Abstract
Ninety percent of anal cancer is associated with human papilloma viruses (HPVs). Using our previously established HPV transgenic mouse model for anal cancer, we tested the role of the individual oncogenes E6 and E7. K14E6 and K14E7 transgenic mice were treated with dimethylbenz[a]anthracene (DMBA) to the anal canal and compared to matched nontransgenic and doubly transgenic K14E6/E7 mice. K14E7 and K14E6/E7 transgenic mice developed anal tumors (papillomas, atypias and carcinomas combined) at significantly higher rates (88% and 100%, respectively) than either K14E6 or NTG mice (18% and 19%, respectively). Likewise, K14E7 and K14E6/E7 transgenic mice developed frank cancer (carcinomas) at significantly higher rates (85% and 85%, respectively) than either K14E6 or NTG mice (18% and 10%, respectively). These findings indicate that E7 is the more potent oncogene in anal cancer caused by HPVs.
Keywords: papillomavirus, E7, E6, anal cancer
Introduction
There are approximately 30,000 cases of anal cancer per year worldwide of which 90% are attributable to infection with high risk HPV (Parkin, 2006). HPV16 is the high risk HPV genotype most commonly found in anal cancer (Cogliano et al., 2005). HPV 16 encodes two oncoproteins that target key cellular tumor suppressors. E6 binds to p53 leading to deregulation of DNA damage and apoptotic pathways. E7 targets pRb for degradation, leading to an increase in cell proliferation and genomic instability. HPV has oncogenic properties in multiple squamous epithelial sites including the cervix, vulva, anus and a subset of head and neck.
The incidence of anal cancer has been rising gradually in the US over the past thirty years, with overall incidence in the general population now at 1.5 persons per 100,000 persons (Ries LAG, 1975–2005, SEER Cancer Statistics Review). The National Cancer Institute estimates that 5,260 people (3,260 women and 2,000 men) were diagnosed with anal cancer in the United States in 2010. HIV seropositivity is among the risk factors for developing anal cancer; others include low CD4 count, persistent high risk HPV infection, infection with multiple HPV types, anoreceptive intercourse, history of cervical cancer or dysplasia, cigarette smoking and immunosuppression for organ allograft (Welton et al., 2004). In the HAART era, HIV-infected patients have an incidence of anal cancer of ~ 40 per 100,000 persons, rising to 78 per 100,000 among HIV-infected men who have sex with men (Patel et al., 2008; Piketty et al., 2008). While 50% of patients present with disease confined to the primary site and these patients have a favorable (80%) five year survival rate, the remainder of the patients present with regional or distant metastasis correlating with less favorable 61% and 21% survival rates, respectively (Ries LAG, 1975–2005). Anal cancer treatment has essentially remained static over the past two decades. The high rate of mortality from advanced anal cancer speaks to the need for more effective clinical interventions for patients with advanced disease.
Prophylactic vaccines against specific HPV genotypes including HPV16 are already making an impact on HPV-associated human disease. After a population wide vaccination program was implemented for young women, there was significantly decreased incidence of high grade cervical squamous intraepithelial lesions from 0.85% down to 0.38% (p<0.01) (Brotherton et al., 2011). For anal cancer, the highest risk groups are men who have sex with men and men with AIDS (Chaturvedi et al., 2009; Cress and Holly, 2003). Prophylactic vaccines have now been shown to be safe and immunogenic in men and have potential to prevent a large portion of HPV-related squamous cell anal carcinoma by preventing the cancer-initiating HPV infection event. (Franceschi and De Vuyst, 2009; Moreira et al., 2011). But prophylactic vaccines will not prevent anal cancers in patients already infected with high risk HPVs or who already have anal cancer. In these groups, therapeutic vaccines or medications may be needed to target oncogenes. Studies on therapeutic vaccines thus far have proven difficult to dissect the relative contributions of the vaccine vs the natural human immune system (Frazer et al., 2011). New medications that can target HPV oncogenes or the cellular pathways they affect could have significant impact on improving clinical outcome for patients with anal cancer, as with other HPV-associated cancers.
