Abstract
The AMP-activated kinase (AMPK) senses the energy status of cells and regulates fuel availability, whereas hypothalamic AMPK regulates food intake. We report that inositol polyphosphate multikinase (IPMK) regulates glucose signaling to AMPK in a pathway whereby glucose activates phosphorylation of IPMK at tyrosine 174 enabling the enzyme to bind to AMPK and regulate its activation. Thus, refeeding fasted mice rapidly and markedly stimulates transcriptional enhancement of IPMK expression while down-regulating AMPK. Also, AMPK is up-regulated in mice with genetic depletion of hypothalamic IPMK. IPMK physiologically binds AMPK, with binding enhanced by glucose treatment. Regulation by glucose of phospho-AMPK in hypothalamic cell lines is prevented by blocking AMPK-IPMK binding. These findings imply that IPMK inhibitors will be beneficial in treating obesity and diabetes.
AMP-activated protein kinase (AMPK) is a principal cellular fuel gauge. In response to energetic stresses, ATP is converted to AMP, which activates AMPK (1–3). AMPK phosphorylates a variety of substrates to alter diverse cellular functions, including fatty acid synthesis and oxidation, cholesterol and glycogen synthesis, mitochondrial biogenesis, gluconeogenesis, and protein synthesis (2). For instance, by phosphorylating acetyl-CoA carboxylase, AMPK inhibits fatty acid synthesis (4). In intact organisms, AMPK plays a major role in hypothalamic signaling that regulates food intake (5, 6).
Inositol polyphosphate multikinase (IPMK) is a key regulatory enzyme in inositol phosphate disposition. It is the principal physiologic generator of inositol pentakisphosphate (IP5) and, thus, is rate-limiting in the formation of the inositol pyrophosphates such as diphosphoinositol pentakisphosphate (IP7) (7–9). IPMK also possesses physiologic PI3-kinase activity, which regulates Akt signaling (10). Acting in a noncatalytic fashion, IPMK binds to mTOR (mammalian target of rapamycin), stabilizing the mTOR-raptor complex and enhancing protein synthesis (11).
Besides influencing carbohydrate and lipid metabolism, AMPK is an important regulator of protein synthesis, an area of possible linkage to IPMK. AMPK phosphorylates and, thereby, activates tuberous sclerosis complex (TSC)2, a GTPase activating protein, which is a negative regulator of mTOR signaling (12). Moreover, AMPK phosphorylates raptor, dissociating the mTOR complex and, thereby, inhibiting protein synthesis (13). mTOR interacts reciprocally with AMPK in influencing food intake with mTOR regulating responses to amino acids, whereas AMPK responds selectively to dietary carbohydrate alterations (2, 5, 14, 15). These interfaces of IPMK and AMPK prompted us to explore their molecular interactions. We show that IPMK binds to AMPK to enhance its signaling. Glucose exposure elicits tyrosine phosphorylation of IPMK, enabling its stimulation of AMPK.
Results
Reciprocal Regulation of IPMK and AMPK in the Hypothalamus and Cell Lines in Response to Nutrients.
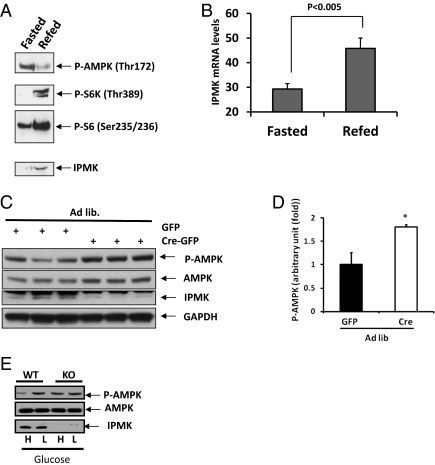
Kahn and coworkers demonstrated dramatic alterations of activated phospho-AMPK in response to fasting and refeeding (5), which we confirm and extend to IPMK regulation (Fig. 1A). We provided 2 h of refeeding to fasted mice, examining phospho-AMPK and protein levels of IPMK, as well as monitoring mTOR signaling via levels of phospho-S6 kinase and phospho-S6. As reported previously, refeeding markedly decreases levels of phospho-AMPK while eliciting major increases in phospho-S6 kinase and phospho-S6 (5, 14). In fasted mice, IPMK levels in the hypothalamus are barely detectable but are increased dramatically by refeeding. Quantitative RT-PCR reveals that refeeding markedly increases mRNA for IPMK (Fig. 1B), indicating that the enhanced levels of IPMK protein reflect transcriptional augmentation.
