Abstract
Genetic studies have associated deficient function of the serine/threonine kinase Akt1 with schizophrenia. This disorder is associated with developmental, structural, and functional abnormalities of the hippocampus that could be traced to abnormal Akt1 function. To establish a closer connection between Akt1 and hippocampal function, mice with a selective deletion of Akt1 (Akt1−/− mice) were examined for physiological and behavioral outcomes dependent on the hippocampus and associated with schizophrenia. Genetic deletion of Akt1 was associated with both impaired proliferative capacity of adult-born hippocampal progenitors and hippocampal long-term potentiation, indicating deficient functions of this brain region associated with neuroplasticity. Moreover, Akt1−/− mice demonstrated impairments in contextual fear conditioning and recall of spatial learning, behaviors known to selectively involve the hippocampus. Akt1−/− mice also showed reduced prepulse inhibition of the acoustic startle response, a sensorimotor gating response that is perturbed in schizophrenia. Postmortem tissue samples from patients with schizophrenia showed significant reductions of phosphorylated Akt levels in hilar neurons of the dentate gyrus, the neurogenic zone of the hippocampus. Taken together, these results implicate the Akt1 isoform in regulating hippocampal neuroplasticity and cognition and in contributing to the etiology of schizophrenia.
Keywords: Akt, learning and memory, cognition, schizophrenia, neurogenesis, hippocampus
INTRODUCTION
Schizophrenia is a severe psychiatric disorder that is produced by abnormalities in the development of the central nervous system (Ross et al., 2006). Recent studies have identified a number of genes associated with increased risk for developing schizophrenia (Consortium, 2008; Stefansson et al., 2008; Walsh et al., 2008). Among genetic factors, an association between schizophrenia and genetic variants for AKT1 has been reported in several studies of large family samples of European or Asian descent (Bajestan et al., 2006; Emamian et al., 2004; Ikeda et al., 2004; Schwab et al., 2005; Thiselton et al., 2008; Xu et al., 2007), although not all studies have measured this association (Ide et al., 2006; Liu et al., 2006; Ohtsuki et al., 2004; Turunen et al., 2007). Additional evidence emerges from decreased protein levels of the serine/threonine kinase Akt and levels of substrate phosphorylation reported in postmortem brain samples from some patients with schizophrenia (Emamian et al., 2004; Zhao et al., 2006).
Akt has been shown to be critical for synaptic development, cell proliferation, and protein trafficking (Coffer et al., 1998; Manning and Cantley, 2007). Akt function is reciprocally coregulated by dopamine type-2 (D2) and serotonin (5-HT) receptor signaling using a mechanism involving glycogen synthase kinase-3 beta and the multifunctional scaffolding protein ß-arrestin-2 (Beaulieu et al., 2009). These receptors and signaling pathways are established targets for antipsychotic and mood-altering drugs. The activation of Akt by trophic factors, such as insulin-like growth factor I and brain-derived neurotrophic factor interacting with their cognate receptors, is essential for the survival of neuronal cell types (Patapoutian and Reichardt, 2001). Akt also promotes cell generation and survival by orchestrating the transcription of many genes involved in cell survival and apoptosis (Brunet et al., 2001). Adult hippocampal neurogenesis is a type of neuroplasticity that is regulated by numerous growth and neurotrophic factors and may be impaired in a variety of neuropsychiatric diseases (Balu and Lucki, 2009; Zhao et al., 2008), including schizophrenia (Reif et al., 2006). Thus, Akt is a critical kinase involved in cell signaling and neural development whose deficiency is associated with the neurobiology of schizophrenia.
In this study, Akt1-deficient (Akt1−/−) mice (Cho et al., 2001; Dummler and Hemmings, 2007) were used to understand the consequences of Akt1 gene deletion on behaviors and neurobiological functions specifically involving the hippocampus. Numerous studies have shown the functions of the hippocampus to be strongly associated with schizophrenia (Harrison, 2004; Weinberger, 1999). Akt1−/− mice showed multiple impairments of behavioral and physiological outcome measures of hippocampal function, as well as perturbations in sensorimotor gating. We also demonstrated significant reductions in phosphorylated Akt in hippocampal tissue from patients with schizophrenia. The convergent loss of hippocampal functions in Akt1−/− mice and reduced phosphorylated Akt in the hippocampus of schizophrenic patients suggest that Akt1 deficiency could be used to model behavioral deficits associated with schizophrenia.
MATERIALS AND METHODS
Animals
Akt1−/− mice were generated for this study in a satellite colony established from progeny of founders generated originally by Dr. Morris Birnbaum at the University of Pennsylvania and bred onto a stable C57BL/6 background for >10 generations (Cho et al., 2001). Mice were bred from multiple heterozygous breeding pairs and genotyped by polymerase chain reaction analysis of DNA samples obtained from the tail. The following primers were used in a single reaction: 851, 5-AGCTCTTCTTCCACCTGTCTC-3; 852, 5-GCTCCATAAGCACACCTTCAGG-3; and 853, 5-GTGGATGTGGAATGTGTGCGAG-3. Mice were assigned to different tests so that no single breeding pair produced a disproportionate number of experimental animals. Adult male wild type (WT) and Akt1−/− mice between 3 and 11 months of age were used in all experiments, except for the Morris water maze (MWM) when data from five males and four females from each group were averaged. The animals were housed in groups of 2–4 in polycarbonate cages and maintained on a 12-h light/dark cycle (lights on at 07:00 h) in a temperature (22°C) and humidity controlled colony. Animal procedures were conducted in accordance with the guidelines published in the National Institutes of Health Guide for Care and Use of Laboratory Animals and all protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Hippocampal Cell Proliferation and Survival
Cell proliferation and survival in Akt1−/− mice were determined by measuring the incorporation of 5-bromo-deoxyuridine (BrdU) into the hippocampus. Mice were injected intraperitoneally with 100 mg/kg BrdU (Roche Applied Sciences Indianapolis, IN) in a volume of 10 ml/kg once daily for 4 consecutive days. To analyze cell proliferation, mice (WT, n = 8; Akt1−/−, n = 8) were sacrificed 24 h after the last BrdU injection and incorporation into the hippocampus was measured using flow cytometry (Balu et al., 2009). To analyze cell survival, mice (WT, n = 8; Akt1−/−, n = 7) were sacrificed 14 days after the last BrdU injection.