Anal cancer is one of the least well-studied HPV-associated cancers because of the absence of laboratory model systems with which to pursue experiments. We have focused over the past few years at establishing mouse models for the purpose of understanding the role of HPVs in anal cancer and identify potential targets for new therapeutic approaches to treat this cancer. Specifically, we generated the first animal model for HPV-associated anal cancer making use of HPV16 transgenic mice that express E6 or E7 in stratified squamous epithelia of various organs (Stelzer et al., 2010c). We first established that HPV16 E6 and E7 are expressed and functional in the anal epithelium of our K14E6 and K14E7 transgenic mice, where they induced hyperproliferation and abrogated DNA damage response. These mice do not spontaneously develop anal cancer indicating that other genetic/epigenetic changes are required for carcinogenesis, much like with other HPV-associated cancers. Because we had previously demonstrated that HPV16 E6 and E7 synergize with the chemical carcinogen DMBA to cause skin cancers (Song et al., 2000), we monitored anal cancer in K14E6/K14E7 double transgenic mice treated topically with DMBA (0.12 umoles administered to the anal canal once per week for twenty weeks), and held an additional eight weeks prior to sacrifice. A strong synergy between DMBA and the viral oncogenes was demonstrated, with a progressive neoplastic disease culminating in malignancy arising within the anal epithelium of the K14E6/K14E7 mice. Biomarker expression paralleled that observed in human anal neoplastic disease, providing further validation of this animal model. Using this model we also identified the MTOR pathway to be activated in anal cancers, and these cancers to be responsive to rapamycin (Stelzer et al., 2010b).
The goal of the present study was to define the relative contributions of HPV16 E6 and E7 in anal cancer. In previously established cervical and head and neck cancer models, E7 had been identified to be the more potent oncogene (Riley et al., 2003a; Strati and Lambert, 2007). HPV induced skin cancer models, however, revealed E6 as the primary contributor to carcinogenesis (Song et al., 2000). We thus sought to discover the contribution of the individual oncogenes in anal cancer using our newly established anal cancer transgenic mouse model (Stelzer et al., 2010c). We evaluated the acute phenotypes of singly transgenic (E6 and E7 only) vs doubly transgenic (E6/E7) and nontransgenic (NTG) anal epithelium. E7 and to lesser degree E6 could induce proliferation within the anal epithelium, while both could abrogate normal DNA damage responses, akin to what has been observed in other epithelial tissues. To evaluate carcinogenesis, cohorts of mice of each genotype had DMBA applied to the perianal area and tumor development was monitored. The E7 only mice and E6/E7 mice had an 85% incidence of anal cancer which was significantly higher than E6 only and NTG mice, 18% and 10% respectively. The tumors that developed in E7 only and E6/E7 mice were larger and appeared earlier. These findings indicate that E7 is the more potent oncogene for anal carcinogenesis.
Results
HPV16 E6 and E7 induce acute phenotypes in the anal epithelium commensurate with their known in vivo activities in other tissues
We previously established by Western analyses that HPV16 E6 and E7 oncoproteins are expressed in the anal epithelium of these mice at levels commensurate with that found in human HPV-positive cancer-derived cell lines (Stelzer et al., 2010c). To assess the function of each oncoprotein in anal tissue, acute phenotypes of E6 only (K14E6) and E7 only (K14E7) mice were compared to doubly transgenic E6/E7 (K14E6/K14E7) mice and NTG mice.
