al., 2008; He and Guo, 2009). The exocyst, a member of the CATCHR family of tethering complexes (including COG, Dsl1, and GARP; Whyte and Munro, 2002; Koumandou et al., 2007; Munson, 2009; Yu and Hughson, 2010), has been proposed to tether secretory vesicles to the plasma membrane; however, there is little direct evidence for tethering activity by any of these complexes.
The exocyst subunit Sec6 plays critical roles in several aspects of exocyst function. As with many of the exocyst subunits, Sec6 was originally discovered as a temperature-sensitive sec mutant of the secretory pathway (Novick et al., 1980). At the restrictive temperature, the sec6-4 mutant strain shows a loss of exocyst stability, with defects in polarized growth and secretion (TerBush and Novick, 1995). Additional temperature-sensitive mutations in conserved residues on the surface of the Sec6 C-terminal domain (Sivaram et al., 2006) led to loss of localization of the exocyst without complex disassembly (Songer and Munson, 2009). These residues are proposed to maintain exocyst localization through interactions with anchoring factor(s) at sites of secretion. Sec6 also binds the reticulon Rtn1, implicating Sec6 in the organization of cortical endoplasmic reticulum structure (De Craene et al., 2006). Moreover, we previously showed that the yeast exocyst subunit Sec6 interacts with the plasma membrane t-SNARE Sec9, inhibiting the formation of Sec9-containing SNARE complexes in vitro (Sivaram et al., 2005). Because the loss of Sec6 function in sec6-4 results in a block in SNARE assembly (Grote et al., 2000), the Sec6–Sec9 interaction we observe may be a critical intermediate in the assembly of SNARE complexes.
The SM protein family is essential for regulating SNARE proteins and SNARE-mediated membrane fusion. Although members of the SM family all bind individual SNARE proteins and/or SNARE complexes, several distinct modes of interaction have been reported, raising the possibility that SM proteins have multiple functions via different mechanisms (Toonen and Verhage, 2003, 2007; Carr and Rizo, 2010). The best-characterized SM protein, Munc18-1 (neuronal Sec1), binds to 1) the “closed” inhibited conformation of the t-SNARE syntaxin-1a (Misura et al., 2000); 2) the N-terminus of syntaxin-1a (Burkhardt et al., 2008); and 3) ternary SNARE complexes containing syntaxin-1a (Dulubova et al., 2007; Shen et al., 2007; Rodkey et al., 2008; Xu et al., 2010). A similar constellation of binding interactions has been reported for the endosomal SM protein Vps45 (Carpp et al., 2006; Furgason et al., 2009). Other SM proteins such as Sly1 appear to bind only the N-terminus of the partner syntaxin (Bracher and Weissenhorn, 2002; Yamaguchi et al., 2002; Peng and Gallwitz, 2004; Arac et al., 2005). In contrast, the yeast Sec1 protein interacts predominantly with assembled ternary SNARE complexes and not with the syntaxin Sso1 (Carr et al., 1999; Togneri et al., 2006). Functionally, several SMs appear to have an inhibitory role in SNARE complex assembly, whereas other studies clearly identified a positive role for SM proteins in SNARE complex assembly and membrane fusion (Gallwitz and Jahn, 2003; Scott et al., 2004; Shen et al., 2007; Toonen and Verhage, 2007). Thus, the functions of SM proteins, the mechanism(s) underlying these functions, and the extent to which these functions are conserved all remain important and incompletely resolved questions.
Although our Sec6–Sec9 binding studies indicated that the exocyst may play a direct role in controlling SNARE complex assembly, the question remained: how is Sec6 inhibition of Sec9 released to promote SNARE complex assembly? Several studies suggested that the exocyst might function with or through the SM protein Sec1 to regulate SNARE complex assembly (Finger and Novick, 2000; Grote et al., 2000; Wiederkehr et al., 2004; Hashizume et al., 2009), and the exocyst and Sec1 are specifically localized to sites of secretion in yeast. Although evidence pointed to a function for Sec1 after SNARE complex assembly by binding assembled SNARE complexes (Carr et al., 1999; Scott et al., 2004; Togneri et al., 2006) and promoting liposome fusion in vitro (Scott et al., 2004), a recent analysis of a large panel of Sec1 mutants demonstrated an additional functional requirement for Sec1 prior to SNARE complex assembly (Hashizume et al., 2009). Furthermore, overexpression of Sec1 resulted in increased levels of SNARE complexes (Wiederkehr et al., 2004). We hypothesized, therefore, that the exocyst and Sec1 work together to directly regulate the SNAREs and SNARE complex assembly. To understand the functional interplay among these proteins, we examined their relationships with one another both in vitro and in vivo.
RESULTS
Sec1 interacts with the exocyst subunit Sec6
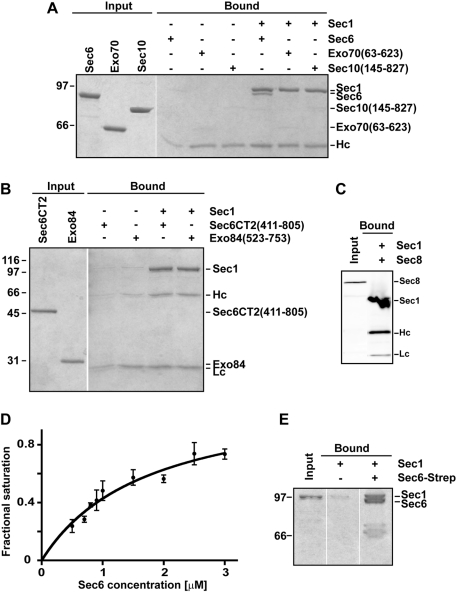
For direct examination of the interactions between the exocyst subunits and Sec1, we purified Sec1-V5-His6 (hereafter called Sec1) from yeast and immobilized it using an α-V5 antibody on Protein G resin (as in Togneri et al., 2006). Purified recombinant exocyst subunits were individually tested for their ability to bind Sec1. Of the eight exocyst subunits, Sec6 (Sivaram et al., 2005), Sec8 (Sivaram et al., 2006), Sec10 (amino acids 145–827; Croteau et al., 2009), Exo70 (63–623; Dong et al., 2005), and the C-terminal region of Exo84 (523–753; Dong et al., 2005) were soluble; the other subunits were not tested. Purified Sec6 was the only subunit to robustly and specifically interact with Sec1 (Figure 1, A–C). The apparent dissociation constant (Kd) between Sec1 and Sec6, based on the pull-down assay, was ∼1.7 ± 0.4 μM (Figure 1D). This affinity is slightly weaker than the apparent Kd for the Sec6–Sec9 interaction, ∼0.5 μM (Sivaram et al., 2005), and for the Sec1–SNARE complex interaction, ∼0.3 μM (Togneri et al., 2006). The Sec6–Sec1 binding interaction was reciprocal; Sec6 immobilized using a C-terminal Strep affinity tag bound to purified Sec1 (Figure 1E). Previous mapping indicated that the N-terminal domain of Sec6 is required for dimerization and for binding to both Sec9 and the exocyst subunit Sec8 (Sivaram et al., 2005, 2006). Conversely, the Sec6 C-terminal domain (411–805) is sufficient for binding the exocyst subunits Sec10 and Exo70 (Sivaram et al., 2006). Here we demonstrate that the Sec6 C-terminal domain is not sufficient for Sec1 binding (Figure 1B), indicating that the N-terminal domain of Sec6 is also necessary for the Sec6–Sec1 complex. The insolubility of the Sec6 N-terminal domain in isolation has thus far precluded direct testing of this region.