Analysis of Brain Monoamine Content
Mice (WT: n = 6; Akt1−/−: n = 4) were decapitated and their brains quickly removed for dissection. The hippocampus, frontal cortex, and amygdala were flash frozen and stored at −80°C until preparation for high performance liquid chromatography analysis. Tissue samples were homogenized in 0.1 N perchloric acid with 100 μM ethylenediaminetetraacetic acid (15 μl/mg of tissue) using a tissuemizer (Tekmar, Cleveland, OH). Samples were centrifuged at 15,000 rpm (23,143g) for 15 min at 2–8°C. The supernatants were filtered using Costar Spin-X™ centrifugal filters (Fisher Scientific, Pittsburgh, PA) and then split into two aliquots. Samples (12 μl) were injected by an autosampler (Sample Sentinel, Bioanalytical Systems, West Lafayette, IN) and analyzed in separate assays for 5-HT, and for norepinephrine and dopamine (DA) content using high performance liquid chromatography coupled with electrochemical detection. Details of the assay procedure have been published previously (O’Leary et al., 2007).
Long-Term Potentiation
Mice where anesthetized with isoflurane and decapitated, their brains removed, and blocked in ice-cold artificial cerebrospinal fluid (ACSF)-sucrose [containing the following (in mM): 130 sucrose, 3 KCl, 1.25 NaHPO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3, and 10 dextrose], where NaCl is replaced with sucrose. Hippocampal slices (350 μm) were cut at 12° off horizontal with a vibrating tissue slicer (VT1000S; Leica, Deerfield, IL). After preparation, slices were transferred into ACSF.
For CA1 long-term potentiation (LTP), Schaffer collateral stimulation was administered with the electrode placed near the border of CA1 and CA3 in stratum radiatum. In dentate gyrus (DG), the medial perforant path was targeted for stimulation and recording. Activation of the medial perforant path was confirmed by the presence of paired-pulse depression. In both cases, baseline stimulation was every 30 s, tetanic stimulation was 100 Hz for 1 s. To record the field response, a patch electrode filled with oxygenated ACSF and an Axoclamp 2D amplifier (Molecular Devices, Sunnyvale, CA) was used. Field excitatory postsynaptic potentials (fEPSPs) were captured into pClamp10 and analyzed using Neuromatic (Jason Rothman) in Igor (Wavemetric, Lake Oswego, OR).
Fear-Conditioned Learning
Mice (WT, n = 11; Akt1−/−, n = 9) were conditioned using a Near Infrared Video Fear Conditioning system (MED Associates, East Fairfield, VT). On the training day, mice were placed in the chambers and were acclimated to the chamber for 280 s, after which they received five trials where a 20-s tone (70 dB, 2 kHz) coterminated with a 2-s shock (0.5 mA), with an 80-s intertrial interval. The mice were tested 24 h later for their freezing responses to the context they were trained in and to the cue (tone). To evaluate contextual fear conditioning, mice were placed in the same chambers they were trained in and the amount of freezing was recorded for 5 min. To assess freezing in response to the cue, mice were placed in a different chamber from which they were trained and three presentations of the tone (70 dB, 2 kHz) were played for 20 s, with an interval of 80 s separating trials. The order of the contextual and cued tests was counterbalanced across both groups. Mice were returned to their home cage between tests for 5 min.
Freezing behavior was scored by a blind observer during the 5-s intervals of the test periods from the video recorded sessions. The percentage of freezing was calculated by scoring the number of intervals freezing relative to the total number of possible freezing events during the test period. Baseline freezing was measured during the period before the presentation of the tones during the training session. Freezing on day 2 to the context was scored during the entire 5-min test period, while freezing to the cue was scored during the three tone presentations.
MWM
The MWM was conducted in a pool (117-cm diameter) filled with opaque water (nontoxic white paint) and maintained at a temperature of 23–25°C. The visual acuity of the mice (WT, n = 9; Akt1, n = 9) was tested on days 1 and 2 before hidden platform training. The platform was made visible during the first 2 days. Each mouse received four trials per day with a 15-min intertrial interval.
For hidden platform training, the platform was submerged 2 cm below the surface of the water. Mice received four training trials per day with a 15-min intertrial interval. If the mouse failed to reach the platform after 60 s, it was placed on the platform by the experimenter. Training was performed for 8 consecutive days.
On day 9, a probe trial was conducted by removing the platform and allowing the mice to swim for 60 s. Each trial was video recorded from above and digital video output was analyzed by an IBM-compatible computer running SMART II Video Tracking System software (San Diego Instruments, San Diego, CA). On day 10, hidden platform training was started again except that the platform was placed in a different quadrant. The mice received four training trials per day for 5 consecutive days.
Startle Response and Prepulse Inhibition
WT (n = 11) and Akt1−/− (n = 8) mice were acclimated to the room for 1 hour before testing. Startle responses and inhibition of startle response after presentation of a nonstartling prepulse were registered by an accelerometer in response to acoustic stimuli delivered by a white noise generator (4–19 kHz) in a four-chamber system (San Diego Instruments). After the mouse was placed in the test chamber, the sessions began with a 5-min acclimation interval to a background white noise of 60 db. This was followed by a block of five 120 db startle pulses in an effort to make the subsequent startle responses less variable. During the next 10-min block, startle responses were measured to 40 ms pulses of 0 (control), 90, 95, 100, 105, 110, 115, and 120-db sound pressure. Each of the intensities was presented five times in random order with an interstimulus interval randomized from 10 to 20 s with a mean of 15 s. The startle portion of the session concluded with an additional block of five 120 db pulses to assess potential effects of habituation. Startle trials were followed by a 10-min block of prepulse inhibition (PPI) trials. Each prepulse trial consisted of a 20 ms prepulse 4, 8, or 16-db above background noise (60 db) followed by a 40 ms pulse of 120 db 100 ms later. Five trials of each prepulse intensity, along with 10 startle-only trials, were presented in random order. Startle responses were collected as 60, 1 ms voltage readings, which were averaged over the collection interval to obtain an average measure for each trial using San Diego Instruments Startle Reflex Software. For PPI, the average startle response was used as the primary dependent measure of the startle reflex. Percentage of prepulse inhibition was calculated using the following formula: [100 − (startle + prepulse/startle alone) × 100], where “startle alone” was obtained from trials conducted in the absence of prepulse stimuli.