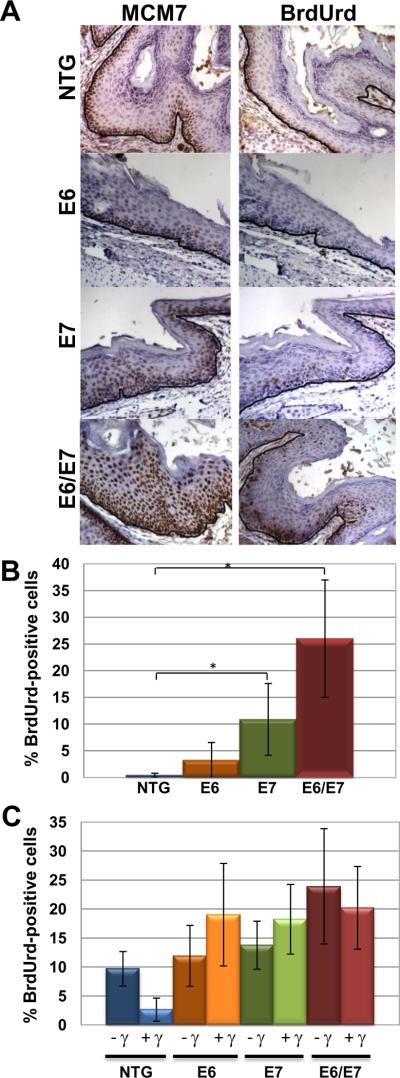
The HPV E7 oncoprotein is known to induce the transcription of E2F-responsive genes through its inactivation of pRb, p107 and p130 (Chellappan et al., 1992; Dyson et al., 1992; McLaughlin-Drubin and Munger, 2009; Phelps et al., 1988). One such E2F-responsive gene is minichromosome maintenance 7 (MCM7), a gene we have previously identified to be an informative marker for HPV-associated cancers of the cervix (Brake et al., 2003), head and neck region (Strati et al., 2006) and anus (Stelzer et al., 2010c). Immunohistochemistry for minichromosome maintenance 7 (MCM7) was performed on anal tissue harvested from mouse genotypes NTG, E6, E7 and E6/E7 that were or were not subjected to radiation one day prior to sacrifice. E7 only mice stained positive for MCM7 similar to E6/E7 mice while E6 only mice resembled NTG mice in their staining pattern (Fig 1A). This was the expected pattern of staining given the importance of E7 in dysregulating E2Fs. Next we monitored levels of proliferation by scoring for the frequency of cells supporting DNA synthesis. For this purpose we injected mice with bromodeoxyuridine (BrdUrd) one hour prior to sacrifice and subjected sections of anal epithelium from mice to BrdUrd-specific immunohistochemistry. E7 and to a lesser degree E6, has been shown to induce DNA synthesis in suprabasal cells of other epithelium for instance of the skin (Gulliver et al., 1997; Song et al., 1999), cervix (Brake et al., 2003; Shai et al., 2007) and tongue (Strati and Lambert, 2007). E7 only (11%) and E6/E7 (26%) mice had significantly higher DNA synthesis in supra basal cells compared to both NTG (0.5%) and E6 only (3%) anal epithelium (Fig 1B). Lastly, we monitored the ability of both E6 and E7 to abrogate normal DNA damage responses in basal cells of the anal epithelium. In other tissues including the skin (Song et al., 1998) and cervix (Shai et al., 2007), both E6 and E7 abrogate the normal response of epithelium to ionizing radiation, which is a cessation of DNA synthesis within the first 24 hours following exposure to ionizing radiation. Only anal epithelium from NTG mice had the appropriate DNA damage response with significantly decreased proliferation 24 hours after radiation exposure (Fig 1C). These results indicate that both E6 and E7 are functional within the anal epithelium, providing us cause to pursue the individual roles of these two viral oncoproteins in anal carcinogenesis.
Figure 1. Acute phenotypes of HPV16 transgenic mouse anal epithelium.
Panel A. Shown is MCM7-specific and BrdUrd immunohistochemical staining of sections from the anus of nontransgenic (NTG), E6, E7 and E6/E7 transgenic mice. Strong induction of MCM7 occurs in E7 and E6/E7 anal epithelium compared to NTG and E6 anal tissue. Similar staining is seen with BrdUrd staining with suprabasal DNA synthesis occurring more frequently in E7 and E6/E7 mice. Brown nuclei indicate positive staining which is expected along the basement membrane (outlined in black) as seen in NTG mice. Panel B. Quantification of the percentage of suprabasal cells with BrdUrd-positive nuclei. Panel C. Response of anal epithelium to radiation-induced growth arrest. Shown is the quantification of the frequency of BrdUrd-positive basal cells in anal epithelia of HPV transgenic mice that were (+) or were not (−) treated with 12Gy (γ) external radiation.