FIGURE 1:
Sec1 interacts with the exocyst subunit Sec6. (A) Purified Sec1-V5-His6 was immobilized with α-V5 antibody and mixed with purified exocyst subunits: Sec6, Exo70 (63–623), and Sec10 (145–827). Input and bound samples were run on an 8% SDS–PAGE gel and stained with Coomassie blue dye. (B) Similar experiments were performed using the C-terminal domain (411–805) of Sec6 and Exo84 (523–753). Input and bound samples were run on a 12% SDS–PAGE gel and stained with Coomassie blue dye. Only full-length Sec6 bound to Sec1. (C) Sec1-V5-His6 was immobilized and incubated with Ni NTA–purified His6-Sec8. In this case, the input and bound proteins (which are all His6 tagged) were analyzed by Western blotting using α-His5 antibody. (D) Representative binding curve for Sec6–Sec1. Sec1 was immobilized on beads and incubated with increasing concentrations of Sec6. The apparent binding constant was 1.7 ± 0.4 μM (mean ± SEM) from fits to three different experiments. (E) The Sec6–Sec1 binding interaction is reciprocal. Purified Strep-tagged Sec6 was immobilized on Strep-Tactin resin and incubated with purified Sec1. Input and bound samples were run on an 8% SDS–PAGE gel and stained with Coomassie blue dye. For all experiments, Hc and Lc refer to the heavy and light chains of the α-V5 antibody, respectively. To avoid leakage of proteins between different lanes, gels were often run with empty lanes between input and bound; empty lanes from the same gel were edited out using Photoshop. For all experiments, representative gels are shown for at least three repetitions.
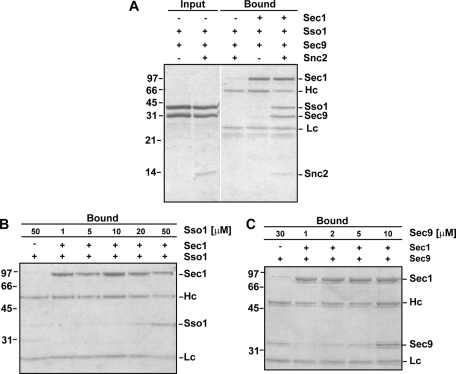
As a positive control, purified Sec1 was analyzed to ensure specific binding of Sec1 to ternary SNARE complexes containing Sec9, its partner t-SNARE Sso1 (the yeast exocytic syntaxin), and the v-SNARE Snc2 (Togneri et al., 2006; Figure 2A). We also found that both Sec9 and Sso1 can weakly bind to Sec1 at high concentrations (≥10 and ≥20 μM, respectively; Figure 2, B and C). These interactions had not been previously observed, likely due to the lower protein concentrations used in those experiments (≤10 μM; Scott et al., 2004; Togneri et al., 2006). However, these interactions are not completely unexpected, because binding of nonsyntaxin SNAREs has been observed for other SM proteins (Peng and Gallwitz, 2004; Carpp et al., 2006; Xu et al., 2010).
FIGURE 2:
Sec1–SNARE interactions. (A) Purified binary and ternary SNARE complexes were incubated with immobilized Sec1-V5-His6. Input and bound samples were run on a 15% SDS–PAGE gel and stained with Coomassie blue dye. (B, C). Titration of increasing concentrations of Sso1 or Sec9 to immobilized Sec1-V5-His6. Bound samples were run on 12% SDS–PAGE gels and stained with Coomassie blue dye. Some background binding of Sec9 to Sec1 is observed at high concentrations of Sec9 (30 μM).
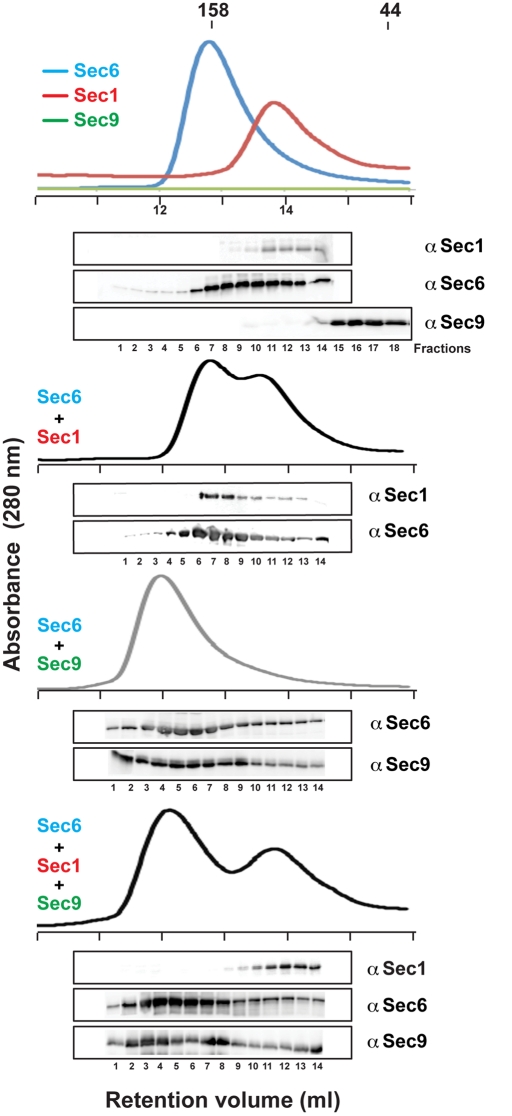
Analytical gel filtration analyses confirmed the Sec6–Sec1 interaction in solution (Figure 3). Sec1 migrates as a monomer, whereas Sec6 runs predominantly as a dimer, with a shoulder in the gel filtration peak that is compatible with a small amount of monomeric Sec6 (including the affinity tags, Sec1 is 89 kDa and Sec6 is 95 kDa). Dimerization of Sec6 was previously reported (Sivaram et al., 2005); however, the presence of the Sec6 monomer was not demonstrated because the method of detection used for the analyses was less sensitive (Coomassie-stained gels vs. Western blots analyzed with a specific α-Sec6 antibody). The Sec6–Sec1 complex, as inferred from the increased apparent molecular weight of Sec1, migrates with an apparent molecular weight comparable to that of the Sec6 dimer (apparent molecular weight ∼180 kDa), suggesting that the Sec6–Sec1 complex has a 1:1 stoichiometry, with Sec1 binding to the monomeric form of Sec6. This is in contrast to the previously characterized 2:2 stoichiometry for the Sec6–Sec9 complex (Figure 3; Sivaram et al., 2005) but is consistent with the stoichiometry determined for the assembled exocyst complex of one subunit each per complex (TerBush et al., 1996).