Fig. 1.
IPMK physiologically regulates AMPK activation in response to nutrients. (A and B) Effects of refeeding on IPMK gene expression in the hypothalamus. Mice fasted for 24 h were refed for 2 h, the whole hypothalami were isolated, and RNA or protein extracts were prepared as described in Materials and Methods. (A) Westerns blots are shown for P-AMPKα, P-S6K, P-S6, and IPMK. (B) Quantification of IPMK mRNA levels in fasted and refed mice (n = 5 per group). (C and D) Loss of hypothalamic IPMK leads to elevated P-AMPKα in ad libitum mice. IPMKlox/lox mice were injected with adenovirus expressing either GFP or Cre recombinase. Mice were fed ad libitum and killed. (C) Westerns blots are shown of P-AMPKα, AMPK, IPMK, and GAPDH. (D) Relative quantifications of P-AMPKα expression levels are shown in GFP-infected (black bar) or GFP-Cre-infected (open bar) mice. Values are corrected for corresponding total AMPK antibody (*Student t test; P < 0.005). (E) IPMK flox/flox (WT) and IPMK−/− (KO) MEFs were incubated with DMEM media containing either high glucose (4.5 g/L) or low glucose (1.5 g/L).
We wondered whether IPMK physiologically regulates AMPK activity in the hypothalamus. We deleted hypothalamic IPMK by injecting the ventral hypothalamus of floxed-floxed IPMK mice with an adenovirus expressing either GFP or GFP-Cre recombinase (Fig. 1 C and D). The injection was targeted to the area of the ventral hypothalamus containing the arcuate nucleus, which is critical for AMPK-mediated food intake. Western blot analysis reveals virtual abolition of IPMK in the ventral hypothalamus following viral treatment (Fig. 1C). By contrast, no alteration in IPMK is evident in the dorsal hypothalamus (Fig. S1 A and B). The IPMK deletion is accompanied by an almost twofold increase in hypothalamic phospho-AMPK, with no changes in AMPK protein level (Fig. 1 C and D).
We substantiated the regulation of phospho-AMPK by IPMK using mouse embryonic fibroblasts (MEFs) from floxed-floxed IPMK mice (Fig. 1E). In WT MEFs, exposure to lowered glucose markedly augments phospho-AMPK. This increase is abolished in IPMK deleted MEFs.
IPMK Physiologically Binds and Regulates AMPK Activity.
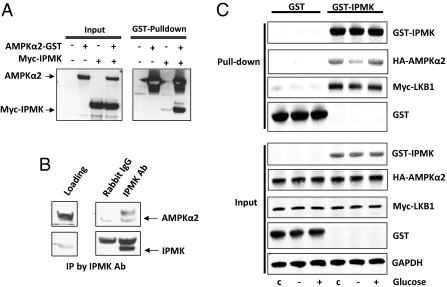
We wondered whether the regulation of AMPK by IPMK derives from direct interactions between the two proteins. In overexpression systems, we demonstrate binding between the two proteins (Fig. 2A). We also show physiologic binding between endogenous IPMK and AMPK in hypothalamic lysates of mouse brain (Fig. 2B). Binding between the two proteins is dynamically regulated by glucose availability (Fig. 2C). Thus, glucose deprivation for 2 h markedly decreases IPMK-AMPK binding, which is fully restored by a 30-min reexposure to glucose.
Fig. 2.