E7 has a strong oncogenic activity in anal carcinogenesis
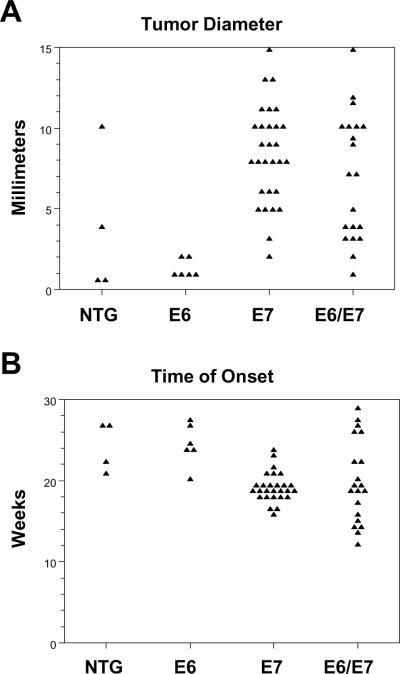
Mice from each genotype, E6/E7, E7, E6 and NTG, were treated with DMBA for 20 weeks using the protocol we had previously established for inducing anal cancer in mice (Stelzer et al., 2010b; Stelzer et al., 2010c). Eight weeks following the completion of DMBA treatments, the entire perianal area was harvested, and scored for overt disease, then formalin fixed, embedded and every 30th 5uM section was stained with hematoxylin and eosin and scored histologically for the worst disease ranging from normal, papilloma, atypia to carcinoma. MKT identified lesions within each section and final histopathological determination was made by HCP, a pathologist experienced in diagnosing the histopathology of mouse tumors. The E7 only mice and E6/E7 mice had an 85% incidence of anal cancer which was significantly higher than E6 only and NTG mice, 18% and 10% respectively (Table 1). Tumors (86% of which were cancers) were larger in E7 and E6/E7 mice compared to E6 and NTG mice (Fig 2A). In addition, they arose at an earlier time after initial DMBA treatment for E7 and E6/E7 mice, 19.1 and 19.8 weeks respectively, compared to E6 and NTG mice, 24.7 and 24.5 weeks (Fig 2B). Cancers that arose in each genotype were of squamous cell origin and, where they were not too large to define their origin, half originated in the anal transition zone and the other half at or near the anal exit (there was no differences between genotypes in this distribution).
Table 1.
E6/E7 and E7 transgenic mice confer significantly higher rates of tumor and carcinoma incidence
| Phenotvpe | Histopathological Classification of Disease | |||||
|---|---|---|---|---|---|---|
| Genotype | n | Overt Tumor | Normal | Papilloma | Atypia | Cancer |
| NTG | 21 | 4 (19%) | 16 | 0 | 3 | 2 (10%) |
| E6 | 33 | 6 (18%) | 24 | 0 | 3 | 6 (18%) |
| E7 | 33 | 29 (88%)* | 2 | 0 | 3 | 28 (85%)† |
| E6/E7 | 20 | 20 (100%)* | 0 | 0 | 3 | 17 (85%)† |
p<0.01 for E7 or E6/E7 tumors compared to E6 or NTG tumors
p<0.01 for E7 or E6/E7 cancers compared to E6 or NTG cancers
P-values were calculated using two-sided Chi square test
Figure 2. Overt tumor characteristics.
Panel A. Tumors more frequently occurred in E7 and E6/E7 mice and were larger in size. Average tumor size was 7mm in E6/E7 mice, 8.3mm in E7 mice while it was 3.75 in NTG mice and 1.3 in E6 mice. Differences in the size of tumors between either the E7 and E6/E7 mice and E6 mice were highly significant (p< 0.01). However there was no significant difference in either criteria between the E6 and the NTG mice (p=1). Panel B. Time of onset of tumors. E6/E7 tumors and E7 only tumors arose earlier at an average of 19.8 and 19.1 weeks after initial DMBA treatment. NTG and E6 mice had significantly (p < 0.05) longer time of average tumor onset at 24.7 and 24.5 weeks, respectively.