Locomotor Activity
WT (n = 10) and Akt1−/− (n = 8) mice were placed in empty mouse cages with no bedding for 30 min. Their behavior was videotaped from above. Digital video output was analyzed by an IBM-compatible computer running SMART II Video Tracking System software (San Diego Instruments). Locomotor activity was defined as the distance traveled over the 30-min test period.
Elevated Zero Maze
The elevated zero maze (Stoelting, Wood Dale, IL) was elevated 24 in. from the ground and consisted of two open areas (wall height, 0.5 in.) and two closed areas (wall height, 12 in.). Mice (WT: n = 10, Akt1−/−: n = 7) were acclimated to the room for 1 hour before testing, after which each mouse was placed in the closed area and the duration of testing was 5 min. The Viewpoint Tracking System (Viewpoint, Champagne au Mont d’Or, France) was used to video record and measure the time spent in the open areas. The test was performed in dim ambient lighting (15 lux).
Phosphorylated Akt-1 Levels in Schizophrenia
A matched-pairs design (n = 18 per group) was used in that each schizophrenia case was matched in sex, age (within 5 y), and hemisphere sampled to a normal control case that was verified using psychiatric and cognitive criteria. All subjects were autopsied at the University of Pennsylvania with consent of their families or guardians, and participated in a longitudinal study of prospectively diagnosed subjects as detailed elsewhere (Talbot et al., 2004). They had met diagnostic criteria for schizophrenia in the Diagnostic and Statistical Manual (DSM-IV) when interviewed before death. None of the cases had an alternate or ambiguous DSM-IV diagnosis nor did they have a history of substance abuse or neurological disorders predating onset of psychiatric symptoms (e.g., epilepsy or traumatic brain injury) or postdating them (e.g., anoxia or stroke). All the cases were free of gross neurodegeneration and did not have abnormal levels of senile plaques, neurofibrillary tangles, or Lewy bodies. The demographic characteristics of the subjects are shown in Table 1. There were no significant group differences between cases and controls in age, postmortem interval, or brain tissue pH.
TABLE 1.
Demographic Characteristics of Controls and Patients with Schizophrenia
| Controls | Schizophrenia | |
|---|---|---|
| Age | 79.4 ± 8.8 | 80.3 ± 7.4 |
| Sex | ||
| Male | 7 | 7 |
| Female | 11 | 11 |
| Total | 18 | 18 |
| Postmortem interval (h) | 11.2 ± 5.2 | 10.8 ± 4.0 |
| Brain tissue pH | 6.34 ± 0.25 | 6.51 ± 0.27 |
| Hemisphere studied (left/right) | 8/10 | 8/10 |
Formalin-fixed tissue from intermediate rostrocaudal levels of the hippocampal formation (HF: consisting of the dentate gyrus + hippocampal fields CA1–3 + subiculum) was embedded in paraffin and sectioned coronally at 6 μm. The sections were reacted immunohistochemically with a standard avidin-biotin-peroxidase method (Talbot et al., 2004) using an antibody specific for Akt phosphorylated at serine 473 (Cell Signaling Technology, Danvers, MA, 4,051) at a dilution of 1:100. All sections were reacted in a single experiment to meet the conditions for quantitative immunohistochemistry. Average levels of neuronal pAkt (S473) in the dentate gyrus hilus and pyramidal layer of CA3 were assessed by quantifying the net optical density (total minus background) of the cellular immunoreactivity. Optical density was measured microscopically using Image-Pro Plus software (Media Cybernetics, Silver Springs, MD).
Statistical Analyses
All comparisons between WT and Akt1−/− mice, except the acquisition of the water maze, LTP, PPI, and startle reactivity, were made using unpaired Student’s t test. For LTP, the Wilcoxon rank test was used to compare responses at 4-min post-tetanus. Repeated measures analysis of variance was used to compare the latencies with find the hidden platform across training days, the % PPI, and startle responses across decibel intensities between WT mice and Akt1−/− mice. The postmortem tissue was analyzed as matched pairs using the Wilcoxon signed ranks test. P < 0.05 was considered statistically significant.
RESULTS
Adult Hippocampal Neurogenesis
Adult WT or Akt1−/− mice were loaded with BrdU (100 mg/kg (i.p.) × 4 days) and then sacrificed either 24 h or 14 days after the last injection to determine the effects of Akt1 deficiency on cell proliferation and survival, respectively. The Akt1−/− mice had significantly lower (45%) levels of hippocampal cell proliferation as compared with WT mice (Fig. 1a; t(14) = 3.12, P = 0.008). Akt1−/− mice demonstrated no differences in the survival of newly born hippocampal progenitors as compared with WT mice measured 2 weeks after labeling with BrdU (Fig. 1b; t(13) = 0.02, P > 0.05).
FIGURE 1.
Akt1 regulates adult hippocampal cell proliferation, but not survival. To assess cell (a) proliferation and (b) survival, WT (black bars; n = 8) and Akt1−/− (white bars; n = 7–8) mice were sacrificed 24 h or 14 days after the last BrdU injection, respectively. Values are expressed as the number of BrdU positive cells per 10 000 7-AAD events. Bars represent mean values + SEM. Asterisk indicates that Akt1−/− mice differed significantly from WT (*P < 0.05).