FIGURE 3:
Sec9 competes with Sec1 for binding Sec6. Analytical gel filtration runs (Superdex 200 column) of purified Sec1, Sec6, Sec9CT, and the various combinations were monitored by the absorbance at 280 nm. Retention volumes of γ-globulin (158 kDa) and ovalbumin (44 kDa) standards are shown for comparison. The presence of each protein in the fractions was determined by Western blot analyses using α-V5 for Sec1, α-Sec6, and α-Sec9 antibodies. Note that the molar extinction coefficient of Sec9 at 280 nm is ∼30-fold less than that of Sec6 and Sec1.
Interestingly, addition of equimolar amounts of Sec1, Sec6, and Sec9 together resulted in a Sec6–Sec9 complex (apparent molecular weight, ∼235 kDa) and monomeric Sec1 (apparent molecular weight, ∼90 kDa). Ternary Sec6-Sec9-Sec1 complexes were not observed. Thus, Sec9 robustly competes with Sec1 for Sec6 binding, suggesting that either the Sec1- and Sec9-binding sites on Sec6 are similar or that Sec1 binding induces an allosteric conformational change in Sec6 that is incompatible with Sec9 binding. Furthermore, Sec1 binding appears to compete with Sec6 dimerization, whereas two molecules of Sec9 bind per Sec6 dimer. These data are supported by our results showing that the N-terminal domain of Sec6 is required for binding both Sec1 and Sec9 and also for dimerization (Figure 1B; Sivaram et al., 2005). However, because Sec1 competes with Sec6 dimerization while Sec9 does not, the binding sites for Sec1 and Sec9 on Sec6 are not identical but likely overlap.
Sec6–exocyst interacts with Sec1, whereas non-exocyst-bound Sec6 binds to Sec9
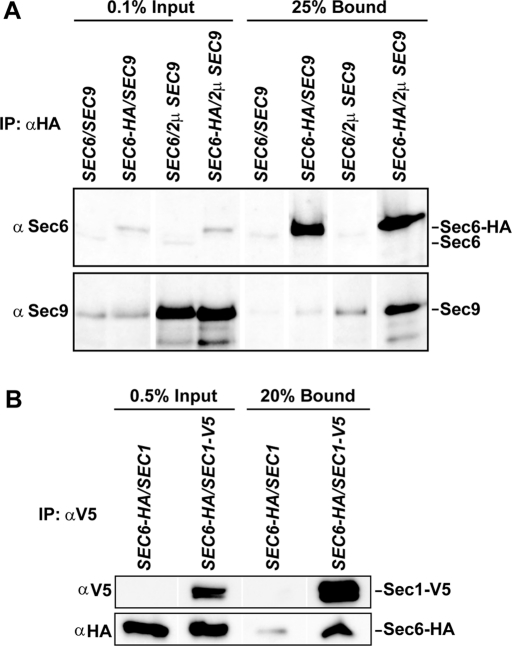
To test Sec6 interactions in vivo, α-HA antibody was used to immunoprecipitate Sec6-HA3 from yeast lysates. A small fraction of Sec9 coimmunoprecipitated with Sec6, unless Sec9 was overexpressed (Figure 4A). Nonspecific binding of Sec1 to the α-HA antibody produced high background levels; therefore, we expressed Sec1-V5 in the Sec6-HA3 strain and immunoprecipitated Sec1 with the α-V5 antibody. Sec6 coimmunoprecipitated with Sec1-V5 (Figure 4B); the amount of Sec6 coimmunoprecipitated was consistently above background levels, although the amount bound was only a fraction (∼1%) of the total Sec6 in the cell. This low percentage was similar to the amount that we observed for the Sec6–Sec9 interaction (Figure 4A). In contrast, the other exocyst subunits readily coimmunoprecipitate with Sec6-HA3 (Songer and Munson, 2009). The Sec1–Sec6 data are consistent with previous results from the Novick lab, in which a comparable amount (∼0.2–0.4%) of Sec1 bound to the exocyst complex, when coimmunoprecipitated with either Sec8-Myc or Sec10-Myc (Wiederkehr et al., 2004). Together, these results suggested that Sec1 can interact with exocyst-bound Sec6.
FIGURE 4:
Sec6 interacts with Sec9 and Sec1 in vivo. (A) Immunoprecipitation of Sec6-HA3 from yeast lysates with α-HA antibody pulls down endogenous full-length Sec9 and Sec9 protein overexpressed from a 2μ plasmid. The input and bound samples were run on 8% SDS–PAGE and immunoblotted for Sec6 and Sec9. (B) Sec1 immunoprecipitated from yeast lysates binds endogenously expressed Sec6-HA3. The input and bound samples were run on 8% SDS–PAGE and immunoblotted for V5 and HA epitope tags.
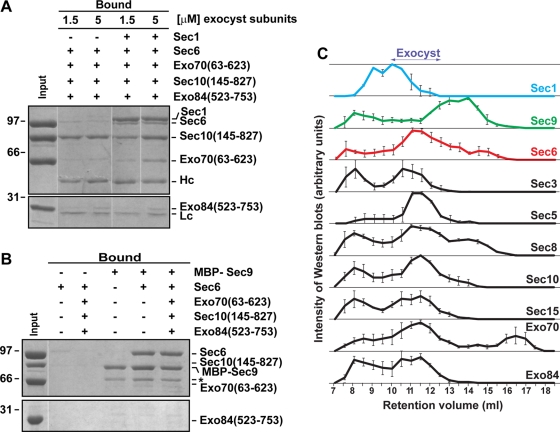
We tested whether recombinant Sec6 could interact with Sec1 while bound to other exocyst subunits in vitro. The pairwise affinities of the exocyst–exocyst interactions appear to be quite weak (estimated >10 μM; Dong et al., 2005; Sivaram et al., 2006). Therefore we analyzed the binding of Sec6–Sec1 to a combination of exocyst subunits. Sec1 was immobilized on the resin and prebound to Sec6. After washing to remove the unbound Sec6, an equimolar mixture of Exo70, Sec10, and Exo84CT was added. The exocyst subunits were able to weakly interact with the Sec6–Sec1 complex (Figure 5A). Exo70 showed the most robust interaction, while Sec10 and Exo84CT also showed some nonspecific binding to the resin. In contrast, Sec6 bound to immobilized MBP-Sec9CT did not interact with the exocyst subunits (Figure 5B). Together, these data indicate that Sec1 can interact with Sec6 in the presence of the other exocyst subunits and may form a weak or transient complex with the exocyst in vivo. In contrast, Sec9 appears to compete with exocyst subunits for Sec6 binding.
FIGURE 5:
Sec1 interacts with exocyst-bound Sec6, whereas Sec9 interacts with non-exocyst-bound Sec6. (A) In vitro, purified exocyst subunits were added simultaneously at 1.5 and 5 μM concentration to preformed immobilized Sec1-V5-His6-Sec6 complexes. After 1 h of incubation at 4°C, input and bound samples were run on 8 and 12% SDS–PAGE and stained with Coomassie blue dye. (B) Similar experiments were performed using MBP-Sec9 immobilized on amylose resin. Purified exocyst subunits (5 μM) were incubated with immobilized Sec9 or preformed Sec9–Sec6 complexes. Bound proteins were run on SDS–PAGE gels and stained with Coomassie blue dye. Asterisk indicates a degradation product of the MBP-Sec9 protein. (C) Superose 6 10/30 analytical gel filtration of yeast lysates was analyzed by Western blot analyses using specific polyclonal antibodies. Band intensities were normalized to the most intense band as visualized by ECL, data points were averaged over three runs, and the error bars represent mean ± SEM.