Biomarker analyses
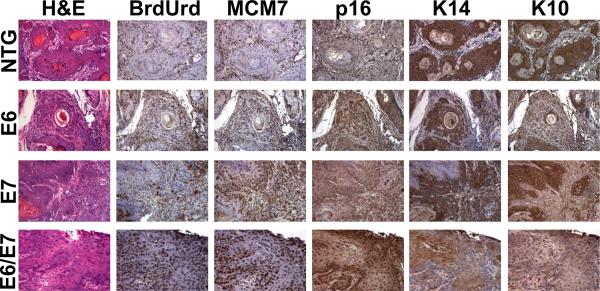
Both MCM7 and p16 are well-validated markers for HPV-associated cancers. We therefore compared the staining patterns of tumors arising in the various mouse genotypes for these two markers along with markers for cell proliferation (BrdUrd) and differentiation (K10 and K14). NTG mouse cancers had BrdUrd and MCM7 staining only at the basement membrane whereas E6, E7 and E6/E7 cancers had BrdUrd and MCM7 positive cells throughout the epithelium and were more highly positive for p16 staining. These results compared favorably with results from biomarker studies in cervical cancers arising in the same mice (Shai et al., 2007). Anal cancers from all genotypes were positive for both K14 and K10, markers for undifferentiated and differentiated epithelia, respectively (Fig 3). This result correlates with the observation based upon analysis of H&E stained sections of these same cancers that by and large they are well-differentiated tumors. An exception are the anal cancers arising in the E6/E7 double transgenic mice in which some (representative example in Figure 3) show comparatively low levels of K10 staining and less evidence for terminal epithelial cell differentiation (i.e. absence of keratin pearls, see H&E stained section).
Figure 3. Histopathology of anal cancer.
Representative images of cancers from each genotype are shown. H&E stained sections showing keratin pearls and large atypical nuclei consistent with squamous cell carcinoma. BrdUrd staining indicative of DNA synthesis is lowest in the NTG cancer as is MCM7 staining consistent with a lack of HPV oncoproteins. Also shown are p16, K14 and K10 staining. See text for full discussion of results.
Discussion
In HPV-positive human cancers two viral genes, E6 and E7, are preferentially expressed owing largely to the integration of the viral genome into the host DNA (zur Hausen, 2009). Elucidating the role of individual HPV oncogenes is vital as targeted therapeutic drugs are developed for these oncoproteins and their specific activities. In this study we show that E7 is the more potent oncogene for anal carcinogenesis. E7 is also the more potent oncogene for our mouse models of cervical and head and neck HPV cancers. Each of these models has a different co-carcinogen (DMBA for anal cancer, estrogen for cervical cancer, and 4-NQO for head/neck cancer). Thus the choice of co-carcinogen is not contributing to the dominance of E7 in causing cancers in these relevant tissue types. Rather, we attribute the dominance of E7 to its interactions with cellular factors within the epithelium of each of these tissues. In head and neck cancer inactivation of pRb partially accounted for E7 ability to induce cancer (Strati and Lambert, 2007); however in the cervix, Rb was less important a target (Balsitis et al., 2006). In contrast, p21 is a relevant target of E7 in the cervix, but again its inactivation by E7 only accounts partially for E7's oncogenic potential (Shin et al., 2009). Which cellular targets of E7 play a critical role in anal cancer remains to be determined.
A role of E6 in anal carcinogenesis was not clearly demonstrated based upon the data from this study. This is not unlike our original findings in the head and neck cancers, wherein E7 was again found to be the dominant oncogene (Strati and Lambert, 2007). However, by reducing the time of treatment with the co-carcinogen used in the head/neck cancer model, we were able to clearly define a role of E6 specifically in synergizing with E7 to cause head/neck cancer (Jabbar et al., 2010). Such a synergy was originally observed in our mouse model for cervical cancer (Riley et al., 2003b), and is highly relevant to HPV-associated human cancers, wherein these two oncogenes are always found to be co-expressed. In the context of cervical cancer studies, mutational analyses of E6 pointed to an importance of E6's interaction with PDZ proteins in mediating E6's synergy with E7 (Shai et al., 2007). This activity of E6 is also largely responsible for its induction of epithelial hyperplasia in the skin (Simonson et al., 2005), a phenotype also observed in our hands in the anal epithelium, on particular in the context of E6's co-expression with E7 (Fig. 1). Lastly, we have identified the MTOR pathway to be highly activated in both anal cancers, be they from humans or our HPV16 transgenic mouse model, and both human and mouse anal cancers responded well to treatment with rapamycin, the MTOR inhibitor (Stelzer et al., 2010a). Importantly, Munger and colleagues determined that E6 specifically activates the MTOR pathway (Spangle and Munger, 2010). Thus there are many reasons to predict that E6 can synergize with E7 to cause anal cancer. Were we to reduce the time of treatment with DMBA, then we might uncover such a synergy in the context of anal cancer, much like we did in the head/neck cancer model.