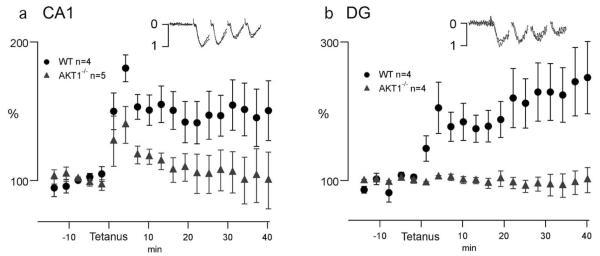
LTP in the Hippocampus
To determine the impact of Akt1 on hippocampal LTP, we examined the ability of the Akt1−/− mice to support LTP in area CA1 and the DG. For probing LTP in the CA1, we activated the CA3 to CA1 synapses in stratum radiatum, whereas in the DG we stimulated the medial perforant path to granule cell synapses. In both areas we used a single train of 100 Hz for 1 second, stimulating at approximately one-third maximal fEPSP amplitude. In the CA1, WT mice responded to Schaffer collateral tetanus with typical fEPSP potentiation lasting over 40 min (Fig. 2a: 150 ± 29.2% of pretetanus levels). In Akt1−/− mice, the tetanus generated short-term potentiation (STP) that was not significantly smaller than WT, yet average LTP was significantly suppressed (Fig. 2a) to 99 ± 26.4% pre-tetanus (P = 0.04). These results demonstrate that loss of Akt1 disrupts long-term plasticity mechanisms in area CA1.
FIGURE 2.
LTP is disrupted in the Akt1−/− mouse. LTP was assessed in area CA1 and dentate in WT (n = 4) and Akt1−/− mice (n = 4–5). A single 1-s 100 Hz stimulus was used that reliably generated LTP in both the CA1 area (a) and dentate gyrus (b) of WT mice (gray triangles). In the CA1 (a), this tetanus generated STP in both the WT and Akt1−/− mice (black, closed circles) during the first 5 min post-tetanus, yet failed to generate LTP in the Akt1−/− mice. The dentate gyrus (b) appeared more severely disrupted, with the tetanus in the Akt1−/− mice unable to generate STP. To show that basic synaptic properties in the Akt1−/− mice remained intact, traces of the first four stimuli from representative experiments were each normalized to their largest peak response and when plotted with artifacts and offset removed (insets) illustrated similar fEPSP properties.
In the DG, the tetanus in slices from Akt1−/− mice was not only unable to induce LTP (Akt1−/−:97 ± 17.0% verses WT 248 ± 52.1%), but in contrast to CA1, the tetanus failed to produce STP (Fig. 2b). The absence of STP is a sign of profound disruption of dentate plasticity mechanisms. Because of this lack of synaptic STP, we analyzed the field response of the stimulus to determine whether the STP suppression was due to lack of stimulation efficacy. Tetanic stimulation produced complex and variable kinetics over the time scale of the whole one-second train that revealed no differences between WT and Akt1−/− mice. Focusing on the first four stimuli of the 100 Hz train that produced consistent responses, no differences were evident between WT and Akt1−/− mice (Fig. 2b) indicating that basic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-dependent synaptic properties were similar in both groups. Thus, the loss of LTP and STP are likely due to disrupted plasticity mechanisms in the DG.
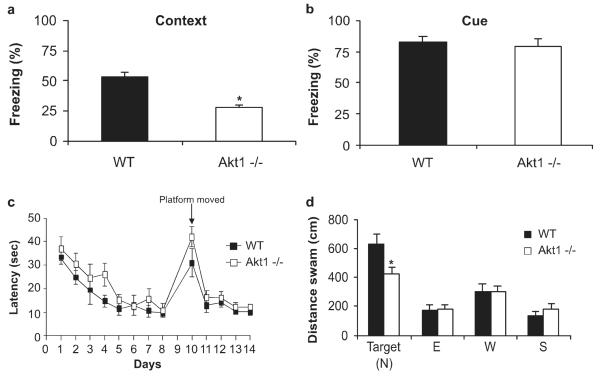
Fear-Conditioned Learning
After contiguous pairing of a tone with electric shock, Akt1−/− mice spent significantly less time freezing in the training context by 50% as compared to WT mice when tested on the day after training (Fig. 3a; t(18) = 4.84, P = 0.001). Baseline freezing behavior did not differ between Akt1−/− and WT mice (t(18) = 1.13, P > 0.05). This response pattern is consistent with impairment in hippocampal function. The animals were also tested for cued fear conditioning, which is dependent on proper amygdala function. There were no differences in cued fear conditioning between WT mice and Akt1−/− mice (Fig. 3b; (t(18) = 0.58, P > 0.05).
FIGURE 3.
Akt1−/− mice demonstrate deficits in contextual fear conditioning and recall of spatial memory. WT (black bars; n = 11) and Akt1−/− (white bars; n = 9), mice were trained for contextual fear conditioning on day 1. (a) Mice were tested on day 2 in their respective training context, and then tested for (b) cued conditioning in a new context. Bars represent the percent time freezing ± SEM during the (a) 5-min context test and (b) the percent time freezing during the tone presentations on day 2. (c) WT (black squares; n = 9) and Akt1−/− (white squares; n = 9) mice were trained in the MWM for 8 days with the hidden platform in the North position. On day 10, the hidden platform was moved to the South position. Values represent the mean (time in seconds taken to reach the platform) ± SEM. (d) On day 9, recall of spatial memory was assessed in the probe trial. Bars (WT, black bars; Akt1−/−, white bars) represent the mean distance swam in each quadrant ± SEM. Asterisk indicates that Akt1−/− mice differed significantly from WT (*P < 0.05). When calculated as the absolute time spent in the target quadrant, Akt1−/− mice again showed a trend toward a significant reduction (WT: 32.6 ± 3.7 s, Akt1−/−: 24.1 ± 2.7 s, P = 0.08).
To evaluate the possibility that decreased contextual fear conditioning was due to reductions in anxiety-like behavior, animals were tested in the elevated zero maze. Akt1−/− mice spent significantly less time in the open areas than WT mice (WT: 121 ± 18, Akt1−/− 67 ± 11; t(15) = 2.24, P = 0.04). This suggests the existence of a modest anxiety phenotype in Akt1−/− mice.