Gel filtration studies also supported this idea. Sec1, Sec9, and the exocyst subunits from yeast lysates were run on an analytical gel filtration column and their mobility monitored by Western blot analyses (Figure 5C). We previously showed that Sec6 is present in both exocyst-bound and non-exocyst-bound fractions, although the magnitudes of the pools varied substantially between different experiments (Sivaram et al., 2005). We originally proposed that the nonexocyst pool contained a dimer of Sec6 because it migrated with approximately twice the molecular weight of Sec6, but we could not rule out other complexes containing Sec6. Here we found that assembled exocyst complexes migrated as a clear peak (∼10–12.5 ml), with an apparent molecular weight of >1 MDa. A nonexocyst pool of Sec6 (93 kDa) is observed (apparent molecular weight ∼250 kDa), which comigrated with a fraction of Sec8 (122 kDa). A non-exocyst-bound pool of Exo70 was also observed (apparent molecular weight ∼70 kDa), consistent with a monomeric pool of Exo70 (molecular weight 71 kDa) that exists at the plasma membrane (Boyd et al., 2004; He et al., 2007). The major peak of Sec1 was found as a high molecular weight species (apparent molecular weight >1 MDa), which partially overlaps with the exocyst complex peak. The mobility of Sec1 is consistent with a small fraction of Sec1 interacting with the exocyst (Wiederkehr et al., 2004), with the majority either interacting with other binding partners or oligomerizing. Conversely, Sec9 was predominantly found as a lower molecular weight species, with mobility overlapping that of the non–exocyst-bound pool of Sec6. The apparent molecular weight (∼600 kDa) of Sec9 is also much larger than expected for a monomer (74 kDa), suggesting oligomerization and/or interactions with other partners. In addition, many of the proteins were present in the void volume (∼8 ml) of the column, indicating that they were forming large oligomers or aggregates. The gel filtration studies support the idea that the Sec6–Sec1 interaction occurs within the exocyst complex, but that Sec6–Sec9 is excluded from the exocyst complex.
Regulation of binary SNARE complex assembly
The rate-limiting step in SNARE complex assembly is Sec9 binding to the syntaxin Sso1 (or its homologue Sso2; Nicholson et al., 1998). Formation of the Sec9–Sso1 complex is inhibited in vitro and in vivo by the N-terminal autoinhibitory domain of Sso1 (Nicholson et al., 1998; Munson et al., 2000; Munson and Hughson, 2002). This Sso1 autoinhibition must be specifically released upon vesicle arrival at sites of secretion to facilitate assembly of the t-SNARE complex. Subsequently, the v-SNARE Snc2 (or its homologue Snc1) binds, leading to membrane fusion (Nicholson et al., 1998). The factor(s) that release the Sso1 inhibition are unknown.
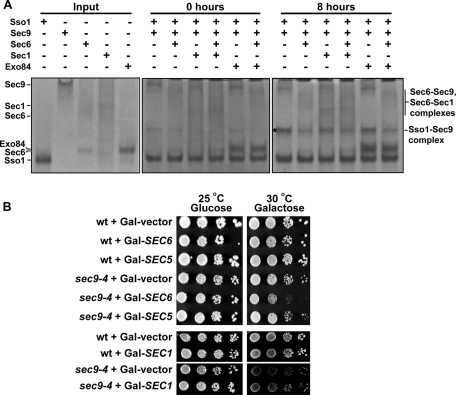
We originally hypothesized that the exocyst subunit Sec6, because it binds Sec9, might be the factor that releases the Sso1 inhibition, opening it for SNARE complex assembly. Instead, addition of purified Sec6 inhibits formation of the binary Sso1–Sec9 SNARE complex by ∼3.5-fold (Sivaram et al., 2005; Figure 6A). The likely explanation is that Sso1 has an infrequent rate of spontaneous opening; when Sso1 does become open, Sec9 is unavailable because it is bound to Sec6, lowering the effective concentration of free Sec9 and slowing the rate of SNARE assembly. This Sec6 inhibition of SNARE complex assembly is supported by yeast genetic experiments. Overexpression of Sec6, which is not toxic to wild-type yeast cells, shows a strong synthetic effect when combined with the sec9-4 temperature-sensitive mutation, inhibiting cell growth (Figure 6B). In contrast, overexpression of either the exocyst subunit Sec5, or Sec1, which is proposed to have a positive role in SNARE complex assembly and membrane fusion, has no effect on the growth of sec9-4 cells.
FIGURE 6:
Sec6 inhibition of SNARE complex assembly. (A) Sec6 inhibits Sec9–Sso1 formation; this is not relieved by addition of Sec1. Purified Sec6 ± Sec1 proteins were incubated at equimolar concentrations with Sso1 and Sec9 for 0 or 8 h at 18°C to allow SNARE complex assembly. Reactions were run on 6% native PAGE gels and stained with Coomassie blue. Representative gels (more than three experiments at four different time points were run) for the 0 h (middle) and 8 h (right) time points are shown; the uncomplexed proteins were run on a separate gel for comparison (left). Asterisk indicates the mobility of the Sso1–Sec9 complex. (B) Overexpression of SEC6, but not SEC5 or SEC1, has a synthetic defect when combined with sec9-4. Tenfold dilution series of wild-type or sec9-4 strains, transformed with vector alone, Gal-SEC6, Gal-SEC5, or Gal-SEC1-V5-His6, were plated on SC-leu or SC-ura plates, respectively, containing either glucose or galactose, and incubated at the indicated temperatures.
One possibility for the requirement of Sec1 prior to SNARE complex assembly (Hashizume et al., 2009) is that Sec1 would bind Sec6 in order to release the inhibited Sec6–Sec9 intermediate, to drive binary SNARE complex assembly. However, consistent with the inability of Sec1 to robustly compete with Sec9 for Sec6 binding (Figure 3), we found that addition of Sec1 to the Sec6-containing reaction did not increase the rate of SNARE assembly (Figure 6A). In fact, addition of Sec1 alone slightly inhibited the reaction, although not to the same extent as Sec6, presumably due to the weakly competing Sec1–Sec9 and Sec1–Sso1 interactions (Figure 2, B and C). In contrast, the Exo84CT subunit, which does not directly interact with Sec6 in the absence of other exocyst subunits, has no effect on the rate of binary SNARE complex assembly. Thus, the Sec1–Sec6 interaction alone is not sufficient to promote the SNARE complex assembly reaction in vitro.
DISCUSSION
SM proteins and tethering complexes are two separate families of proteins known to be intimately involved in the final stages of membrane trafficking, through both their recognition of vesicle and target membranes and their interactions with the membrane-fusing SNARE proteins. However, their mechanisms of action have been enigmatic (Carr and Rizo, 2010; Yu and Hughson, 2010), possibly because these proteins have been studied independently.