Materials and Methods
Mice
Generation of K14E6 and K14E7 mice has been previously described (Herber et al., 1996; Song et al., 1999). These mice were maintained on the inbred FVB/N genetic background. E6/E7 transgenic mice were generated by crossing K14E6 females with K14E7 males. All mice were kept in American Association for Accreditation of Laboratory Animal Care-approved McArdle Laboratory Cancer Center Animal Care Facility and studies with them were carried out in accordance to an approved animal protocol.
Acute Phenotypes
All mice received intraperitoneal injections of bromo deoxyuracil (BrdUrd)12.5 mg/mL in PBS at 10 μL/g body weight one hour prior to sacrifice. Mice did not receive any type of perianal treatment prior to sacrifice. All mice were between 6 and 8 weeks of age. Irradiated mice were exposed to 12 Gy of ionizing radiation from a 137Cs source twenty-four hours prior to sacrifice. Nuclei in anal epithelium was scored as positive (brown) or negative (blue) for BrdUrd based upon BrdUrd-specific immunohistochemistry. A minimum of five high power fields were counted for each group.
Immunohistochemistry
Immunohistochemistry staining was carried out as previously described (Balsitis et al., 2003). Primary antibodies were applied overnight at 4°C at the following concentrations in 2.5% horse serum (1:50 BrdUrd, Calbiochem; 1:200 antiMCM7, Neomarkers; 1:50 p16 (M156), Santa Cruz; 1:500 K10, Covance, 1:1000 K14, Covance).
DMBA Induced Anal Carcinogenesis
Anal carcinogenesis was achieved as previously described using the DMBA (dimethylbenz[a]anthracene) dose of 0.12μmoles (Stelzer et al., 2010b; Stelzer et al., 2010c). Mice were monitored weekly for appearance of overt tumors and size (diameter in mm) was measured at sacrifice.
Histological Analysis
The anal canal tissue was fixed in 10% formalin, paraffin embedded, and serially sectioned at 5μm. Every 30th section was stained with H&E and histopathologically analyzed for hyperplasia, papilloma, atypia, or carcinoma.
Statistical Analysis
Two-sided Wilcoxon rank-sum test was used to determine significant differences in BrdUrd counts, tumor onset and tumor size at sacrifice. Two-sided Chi square test was used to determine differences in rates of disease among mouse genotypes.
Acknowledgements
This study was supported by NIH grants DE017315, CA098428, and CA022443 to PFL. MKT was supported by a NIH training grant (NIH/NCI T32 CA090217) for clinical fellows.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Balsitis S, Dick F, Dyson N, Lambert PF. Critical roles for non-pRb targets of human papillomavirus type 16 E7 in cervical carcinogenesis. Cancer Res. 2006;66:9393–9400. doi: 10.1158/0008-5472.CAN-06-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis SJ, Sage J, Duensing S, Munger K, Jacks T, Lambert PF. Recapitulation of the effects of the human papillomavirus type 16 E7 oncogene on mouse epithelium by somatic Rb deletion and detection of pRb-independent effects of E7 in vivo. Mol Cell Biol. 2003;23:9094–9103. doi: 10.1128/MCB.23.24.9094-9103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake T, Connor JP, Petereit DG, Lambert PF. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–8180. [PubMed] [Google Scholar]
- Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–2092. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A, Madeleine M, Biggar R, Engels E. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, Nevins JR. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Cancer W.I.A.f.R.o. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- Cress R, Holly E. Incidence of anal cancer in California: increased incidence among men in San Francisco, 1973–1999. Prev Med. 2003;36:555–560. doi: 10.1016/s0091-7435(03)00013-6. [DOI] [PubMed] [Google Scholar]
- Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66:6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, De Vuyst H. Human papillomavirus vaccines and anal carcinoma. Curr Opin HIV AIDS. 2009;4:57–63. doi: 10.1097/COH.0b013e32831b9c81. [DOI] [PubMed] [Google Scholar]
- Frazer IH, Leggatt GR, Mattarollo SR. Prevention and treatment of papillomavirus-related cancers through immunization. Annu Rev Immunol. 2011;29:111–138. doi: 10.1146/annurev-immunol-031210-101308. [DOI] [PubMed] [Google Scholar]