MWM
After 8 days of training in the MWM, there was no difference in the rate at which the Akt1−/− mice learned to find the hidden platform compared with WT mice (Fig. 3c; genotype: F1,18 = 2.58, P > 0.05), or when the hidden platform was moved to a new location in the pool (Fig. 3c; genotype: F1,18 = 2.68, P > 0.05). However, during the probe trial, Akt1−/− mice swam significantly less (33%) than WT mice (Fig. 3d; t(16) = 2.36, P = 0.03). Akt1−/− mice also showed a trend for less percentage of time spent in the target quadrant (WT: 54 ± 6, Akt1−/−:39 ± 4; t(16) = 2.04, P = 0.08) and a greater percentage time spent in nontarget quadrants during the probe trial, suggesting deficiencies in spatial memory. The reduced distance traveled in the target quadrant by Akt1−/− mice was not due to impaired swimming performance based on swimming speed (WT: 21.0 ± 1.0, Akt1−/−: 18.6 ± 0.7; t(16) = 2.04, P > 0.05) or total distance traveled (WT: 1,221 cm ± 63, Akt1−/−: 1,095 cm ± 41; t(16) = 1.76, P > 0.05). This suggests that Akt1−/− mice demonstrated deficits in spatial memory when tested for recall following training in the MWM.
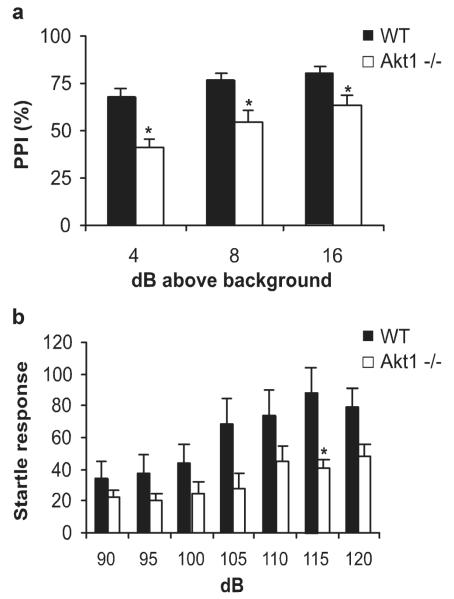
Prepulse Inhibition
As compared with WT mice, Akt1−/− mice had a significantly attenuated PPI response to all three prepulse intensities (Fig. 4a; intensity: F2,17 = 12.56, P < 0.0001; genotype: F1,17 = 18.25, P = 0.0005). Akt1−/− mice did not differ significantly in overall startle reactivity as compared with WT mice (Fig. 4b; intensity: F6,17 = 13.66, P < 0.0001; genotype: F1,17 = 3.58, P > 0.05; genotype × intensity: F6,17 = 2.48, P = 0.028), except at the 115 dB pulse (t(17) = 2.58, P = 0.02).
FIGURE 4.
Akt1−/− mice have abnormalities in prepulse inhibition. (a) Baseline levels of PPI were compared between WT (black bars; n = 11) and Akt1−/− mice (white bars; n = 8) across three prepulses of increasing intensities (64 dB, 68 dB, 76 dB). Bars represent the mean (% PPI) ± SEM. (b) Startle reactivity was measured across increasing pulse intensities. Bars represent the mean (startle response) ± SEM. Asterisk indicates that Akt1−/− mice differed significantly from WT (*P < 0.05).
A follow-up analysis examined whether reductions of PPI were associated with lower startle intensities in subset of mice that did not differ in startle response. Using a subset of WT mice that did not differ in baseline startle at 120 dB from Akt1−/− mice (WT = 57 ± 4, n = 7; Akt1−/− = 48 ± 8, n = 8; P = 0.34), PPI still differed significantly between genotypes (F1,13 = 10.14, P < 0.007).
Locomotor Activity
There was no significant difference in the distance traveled between WT and Akt1−/− mice during a 30-min test (WT: 1,173 ± 94 cm, Akt1−/−: 1,230 ± 74 cm; t(16) = 0.47, P > 0.05).
Tissue Monoamine Content
There were no significant differences between WT and Akt1−/− mice in tissue content for dopamine, serotonin or norepinephrine in the hippocampus, frontal cortex, amygdala, and brain stem (Table 2).
TABLE 2.
Monoamine Content in Different Brain Regions of WT and Akt1−/− Mice
| Dopamine |
Serotonin |
Norepinephrine |
||||
|---|---|---|---|---|---|---|
| Brain region | WT | Akt1−/− | WT | Akt1−/− | WT | Akt1−/− |
| Hippocampus | 23 ± 2 | 21 ± 2 | 356 ± 47 | 397 ± 16 | 516 ± 17 | 608 ± 40 |
| Frontal cortex | 97 ± 19 | 122 ± 29 | 212 ± 16 | 163 ± 17 | 548 ± 23 | 575 ± 30 |
| Amygdala | 110 ± 5 | 94 ± 6 | 291 ± 18 | 254 ± 28 | 316 ± 30 | 345 ± 65 |
| Brainstem | 141 ± 7 | 145 ± 29 | 393 ± 33 | 376 ± 38 | 746 ± 36 | 829 ± 29 |
Values are expressed as pg/mg fresh tissue weight ± SEM.
N per group: wild type (WT), 6; Akt1−/−, 3-4.