Here we uncover a molecular link between these families—the exocyst subunit Sec6 directly binds the exocytic SM protein Sec1. Importantly, Sec6 is the exocyst subunit that binds the t-SNARE Sec9, thus inhibiting Sec9 from forming SNARE complexes with its partner syntaxin Sso1 in vitro (Sivaram et al., 2005). Sec6–Sec1 is compatible with Sec6–exocyst interactions, whereas Sec6 and Sec9 interact in the absence of the other exocyst subunits (Figure 5). Furthermore, the N-terminal domain of Sec6 is required for both Sec6–Sec1 and Sec6–Sec9 (Sivaram et al., 2005; Figure 1B), suggesting that the binding sites on Sec6 may be overlapping for Sec1 and Sec9. Consistent with these data, we were unable to detect a Sec6-Sec1-Sec9 ternary complex (Figure 3) or any Sec6–Sec1 complexes in the presence of Sec9 (Figure 3). These data suggest a model in which Sec6–exocyst coordinates with Sec1 to regulate SNARE complex assembly.
Sec1 had appeared to be an outlier of the SM protein family; unlike other SM proteins, it did not seem to interact with individual SNARE proteins (Carr et al., 1999; Scott et al., 2004; Togneri et al., 2006), but instead bound ternary Sso1-Sec9-Snc2 complexes (Togneri et al., 2006). In fact, Sec1 and Sso1 lack the conserved binding sites observed for SM proteins bound to the N-peptides of their cognate syntaxins (Bracher and Weissenhorn, 2002; Peng and Gallwitz, 2004; Arac et al., 2005; Carpp et al., 2006; Hu et al., 2007; Burkhardt et al., 2008; Furgason et al., 2009; Xu et al., 2010). These data suggested that Sec1 has a role downstream of SNARE complex assembly, perhaps driving SNARE-mediated membrane fusion (Scott et al., 2004). However, characterization of novel Sec1 mutants recently uncovered a role for Sec1 prior to SNARE complex assembly (Hashizume et al., 2009). Our in vitro binding assays revealed weak interactions with both Sso1 and Sec9 (Kd > 10 μM; Figure 2), supporting the idea that Sec1 and Sec6–exocyst may work together to facilitate proper SNARE complex assembly.
We propose that the Sec6–Sec1 interaction is critical for targeting Sec1 to sites of polarized secretion. The weak interactions between Sec1 and the individual SNARE proteins are not likely to recruit Sec1; furthermore, neither Sso1 nor Sec9 localizes specifically to sites of secretion on the plasma membrane (Brennwald et al., 1994). In contrast, Sec6 is critical for anchoring the exocyst complex at sites of secretion on the plasma membrane (Songer and Munson, 2009). We propose that exocyst-bound Sec6 recruits Sec1 to sites of secretion, where it is handed off to newly formed ternary SNARE complexes for membrane fusion (Scott et al., 2004). Consistent with this idea, destabilization or mislocalization of the exocyst complex, for example, in the sec6-4 mutant, leads to loss of Sec1 localization (Grote et al., 2000).
In contrast to Sec1 recruitment, Sec6 is not a likely candidate to be the factor that recruits Sec9 to the plasma membrane. Unlike its homologue SNAP-25, Sec9 has no lipid modifications to anchor it and thus requires a binding partner on the plasma membrane. Moreover, Sec9 is dispersed throughout the plasma membrane, whereas Sec6 and Sec1 are specifically polarized to sites of secretion. Instead, we propose that Sec6–Sec9 holds Sec9 in an inactive state (perhaps an assembly intermediate) at sites of secretion, where Sso1 becomes activated, to prevent premature or inappropriate SNARE assembly and vesicle fusion. The small amount of Sec6 that is Sec9 bound (Figure 4) is consistent with the low abundance of Sec9 localized to sites of exocytosis.
What releases Sec6 from Sec9 to drive SNARE complex assembly? At sites of secretion, a small amount of unbound Sso1 and Sec9 would be present. The factor that opens Sso1 would also be localized to these sites; any SNARE complex assembly caused by premature opening of Sso1 at these sites would be blocked by the Sec6–Sec9 interaction. Vesicle arrival would then trigger exocyst assembly (Boyd et al., 2004), leading to the release of Sec6 from Sec9, concomitant with Sso1 opening. These changes may also occur concurrently with Sec1 recruitment to provide coordinated regulation of vesicle arrival, tethering, and production of fusion-ready SNARE complexes. Individually, the Sec6–exocyst interactions are weak and do not compete with Sec9 for binding Sec6; however, the multivalent combination of exocyst subunits would likely be strong enough to release Sec9. Furthermore, it is possible that in the cell, Sec1 may exist at a high enough local concentration at sites of secretion to drive Sec9 release, likely in conjunction with exocyst assembly.
The exocyst and Sec1 are also likely to interact with the Sso1 opener, whose identity is unknown. Evidence suggests that Caenorhabditis elegans Unc13 and the mammalian Munc13 may provide this function for Munc18-bound syntaxin-1 (Betz et al., 1997; Richmond et al., 2001; Guan et al., 2008; Ma et al., 2011). There is no obvious homologue for Munc13 in yeast; the most likely candidate(s) are members of the exocyst complex, which were recently shown to be structurally homologous to the MUN domain of Munc13 (Li et al., 2011). However, no opening activity has been directly observed for any exocyst subunit or the complex.
The cooperation between Sec1 and the exocyst represents a general phenomenon for SM proteins and their partner tethering complexes. For example, the vacuolar SM protein Vps33 is an essential component of the class C/HOPS complex (Sato et al., 2000; Seals et al., 2000). Sly1, the SM protein that regulates traffic between the endoplasmic reticulum and Golgi, was recently shown to interact with the assembled COG complex (Laufman et al., 2009). Interactions between tethering complexes and SM proteins have been shown to be important for proofreading SNARE complexes and/or for SNARE complex formation (Koumandou et al., 2007; Starai et al., 2008; Laufman et al., 2009), suggesting that Sec1-Sec6-exocyst may add further regulation and specificity to the fusion process.
Elucidation of these interactions is a critical step toward understanding the molecular mechanism(s) underpinning the regulation of specific SNARE complex assembly. Future studies will require reconstitution and testing of assembled exocyst complexes and generation of novel separation-of-function mutants to further characterize these events. In addition, tethering complexes and SM proteins are not the only regulators at these sites; in yeast, the tomosyn homologue Sro7 interacts with Sec9 and the exocyst to form a parallel regulatory pathway (Lehman et al., 1999; Zhang et al., 2005; Grosshans et al., 2006), and a fungal-specific Sec1 cofactor, Mso1, may bridge Sec1 and Sso1 to facilitate SM recruitment and SNARE regulation (Castillo-Flores et al., 2005; Weber et al., 2010; Weber-Boyvat et al., 2011). Their precise role in these processes and their potential cooperation with the exocyst and Sec1 require further study. Identification of the factor responsible for triggering the open conformation of Sso1, in combination with the known regulatory proteins and SNAREs, will lead to a more complete picture of how SNAREs are inhibited and then released at the proper time and place for specific vesicle docking and fusion.