IPMK regulates AMPK activation through interaction of IPMK-AMPK in response to glucose availability. (A) IPMK interacts with AMPKα2. GST-AMPKα2 was cotransfected in HEK293T cells with myc-IPMK, followed by GST pull-down assay. The precipitated proteins and the input proteins were detected by immunoblotting with antibodies to GST or myc. (B) Hypothalamic lysate (500 μg) was used for immunoprecipitation (IP) against Rabbit IgG antibody or IPMK antibody to determine the physiological binding of AMPKα2. (C) HA-AMPKα2 and Myc-LKB1 were cotransfected in GT1-7 cells with GST or GST-IPMK as indicated. Cells were deprived of glucose for 3 h and stimulated with glucose for 30 min. GST pull-down assay was performed to determine IPMK and AMPKα2 interaction.
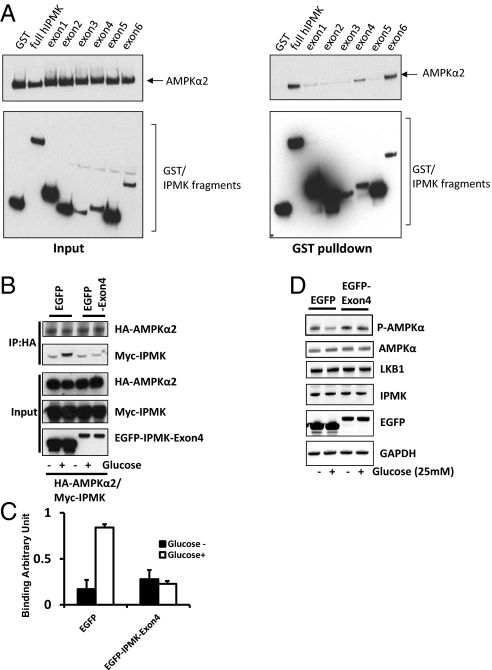
We developed a dominant-negative construct of IPMK as a probe to elucidate functional consequences of IPMK-AMPK binding. We mapped sites on IPMK that are required for AMPK binding (Fig. 3A). Two portions of IPMK appear to be critical for binding: (i) exon 4, which occurs in the linker region between the inositol phosphate binding site and the kinase domain; and (ii) exon 6, which is part of the kinase domain of IPMK. We used exon 4, which codes for amino acids 125–182 of IPMK, as a dominant-negative in the GT1-7 cell line, which derives from hypothalamic neurons (16) (Fig. 3). Overexpression of IPMK-exon 4 markedly disrupts IPMK-AMPK binding. The considerable enhancement of IPMK-AMPK binding elicited by glucose is abolished in preparations treated with IPMK-exon 4 (Fig. 3 B and C). We then investigated whether IPMK-AMPK binding is responsible for the regulation of phospho-AMPK by glucose. The striking decrease in phospho-AMPK elicited by glucose is abolished by treatment with IPMK-exon 4 (Fig. 3D). These findings establish that interactions of IPMK with AMPK are responsible for the regulation of phospho-AMPK by glucose.
Fig. 3.
Physical interaction between IPMK and AMPK is required for IPMK-mediated modulation of AMPK. (A) Mapping of binding region of IPMK responsible for AMPK interaction. GST, GST-IPMK or GST–IPMK exon fragments (exon 1: 1–62, exon 2: 63–92, exon 3: 93–124, exon 4: 125–182, exon 5: 183–208 and exon 6: 209–416) were pull-downed from HEK293T cells cotransfected with AMPKα2. Coimmunoprecipitation of AMPKα2 was determined by Western blots. (B–D) Dominant-negative IPMK exon 4 disrupts the IPMK-AMPK interaction. (B) HA-AMPKα2, Myc-IPMK, and EGFP or EGFP-IPMK exon 4 were cotransfected into GT1-7 cells as indicated. Cells were deprived of glucose for 3 h and stimulated with glucose for 30 min before lysis. HA-AMPKα2 was immunoprecipitated and coimmunoprecipitates of Myc-IPMK were determined by Western blot (C) Relative quantifications of bound AMPKα2 and IPMK levels are shown. Values are expressed as means ± SD of three determinations (*Student t test; P < 0.005). (D) EGFP or EGFP-exon 4 was transfected into HEK293 cells as indicated. Cells were deprived of glucose for 3 h and stimulated with or without glucose for 30 min before lysis. Proteins were extracted and analyzed by Western blotting.