Akt Expression in Schizophrenia
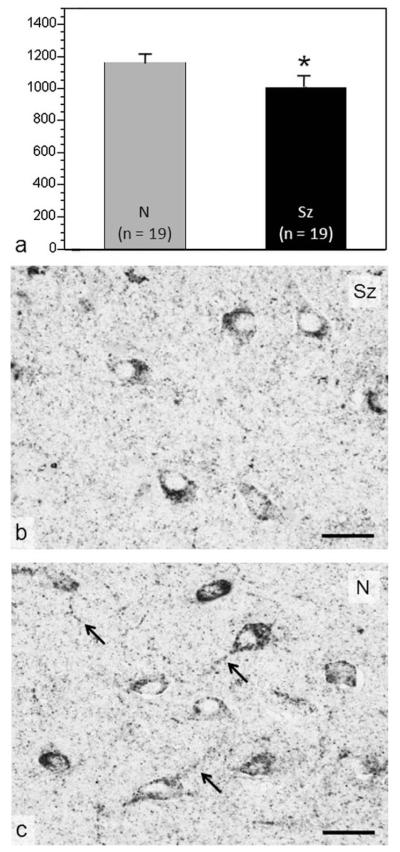
The mean levels of total Akt and endogenously activated Akt, reflected as phosphorylated at serine 473 (pAkt [S473]), were compared in hilar neurons of the DG in postmortem tissue from schizophrenia cases and controls matched for age, sex, and hemisphere sampled. Although no differences were detected in the levels of Akt itself (data not shown), a significant reduction was found in the levels of pAkt (S473) (P = 0.043), as shown in Figure 5a. Reductions averaging 18.4% were detected in 15 of the 18 matched pairs (i.e., 83% of the schizophrenia cases). These reductions can be seen in the average density of immunoreactivity within the cell bodies and proximal dendrites of a typical matched pair illustrated in Figure 5b,c. Interestingly, the observed reductions likely reflect a chronic impairment in Akt activation in patients with schizophrenia, because acute protein phosphorylation cannot be detected at the postmortem intervals involved in human studies. No differences were detected in neuronal Akt or pAkt (S473) in the CA3 field of the hippocampus adjacent to the hilus of the DG (data not shown).
FIGURE 5.
Reduced Akt phosphorylation at serine 473 in postmortem DG hilar neurons of patients with schizophrenia (Sz) compared with normal (N) controls. (a) The amount of pAkt (S473) reactivity in cell bodies and their processes of DG neurons from patients with Sz (black bar; n = 18) is lower than in N controls (gray bar; n = 18). The photomicrographs from a typical Sz case (b) and its matched control (c) show that moderate to high levels of pAkt (S473) occupy a lower proportion of neuronal cell bodies and their processes in the DG hilus of patients with Sz. Note that pAkt (S473) in neuronal processes (arrowed in c) is virtually absent in the patient with Sz. Scale bars = 100 μm. Asterisk indicates that Sz differed significantly from N (*P < 0.05).
The lower levels of phosphorylated Akt in the postmortem brain from schizophrenia cases were unlikely to be caused by antipsychotic medication. A previous study found that chronic clozapine administration to mice did not significantly affect levels of Akt pS473 (Zhao et al. 2006). Moreover, we found no significant correlation between levels of Akt pS473 in DG hilar neurons of the patients and their levels of antipsychotic medication (in chlorpromazine equivalents) a month before death (r = 0.336, df = 13, P = 0.239). There was no data on medication levels in four of the 18 cases.
DISCUSSION
Recent genetic evidence has highlighted the potential importance of an AKT1 haplotype associated with deficient protein levels in the transmission of schizophrenia (Emamian et al., 2004; Ikeda et al., 2004; Schwab et al., 2005; Thiselton et al., 2008; Xu et al., 2007). Further support derives from deficient Akt1 signaling reported in the postmortem prefrontal cortex of patients with schizophrenia (Emamian et al., 2004; Thiselton et al., 2008; Zhao et al., 2006). The finding in the present study of reduced levels of phosphorylated Akt in postmortem hippocampus from patients with schizophrenia is in agreement with the view that chronic impairments caused by deficient Akt1 levels in this region may have neuropathological significance (Emamian et al., 2004). The prefrontal cortex (Lewis and Gonzalez-Burgos, 2008) and hippocampus (Harrison, 2004) are the two brain regions most strongly linked to the pathophysiology of schizophrenia. In addition, drugs that are clinically used for the treatment of schizophrenia, such as haloperidol, enhance Akt signaling in vivo (Emamian et al., 2004). Akt is a common mediator of cell signaling for a number of genes showing the strongest association with schizophrenia, such as neuregulin, dysbindin and DISC1 (Harrison and Weinberger, 2005; Li et al., 2003; Numakawa et al., 2004). It is therefore a central part of functionally linked networks involved in regulating the normal growth and maturation of neurons and is critical to many neural signaling pathways whose common dysfunction could lead to the development of schizophrenia (Kalkman, 2006).
Mouse mutants (Akt1−/− mice) were used to understand how a selective deficiency in Akt1 signaling would impact the progenitor cell population in the adult hippocampus. The DG of the hippocampus is one of the few brain regions that generates neurons throughout the lifespan, and this process has been shown to be critical when engaged for the maintenance of neuroplasticity, including responses to stress and learning (Balu and Lucki, 2009). Mice with Akt1 deficiency demonstrated reduced proliferation of newborn cells in the hippocampus, consistent with what has been observed in postmortem schizophrenic brains (Reif et al., 2006). Although the precise mechanism of reduced hippocampal neurogenesis is unknown, deficient Akt signaling would be expected to impair the cellular effects of a number of neurotrophins, such as brain derived neurotrophic factor, that activate TrkB receptors located on neural progenitor cells and directly regulate cell proliferation (Li et al., 2008). Although most newborn cells in the mouse hippocampus die shortly after generation, ~90% of surviving cells will develop eventually into neurons that are incorporated into behavioral circuits (Snyder et al., 2009). The reduced proliferative capability in the hippocampus in Akt1 deficient mice could be a component of the neuropathology responsible for particular behavioral deficits, such as impaired learning and cognition.