- Gulliver GA, Herber RL, Liem A, Lambert PF. Both conserved region 1 (CR1) and CR2 of the human papillomavirus type 16 E7 oncogene are required for induction of epidermal hyperplasia and tumor formation in transgenic mice. J Virol. 1997;71:5905–5914. doi: 10.1128/jvi.71.8.5905-5914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar S, Strati K, Shin MK, Pitot HC, Lambert PF. Human papillomavirus type 16 E6 and E7 oncoproteins act synergistically to cause head and neck cancer in mice. Virology. 2010;407:60–67. doi: 10.1016/j.virol.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Munger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384:335–344. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira ED, Palefsky JM, Giuliano AR, Goldstone S, Aranda C, Jessen H, Hillman RJ, Ferris D, Coutlee F, Vardas E, Marshall JB, Vuocolo S, Haupt RM, Guris D, Garner EI. Safety and reactogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 viral-like-particle vaccine in older adolescents and young adults. Hum Vaccin. 2011;7 doi: 10.4161/hv.7.7.15579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, Holmberg SD, Brooks JT. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- Phelps WC, Yee CL, Munger K, Howley PM. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988;53:539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, Costagliola D, Mary-Krause M. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008;22:1203–1211. doi: 10.1097/QAD.0b013e3283023f78. [DOI] [PubMed] [Google Scholar]
- Ries LAG MD, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2005. [Google Scholar]
- Riley R, Duensing S, Brake T, Münger K, Lambert P, Arbeit J. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003a;63:4862–4871. [PubMed] [Google Scholar]
- Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003b;63:4862–4871. [PubMed] [Google Scholar]
- Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MK, Balsitis S, Brake T, Lambert PF. Human papillomavirus E7 oncoprotein overrides the tumor suppressor activity of p21Cip1 in cervical carcinogenesis. Cancer Res. 2009;69:5656–5663. doi: 10.1158/0008-5472.CAN-08-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson SJ, Difilippantonio MJ, Lambert PF. Two distinct activities contribute to human papillomavirus 16 E6's oncogenic potential. Cancer Res. 2005;65:8266–8273. doi: 10.1158/0008-5472.CAN-05-1651. [DOI] [PubMed] [Google Scholar]
- Song S, Gulliver GA, Lambert PF. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci U S A. 1998;95:2290–2295. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000;267:141–150. doi: 10.1006/viro.1999.0106. [DOI] [PubMed] [Google Scholar]
- Song S, Pitot HC, Lambert PF. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J Virol. 1999;73:5887–5893. doi: 10.1128/jvi.73.7.5887-5893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangle JM, Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84:9398–9407. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer MK, Pitot HC, Liem A, Lee D, Kennedy GD, Lambert PF. Rapamycin inhibits anal carcinogenesis in two preclinical animal models. Cancer Prev Res (Phila) 2010a;3:1542–1551. doi: 10.1158/1940-6207.CAPR-10-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer MK, Pitot HC, Liem A, Lee D, Kennedy GD, Lambert PF. Rapamycin inhibits anal carcinogenesis in two preclinical animal models. Cancer Prev Res (Phila) 2010b;3:1542–1551. doi: 10.1158/1940-6207.CAPR-10-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer MK, Pitot HC, Liem A, Schweizer J, Mahoney C, Lambert PF. A mouse model for human anal cancer. Cancer Prev Res (Phila) 2010c;3:1534–1541. doi: 10.1158/1940-6207.CAPR-10-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati K, Lambert PF. Role of Rb-dependent and Rb-independent functions of papillomavirus E7 oncogene in head and neck cancer. Cancer Res. 2007;67:11585–11593. doi: 10.1158/0008-5472.CAN-07-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc Natl Acad Sci U S A. 2006;103:14152–14157. doi: 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton M, Sharkey F, Kahlenberg M. The etiology and epidemiology of anal cancer. Surg Oncol Clin N Am. 2004;13:263–275. doi: 10.1016/j.soc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]