MATERIALS AND METHODS
Protein expression and purification
Recombinant Sec6 (1–805; Sivaram et al., 2005), Exo70 (63–623; Dong et al., 2005), Exo84CT (523–753; Dong et al., 2005), Sec10 (145–827; Croteau et al., 2009), Sec8 (Sivaram et al., 2006), and the cytoplasmic regions of the SNARE proteins Sso1, Snc2, and Sec9CT (416–651) (Nicholson et al., 1998) were overexpressed in Escherichia coli and purified as described. Note that this C-terminal Sec9 construct used in all the in vitro studies is the domain of Sec9 homologous to the mammalian SNAP-25 protein and is sufficient for Sec9 function in yeast (Brennwald et al., 1994).
C-terminally V5-His6 tagged Sec1 was expressed in Saccharomyces cerevisiae from the pYES/CT Sec1-V5-His6 plasmid (Togneri et al., 2006). After induction with 2% galactose for 8–10 h, cells were harvested and resuspended in wash buffer (20 mM Tris, 20 mM NaF, 20 mM NaN3, pH 7.5). Lysis was performed using a Microfluidizer 110S (Microfluidics, Newton, MA) cell disruptor in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 150 mM KCl, 15 mM imidazole, and 10% glycerol, pH 7.4, in the presence of phenylmethylsulfonyl fluoride (PMSF; 1 mM), β-mercaptoethanol (5 mM), and protease inhibitor tablets (Roche, Indianapolis, IN). Lysate was clarified by centrifugation (13,000 rpm, 30 min), and the supernatant was ultracentrifuged to remove aggregated particles (37,000 rpm, 40 min, in a Beckman 50.2 Ti rotor [Beckman Coulter, Brea, CA]). Sec1 was purified on nickel-nitriloacetic acid (Ni NTA) resin (Qiagen, Valencia, CA) and eluted with 200 mM imidazole. Fractions were analyzed by SDS–PAGE and Coomassie blue staining; those fractions containing pure Sec1 were pooled and exchanged into 50 mM HEPES, 250 mM KCl, 10% glycerol, and 1 mM dithiothreitol (DTT), pH 7.4 buffer, using a PD10 column (GE Healthcare Piscataway, NJ). All proteins (except Sec8, which is sparingly soluble) were >90% pure, as assayed by SDS–PAGE. Protein concentrations were determined by quantitative ninhydrin protein assay (Rosen, 1957).
In vitro binding assays
All binding experiments were repeated at least three times, and representative data are shown for each. For in vitro binding of Sec1 to recombinant proteins, 0.8 μg (∼0.2 μM) of purified Sec1-V5-His6 was immobilized on protein G beads using α-V5 antibody (Invitrogen, Carlsbad, CA) and washed with binding buffer (50 mM HEPES, 150 mM KCl, 10% glycerol, 0.5% NP-40, 1 mM DTT, pH 7.4) lacking protease inhibitors. Purified proteins or purified protein complexes (1–2 μM) (purified exocyst subunits and SNAREs) were then added to the immobilized Sec1, and the mixture was incubated for 2 h at 4°C with mixing. Beads were washed with binding buffer, and the bound beads and supernatant were run on SDS–PAGE gels. Gels were either stained with Coomassie blue or analyzed by immunoblotting with α-His5 antibody (Qiagen).
For in vitro binding of Sec9 to exocyst subunits (individually or in combinations), MBP-tagged Sec9 protein was purified using amylose agarose affinity chromatography (New England BioLabs, Ipswich, MA). The purified protein immobilized on the amylose beads was then washed in potassium phosphate buffer and incubated for 1 h at 4°C with equimolar concentration of purified proteins. Beads were washed with the binding buffer, and then beads and supernatant were separated by SDS–PAGE and stained with Coomassie blue.
Similarly, C-terminally Strep-tagged Sec6 (WSHPQFEK) was immobilized on Strep-Tactin beads (engineered streptavidin from IBA GmbH, Göttingen, Germany) and washed with binding buffer (50 mM HEPES, 150 mM KCl, 10% glycerol, 0.5% NP-40, 1 mM DTT, pH 7.4). Equimolar purified His-V5-Sec1 (1 μM) was added to the Strep-Tactin beads alone and to the beads with the immobilized Sec6 and incubated for 1 h at 4°C. Beads were washed with the binding buffer, and then beads and supernatant were separated by SDS–PAGE and stained with Coomassie blue.
The apparent binding affinity for the Sec1–Sec6 complex was estimated by using the in vitro V5 pull-down assay. Increasing concentrations of purified Sec6 (from 0.5 to 3 μM) were added to immobilized V5-Sec1 on protein G resin. The apparent Kd was estimated from a one-site binding curve fit to the data (GraphPad Prism 5 Software, La Jolla, CA), obtained by plotting the fractional saturation of Sec1 as a function of the total concentration of Sec6 in the binding reaction (apparent Kd is average ± SE from three experiments). Each data point represents a normalized Coomassie-stained band intensity determined by densitometry.
Immunoprecipitations
For the Sec6–Sec9 immunoprecipitations, the yeast strains were grown at 30°C until OD600 of 1.5–2.0. One hundred fifty OD600 units were pelleted and stored at −80°C until use. Pellets were thawed into and washed in 50 ml of 50 mM NaPO4, pH 7.4, resuspended in 5 ml of spheroplasting buffer (50 mM NaPO4 pH 7.4, 1.5 M sorbitol, 10 mM NaN3, 35 mM β-mercaptoethanol), and incubated at 35°C for 40 min at 80 rpm. The resulting spheroplasts were layered onto 5 ml of sorbitol solution (50 mM NaPO4, pH 7.4, 1.5 M sorbitol) and spun at 1000 × g for 10 min. Spheroplasts were lysed using 0.4 ml of lysis buffer (20 mM HEPES, pH 7.4, 150 mM KCl, 1 mM EDTA, 1 mM DTT, 0.5% NP-40, and protease inhibitors), and lysate was cleared at 15,600 × g for 15 min at 4°C. Lysate protein levels were equalized using a Bio-Rad (Hercules, CA) protein concentration assay. Equal protein levels were diluted to 250 μl with lysis buffer and incubated with 40 μl of protein G–coupled beads and 1:200 dilution α-HA antibody for 1 h at 4°C. Beads were washed in 300 μl of lysis buffer, and the bound proteins versus input were separated by SDS–PAGE. Sec6 and Sec9 were detected by immunoblotting with the appropriate primary antibody, α-rabbit IgG (Roche), and then developed with ECL (Amersham, Piscataway, NJ) and imaged using the LAS-3000 (Fujifilm, Tokyo, Japan) and image reader LA-3000 (Fujifilm).
For the V5-Sec1 immunoprecipitations, yeast expressing His6-V5-Sec1 were induced in SC-URA plus 2% galactose shaking for 7 h at 30°C. Pellets, 100 OD, were frozen and stored at −80°C. Each 100-OD pellet was resuspended in 400 μl of lysis buffer (50 mM HEPES, 100 mM NaCl, 1 mM EDTA, 1 mM PMSF, 0.5 mM DTT, 0.5% NP-40, Complete Mini Protease Inhibitor [Roche]) and lysed by vortexing with 50% slurry of 0.5-mm zirconia/silica beads (BioSpec, Bartlesville, OK). Lysates were centrifuged at 13,000 rpm and then at 100,000 × g (Beckman TLA100) to clear cellular debris. The supernatant was then precleared with 15-μl bed volume of protein G–Sepharose beads (Roche) for 30 min, and the protein concentration was determined by Bio-Rad protein assay. Lysates were equalized for total protein content and then incubated with α-V5 (Invitrogen) and 20-μl bed volume of protein G–Sepharose beads for 1.5 h. Beads were washed three times with 500 μl of chilled lysis buffer. Beads were resuspended in 40 μl of 1× SDS–PAGE loading buffer, separated by SDS–PAGE, and analyzed by Western blotting.