Activation of the PI3K/Akt signaling pathway is necessary for the induction of dentate gyral LTP, a process that requires intact hippocampal neurogenesis (Saxe et al., 2006). The profound reduction in dentate STP and LTP, suggests a predominate impairment of dentate function in the Akt1−/− mice. Although the LTP studies do not pinpoint the dentate plasticity mechanisms impacted by the loss of Akt1 activity, the lack of STP at the perforant path granule cell synapse, in contrast to the maintained CA1 STP, points to disruption of plasticity properties specific to the DG. Despite the fact that short-term processes are likely as important as LTP mechanisms to DG function, the mechanisms of STP are not as well studied as LTP. STP is often posited to be a presynaptic; or, if postsynaptic, an N-methyl-d-aspartate-dependent process. In the dentate, the N-methyl-d-aspartate-dependent process of STP (also called post-tetanic potentiation) has been shown to occur in less then a minute and thus is not likely assayed by our LTP paradigm (Xie et al., 1997). Additionally, in the DG, AMPA responses have been described to mediate STP within the first minutes after the tetanus (Xie et al., 1997). Thus, loss of Akt1 may disrupt presynaptic mechanisms of STP, as well as short-term postsynaptic AMPA potentiation. Future studies dissecting the involvement of Akt1 in both STP and LTP, either directly or via tertiary effects such as loss of dentate proliferative capacity, will be necessary to fully understand how Akt1 uniquely impacts dentate function. Nevertheless, this work demonstrates loss of hippocampal plasticity to a degree likely to impact hippocampal-dependent learning.
Our results also demonstrated that genetic deficiency in Akt1 disrupted the performance of behaviors in adult mice that are normally associated with the hippocampus. Akt1−/− mice showed deficient acquisition of contextual fear conditioning, a hallmark behavior associated with hippocampal function (Sanders et al., 2003). Intact cued fear conditioning, a behavior thought to be mediated by other brain regions such as the amygdala, suggests that the behavioral deficits in Akt1−/− mice were specific to contextual processing. Reduced anxiety could have decreased fear conditioning, but the presence of a modest anxiety phenotype in Akt1−/− mice does not support this motivational explanation of the fear conditioning data. Akt1−/− mice were also deficient in the recall of spatial learning in the MWM on a probe performance trial. The normal rates of acquisition and reversal of maze learning suggests that subtle elements of performance differences involving the manner that the Akt1−/− mice respond to the learning task remain to be understood. Because neurogenesis is involved in the normal performance of certain types of hippocampal-dependent behaviors, such as contextual fear conditioning (Saxe et al., 2006; Winocur et al., 2006) and spatial memory formation in the MWM (Dupret et al., 2008), these behavioral deficits in Akt1−/− mice could be associated with persistent deficits of hippocampal neurogenesis. However, it is also possible for the behavioral deficits to be associated directly with deficient Akt1 signaling in the hippocampus.
PPI, the attenuation of a reflex startle response by a weak prepulse stimulus, is diminished in patients with schizophrenia (Cadenhead et al., 2000), and has been used widely in rodent studies addressing the neurobiology of sensorimotor gating in this disease (Harrison, 2004; Swerdlow and Geyer, 1998). We found that Akt1−/− mice exhibited reduced PPI, an intermediate phenotype common to schizophrenia, suggesting that Akt1 signaling is important for proper functioning of the neural circuitry mediating this behavior. The hippocampus is an important brain region for the regulation of sensory gating and PPI (Bast and Feldon, 2003). The reductions in the proliferative capacity and synaptic plasticity of the DG in Akt1−/− mice might, in part, be responsible for the sensorimotor gating abnormalities in these animals. It is also possible that deficient Akt1 signaling in other brain regions involved in PPI is contributing to the phenotype. Our finding of baseline differences in PPI of Akt1−/− mice contrasts with a previous report that did not report differences (Emamian et al., 2004). The discrepancy between studies could be due to alternate housing conditions (single vs. group), differences in the PPI protocol and that the mice in this study were experimentally naive.
Previous studies had showed that Akt1−/− mice demonstrated a number of phenotypes, such as greater sensitivity to dopamine-mediated disruptions of sensorimotor gating, enhanced locomotor activity produced by amphetamine, and altered dopamine-mediated regulation of delayed continuous alternation, a behavior associated with the function of the prefrontal cortex (Lai et al., 2006). These phenotypes evoked by dopamine receptor activation are consistent with a role for Akt1 in schizophrenia based on the dopaminergic hypothesis of this disease, even if we did not find altered tissue levels of dopamine in Akt1−/− mice. The present study expanded on these findings by demonstrating the importance of Akt1 in regulating neuroplasticity and behaviors relevant to the hippocampus. Deficits of hippocampal function may help explain the common impairment of spatial memory found in schizophrenia (Barch, 2005; Sharma and Antonova, 2003), including deficits in a virtual MWM (Hanlon et al., 2006), given that (a) DG hilar neurons can prime or recruit large numbers of granule cells to respond to entorhinal cortex input in a manner supporting LTP in granule cells (Hetherington et al., 1994) and (b) that levels of pAkt (Ser473) increase rapidly in DG slice preparations under conditions promoting LTP in response to entorhinal cortex input (Horwood et al., 2006). Taken together, these studies support the development of behavioral changes in mice with Akt1 deficiency mediated by key brain regions that are associated with behavioral deficits reported in patients with schizophrenia.
In summary, these data support consideration of Akt1 function as a potential etiological cause and target for therapeutic intervention in schizophrenia. The identification of multiple phenotypic changes of hippocampal function caused by disrupting a key cell signaling pathway in mice that may be similar to those underlying the neurobiology of schizophrenia allows us to better understand the mechanisms for how molecular events could lead to the development of this disease. Better treatments for schizophrenia may emerge by testing pharmaceuticals using a genetic mouse model that displays homologous deficits in disease-related pathologies and behavior.
Acknowledgments
We thank Dr. Steven Siegel for the use of his fear conditioning and PPI equipment and Noam Rudnick for his technical assistance.
Grant sponsor: National Institutes of Health; Grant number: U01 MH72832 (for a National Cooperative Drug Discovery Group in Mood Disorders to I.L.), P50 MH064045 (to K.T.), and R01-MH072880 and R01-DK56886 (to M.J.B.). Wyeth was an industry collaborator on U01 MH72832.
Abbreviations used
- ACSF
artificial cerebrospinal fluid
- BrdU
5-bromo-deoxyuridine
- DG
dentate gyrus
- fEPSPs
field excitatory postsynaptic potential
- LTP
long-term potentiation
- MWM
Morris water maze
- STP
short-term potentiation
- WT
wild type
REFERENCES
- Bajestan SN, Sabouri AH, Nakamura M, Takashima H, Keikhaee MR, Behdani F, Fayyazi MR, Sargolzaee MR, Bajestan MN, Sabouri Z, Khayami E, Haghighi S, Hashemi SB, Eiraku N, Tufani H, Najmabadi H, Arimura K, Sano A, Osame M. Association of AKT1 haplotype with the risk of schizophrenia in Iranian population. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:383–386. doi: 10.1002/ajmg.b.30291. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Hill TE, Ho N, Rahman Z, Bender CN, Ring RH, Dwyer JM, Rosenzweig-Lipson S, Hughes ZA, Schechter LE, Lucki I. Flow cytometric analysis of BrdU incorporation as a high-throughput method for measuring adult neurogenesis in the mouse. J Pharmacol Toxicol Methods. 2009;59:100–107. doi: 10.1016/j.vascn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Prog Neurobiol. 2003;70:319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: Evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335(Pt 1):1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium IS. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35(Pt 2):231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Weisend MP, Hamilton DA, Jones AP, Thoma RJ, Huang M, Martin K, Yeo RA, Miller GA, Canive JM. Impairment on the hippocampal-dependent virtual Morris water task in schizophrenia. Schizophr Res. 2006;87:67–80. doi: 10.1016/j.schres.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hetherington PA, Austin KB, Shapiro ML. Ipsilateral associational pathway in the dentate gyrus: An excitatory feedback system that supports N-methyl-D-aspartate-dependent long-term potentiation. Hippocampus. 1994;4:422–438. doi: 10.1002/hipo.450040405. [DOI] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Ide M, Ohnishi T, Murayama M, Matsumoto I, Yamada K, Iwayama Y, Dedova I, Toyota T, Asada T, Takashima A, Yoshikawa T. Failure to support a genetic contribution of AKT1 polymorphisms and altered AKT signaling in schizophrenia. J Neurochem. 2006;99:277–287. doi: 10.1111/j.1471-4159.2006.04033.x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Inada T, Ozaki N. Association of AKT1 with schizophrenia confirmed in a Japanese population. Biol Psychiatry. 2004;56:698–700. doi: 10.1016/j.biopsych.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Kalkman HO. The role of the phosphatidylinositide 3-kinase-protein kinase B pathway in schizophrenia. Pharmacol Ther. 2006;110:117–134. doi: 10.1016/j.pharmthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Lai WS, Xu B, Westphal KG, Paterlini M, Olivier B, Pavlidis P, Karayiorgou M, Gogos JA. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc Natl Acad Sci U S A. 2006;103:16906–16911. doi: 10.1073/pnas.0604994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Li BS, Ma W, Jaffe H, Zheng Y, Takahashi S, Zhang L, Kulkarni AB, Pant HC. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J Biol Chem. 2003;278:35702–35709. doi: 10.1074/jbc.M302004200. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Fann CS, Liu CM, Wu JY, Hung SI, Chan HY, Chen JJ, Pan CC, Liu SK, Hsieh MH. Absence of significant associations between four AKT1 SNP markers and schizophrenia in the Taiwanese population. Psychiatr Genet. 2006;16:39–41. doi: 10.1097/01.ypg.0000180681.80546.f3. others. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, Ozaki N, Taguchi T, Tatsumi M, Kamijima K, Straub RE, Weinberger DR, Kunugi H, Hashimoto R. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13:2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- O’Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berl) 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T, Inada T, Arinami T. Failure to confirm association between AKT1 haplotype and schizophrenia in a Japanese case-control population. Mol Psychiatry. 2004;9:981–983. doi: 10.1038/sj.mp.4001559. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: Mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SG, Hoefgen B, Hanses C, Hassenbach MB, Albus M, Lerer B, Trixler M, Maier W, Wildenauer DB. Further evidence for association of variants in the AKT1 gene with schizophrenia in a sample of European sib-pair families. Biol Psychiatry. 2005;58:446–450. doi: 10.1016/j.biopsych.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Sharma T, Antonova L. Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26:25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, Möller HJ, Hartmann A, Shianna KV, Ge D, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, Mühleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, GROUP. Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, Nöthen MM, Peltonen L, Collier DA, St Clair D, Stefansson K. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, Hahn CG, Siegel SJ, Trojanowski JQ, Gur RE, Blake DJ, Arnold SE. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiselton DL, Vladimirov VI, Kuo PH, McClay J, Wormley B, Fanous A, O’Neill FA, Walsh D, Van den Oord EJ, Kendler KS, Riley BP. AKT1 is associated with schizophrenia across multiple symptom dimensions in the Irish study of high density schizophrenia families. Biol Psychiatry. 2008;63:449–457. doi: 10.1016/j.biopsych.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen JA, Peltonen JO, Pietilainen OP, Hennah W, Loukola A, Paunio T, Silander K, Ekelund J, Varilo T, Partonen T, Lönnqvist J, Peltonen L. The role of DTNBP1, NRG1, and AKT1 in the genetics of schizophrenia in Finland. Schizophr Res. 2007;91:27–36. doi: 10.1016/j.schres.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Xie X, Liaw JS, Baudry M, Berger TW. Novel expression mechanism for synaptic potentiation: alignment of presynaptic release site and postsynaptic receptor. Proc Natl Acad Sci U S A. 1997;94:6983–6988. doi: 10.1073/pnas.94.13.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MQ, Xing QH, Zheng YL, Li S, Gao JJ, He G, Guo TW, Feng GY, Xu F, He L. Association of AKT1 gene polymorphisms with risk of schizophrenia and with response to antipsychotics in the Chinese population. J Clin Psychiatry. 2007;68:1358–1367. doi: 10.4088/jcp.v68n0906. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM. Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr Res. 2006;84:1–14. doi: 10.1016/j.schres.2006.02.009. [DOI] [PubMed] [Google Scholar]