Gel filtration analyses
For in vitro analyses using purified Sec6, Sec1, and Sec9 proteins, 200 μl of 5 μM each (individually and in combinations) was loaded on a Superdex 200 30/10 column (GE Healthcare) equilibrated in K phosphate buffer (10 mM potassium phosphate, pH 7.4, 140 mM KCl, 1 mM DTT; bed volume, 24 ml). Eluted proteins were located by monitoring the absorbance at 280 nm. The gel filtration column was previously calibrated using standards (Bio-Rad).
For analyses of exocyst subunits, Sec1 and Sec9 proteins from yeast lysates, MMY205 (MATα sec6Δ::KanMX-4 his3Δ1 leu2Δ0 ura3Δ0 [LEU2 CEN SEC6]) cells were grown to OD600 1.5–2.0 and stored as 150-OD600 pellets at −80°C. Pellets were thawed into and washed in 50 ml of 50 mM NaPO4, pH 7.4, resuspended in 5 ml of spheroplasting buffer (50 mM NaPO4, pH 7.4, 1.5 M sorbitol, 10 mM NaN3, 35 mM β-mercaptoethanol), and incubated at 35°C for 40 min, gently shaking at 80 rpm. The resulting spheroplasts were layered onto a 5 ml of sorbitol solution (50 mM NaPO4, pH 7.4, 1.5 M sorbitol) and spun at 1000 × g for 10 min. Spheroplasts were lysed using 0.3 ml of lysis buffer (20 mM HEPES, pH 7.4, 150 mM KCl, 1 mM EDTA, 1 mM DTT, 0.5% NP-40, and protease inhibitors), and lysate was cleared at 15,000 × g for 40 min at 4°C. Lysate, 200 μl, was loaded into a Superose 6 gel filtration column (GE Healthcare) in lysis buffer without protease inhibitors. Fractions were analyzed for the presence of each protein by immunoblotting using specific rabbit antibodies. Band intensities were determined in Photoshop CS5 (Adobe, San Jose, CA) by taking the average pixel intensity of the band, subtracting out the average pixel intensity of a background sample of equal size from the same lane, and normalizing to a loading control band. Each protein was normalized to the highest band intensity for averaging and SE calculations. Apparent molecular weights were calculated using standard curves generated using known molecular weight standards (Bio-Rad).
SNARE complex assembly assays
Native gel mobility shift assays were performed essentially as described (Nicholson et al., 1998; Sivaram et al., 2005). Purified proteins were incubated together at equimolar concentrations (2 μM) in binding buffer (50 mM HEPES, 150 mM KCl, pH 7.5) at 18°C for 0–24 h (0 and 8 h are shown). Proteins were loaded onto 6% native polyacrylamide gels and buffered at pH 7.4 with 215 mM imidazole and 175 mM HEPES for native gel mobility shift assays. The gels were run at 30 mA for 85 min at 4°C and stained with Coomassie blue.
Yeast methods
SEC6 and SEC5 were overexpressed from the Gal-expression vector pPP450 (Peter Pryciak, University of Massachusetts Medical School, Worcester, MA) in wild-type (BY4741; Open Biosystems, Thermo Biosystems, Huntsville, AL) and sec9-4 (MMY623; Songer and Munson, 2009) strains. Sec1-V5-His6 was overexpressed from pYES/CT (Invitrogen) in wild-type (NY179; Novick et al., 1980) and sec9-4 (BY68; Brennwald et al., 1994) strains. Media, growth conditions, and genetic methods for yeast were used as described (Munson et al., 2000).
Acknowledgments
We thank Pat Brennwald (University of North Carolina, Chapel Hill, NC) for exocyst antibodies and Peter Novick (University of California, San Diego, La Jolla, CA) and Peter Pryciak (University of Massachusetts Medical School) for yeast strains and advice. We acknowledge John Togneri (University of Medicine and Dentistry of New Jersey, Piscataway, NJ) for his initial observations of the Sec6–Sec1 interaction. We are deeply grateful to Jen Songer (1979–2008) for all of her advice, technical assistance, and friendship. We thank Fred Hughson, Reid Gilmore, Nia Bryant, Lois Weisman, Sean Ryder, and members of the Munson lab for critical reading of the manuscript and advice. This work was supported by National Institutes of Health Grant GM068803 to M.M.
Abbreviations used:
- SM
Sec1/Munc18
- SNARE
soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-08-0670) on November 23, 2011.
REFERENCES
- Arac D, Dulubova I, Pei J, Huryeva I, Grishin NV, Rizo J. Three-dimensional structure of the rSly1 N-terminal domain reveals a conformational change induced by binding to syntaxin 5. J Mol Biol. 2005;346:589–601. doi: 10.1016/j.jmb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Baek K, Knodler A, Lee SH, Zhang X, Orlando K, Zhang J, Foskett TJ, Guo W, Dominguez R. Structure-function study of the N-terminal domain of exocyst subunit Sec3. J Biol Chem. 2010;285:10424–10433. doi: 10.1074/jbc.M109.096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J Biol Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- Boyd C, Hughes T, Pypaert M, Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher A, Weissenhorn W. Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J. 2002;21:6114–6124. doi: 10.1093/emboj/cdf608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpp LN, Ciufo LF, Shanks SG, Boyd A, Bryant NJ. The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes. J Cell Biol. 2006;173:927–936. doi: 10.1083/jcb.200512024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Rizo J. At the junction of SNARE and SM protein function. Curr Opin Cell Biol. 2010;22:488–495. doi: 10.1016/j.ceb.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Flores A, Weinberger A, Robinson M, Gerst JE. Mso1 is a novel component of the yeast exocytic SNARE complex. J Biol Chem. 2005;280:34033–34041. doi: 10.1074/jbc.M507142200. [DOI] [PubMed] [Google Scholar]
- Croteau NJ, Furgason ML, Devos D, Munson M. Conservation of helical bundle structure between the exocyst subunits. PLoS ONE. 2009;4:e4443. doi: 10.1371/journal.pone.0004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene JO, Coleman J, Estrada de Martin P, Pypaert M, Anderson S, Yates JR, 3rd, Ferro-Novick S, Novick P. Rtn1p is involved in structuring the cortical endoplasmic reticulum. Mol Biol Cell. 2006;17:3009–3020. doi: 10.1091/mbc.E06-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Hutagalung AH, Fu C, Novick P, Reinisch KM. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat Struct Mol Biol. 2005;12:1094–1100. doi: 10.1038/nsmb1017. [DOI] [PubMed] [Google Scholar]
- Dulubova I, Khvotchev M, Liu S, Huryeva I, Sudhof TC, Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci USA. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger FP, Novick P. Synthetic interactions of the post-Golgi sec mutations of Saccharomyces cerevisiae. Genetics. 2000;156:943–951. doi: 10.1093/genetics/156.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furgason ML, MacDonald C, Shanks SG, Ryder SP, Bryant NJ, Munson M. The N-terminal peptide of the syntaxin Tlg2p modulates binding of its closed conformation to Vps45p. Proc Natl Acad Sci USA. 2009;106:14303–14308. doi: 10.1073/pnas.0902976106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D, Jahn R. The riddle of the Sec1/Munc-18 proteins—new twists added to their interactions with SNAREs. Trends Biochem Sci. 2003;28:113–116. doi: 10.1016/S0968-0004(03)00028-8. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Andreeva A, Gangar A, Niessen S, Yates JR, 3rd, Brennwald P, Novick P. The yeast lgl family member Sro7p is an effector of the secretory Rab GTPase Sec4p. J Cell Biol. 2006;172:55–66. doi: 10.1083/jcb.200510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Dai H, Rizo J. Binding of the Munc13-1 MUN domain to membrane-anchored SNARE complexes. Biochemistry. 2008;47:1474–1481. doi: 10.1021/bi702345m. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume K, Cheng YS, Hutton JL, Chiu CH, Carr CM. Yeast Sec1p functions before and after vesicle docking. Mol Biol Cell. 2009;20:4673–4685. doi: 10.1091/mbc.E09-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007;26:4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SH, Latham CF, Gee CL, James DE, Martin JL. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc Natl Acad Sci USA. 2007;104:8773–8778. doi: 10.1073/pnas.0701124104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumandou VL, Dacks JB, Coulson RM, Field MC. Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol Biol. 2007;7:29. doi: 10.1186/1471-2148-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufman O, Kedan A, Hong W, Lev S. Direct interaction between the COG complex and the SM protein, Sly1, is required for Golgi SNARE pairing. EMBO J. 2009;28:2006–2017. doi: 10.1038/emboj.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman K, Rossi G, Adamo JE, Brennwald P. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J Cell Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma C, Guan R, Xu Y, Tomchick DR, Rizo J. The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle tethering factors. Structure. 2011;19:1443–1455. doi: 10.1016/j.str.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat Struct Mol Biol. 2011;18:542–549. doi: 10.1038/nsmb.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Munson M. Tip20p reaches out to Dsl1p to tether membranes. Nat Struct Mol Biol. 2009;16:100–102. doi: 10.1038/nsmb0209-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M, Chen X, Cocina AE, Schultz SM, Hughson FM. Interactions within the yeast t-SNARE Sso1p that control SNARE complex assembly. Nat Struct Biol. 2000;7:894–902. doi: 10.1038/79659. [DOI] [PubMed] [Google Scholar]
- Munson M, Hughson FM. Conformational regulation of SNARE assembly and disassembly in vivo. J Biol Chem. 2002;277:9375–9381. doi: 10.1074/jbc.M111729200. [DOI] [PubMed] [Google Scholar]
- Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Casanova JE. Salmonella-directed recruitment of new membrane to invasion foci via the host exocyst complex. Curr Biol. 2010;20:1316–1320. doi: 10.1016/j.cub.2010.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KL, Munson M, Miller RB, Filip TJ, Fairman R, Hughson FM. Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat Struct Biol. 1998;5:793–802. doi: 10.1038/1834. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Peng R, Gallwitz D. Multiple SNARE interactions of an SM protein: Sed5p/Sly1p binding is dispensable for transport. EMBO J. 2004;23:3939–3949. doi: 10.1038/sj.emboj.7600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodkey TL, Liu S, Barry M, McNew JA. Munc18a scaffolds SNARE assembly to promote membrane fusion. Mol Biol Cell. 2008:5422–5434. doi: 10.1091/mbc.E08-05-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957;67:10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Sato TK, Rehling P, Peterson MR, Emr SD. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Scott BL, Van Komen JS, Irshad H, Liu S, Wilson KA, McNew JA. Sec1p directly stimulates SNARE-mediated membrane fusion in vitro. J Cell Biol. 2004;167:75–85. doi: 10.1083/jcb.200405018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Sivaram MV, Furgason MLM, Brewer DN, Munson M. The structure of the exocyst subunit Sec6p defines a conserved architecture with diverse roles. Nat Struct Mol Biol. 2006;13:555–556. doi: 10.1038/nsmb1096. [DOI] [PubMed] [Google Scholar]
- Sivaram MV, Saporita JA, Furgason MLM, Boettcher AJ, Munson M. Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry. 2005;44:6302–6311. doi: 10.1021/bi048008z. [DOI] [PubMed] [Google Scholar]
- Songer JA, Munson M. Sec6p anchors the assembled exocyst complex at sites of secretion. Mol Biol Cell. 2009;20:973–982. doi: 10.1091/mbc.E08-09-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togneri J, Cheng YS, Munson M, Hughson FM, Carr CM. Specific SNARE complex binding mode of the Sec1/Munc-18 protein, Sec1p. Proc Natl Acad Sci USA. 2006;103:17730–17735. doi: 10.1073/pnas.0605448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonen RF, Verhage M. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol. 2003;13:177–186. doi: 10.1016/s0962-8924(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Toonen RF, Verhage M. Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30:564–572. doi: 10.1016/j.tins.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Weber-Boyvat M, Aro N, Chernov KG, Nyman T, Jantti J. Sec1p and Mso1p C-terminal tails cooperate with the SNAREs and Sec4p in polarized exocytosis. Mol Biol Cell. 2011;22:230–244. doi: 10.1091/mbc.E10-07-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Chernov K, Turakainen H, Wohlfahrt G, Pajunen M, Savilahti H, Jantti J. Mso1p regulates membrane fusion through interactions with the putative N-peptide-binding area in Sec1p domain 1. Mol Biol Cell. 2010;21:1362–1374. doi: 10.1091/mbc.E09-07-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A, De Craene JO, Ferro-Novick S, Novick P. Functional specialization within a vesicle tethering complex: bypass of a subset of exocyst deletion mutants by Sec1p or Sec4p. J Cell Biol. 2004;167:875–887. doi: 10.1083/jcb.200408001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Su L, Rizo J. Binding of Munc18-1 to synaptobrevin and to the SNARE four-helix bundle. Biochemistry. 2010;49:1568–1576. doi: 10.1021/bi9021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Sudhof TC. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell. 2002;2:295–305. doi: 10.1016/s1534-5807(02)00125-9. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Kurokawa K, Sato Y, Yamagata A, Mimura H, Yoshikawa A, Sato K, Nakano A, Fukai S. Structural basis for the Rho- and phosphoinositide-dependent localization of the exocyst subunit Sec3. Nat Struct Mol Biol. 2010;17:180–186. doi: 10.1038/nsmb.1722. [DOI] [PubMed] [Google Scholar]
- Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang P, Gangar A, Zhang J, Brennwald P, TerBush D, Guo W. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J Cell Biol. 2005;170:273–283. doi: 10.1083/jcb.200502055. [DOI] [PMC free article] [PubMed] [Google Scholar]