Tyrosine Phosphorylation of IPMK Determines Its Regulation of AMPK.
Regulation by glucose of IPMK-AMPK binding and AMPK activity raises questions about mechanisms that might determine IPMK binding to AMPK. Catalytic activity of the two proteins does not appear to be critical. Thus, AMPK-K45R, which is catalytically dead, and AMPK-T172D, which is constitutively active, do not display altered binding to IPMK (Fig. S2A). Also, kinase-dead IPMK binds normally to AMPK (Fig. S2B).
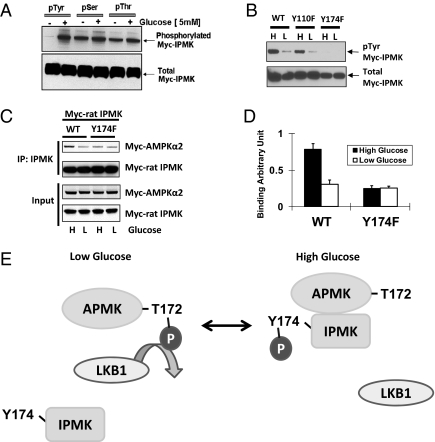
We wondered whether phosphorylation of IPMK might influence its interactions with AMPK. Our earlier studies established that phosphorylation of IPMK plays a role in its regulation of Akt, but specific kinases influencing IPMK were not identified (10). We immunoprecipitated with antibodies to phospho-tyrosine, phospho-serine, and phospho-threonine, using cells maintained in the absence or presence of glucose (Fig. 4A). This model of stringent glucose starvation and repletion is typically used to highlight actions of glucose on AMPK, whereas comparisons of high- and low-glucose media are often used to mimic physiologic and mild modulation. Total levels of IPMK protein are the same under all conditions. By contrast, tyrosine phosphorylation of IPMK, absent in preparations not exposed to glucose, displays robust levels in glucose treated samples. Although we detect phosphorylation of IPMK at serines and threonines, we fail to observe major changes with glucose treatment. To determine the site of tyrosine phosphorylation on IPMK, we used Scansite (www.mit.edu) analysis, which suggested tyrosines 110 and 174 as candidates. Mutation to phenylalanine of tyrosine 174 abolishes the glucose-stimulated tyrosine phosphorylation of IPMK, whereas mutation of tyrosine 110 has no effect (Fig. 4B). Thus, tyrosine phosphorylation of IPMK can be fully attributed to tyrosine 174. Mutation of tyrosine 174 to phenylalanine does not alter IPMK catalytic activity (Fig. S3B).
Fig. 4.
IPMK phosphorylation is required for AMPK interaction on the high glucose. (A) High glucose induces IPMK phosphorylation. GT1-7 cells were transfected with Myc rat-IPMK. Cells were starved of glucose for 3 h and resupplied with glucose (5 mM) for 30 min. Phosphorylated protein was immunoprecipitated with phospho-tyrosine, phospho-serine, and phospho-threonine antibodies, respectively. Phosphorylated (top) and total (bottom) IPMK was determined by Western blot. (B) Comparisons of tyrosine phosphorylated IPMK in cells overexpressed WT and tyrosine mutants of rat IPMK. WT or two tyrosine mutants (Y110F and Y174F) were transfected into GT1-7 cells and incubated with DMEM containing high glucose (H) or low glucose (L) for 24 h. Immunoprecipitation was done with phospho-tyrosine antibody to the indicated proteins from lysates and Western blots of tyrosine-phosphorylated IPMK. (C) Myc-AMPKα2 and Myc-IPMK were cotransfected into GT1-7 cells as indicated. Cells were incubated with DMEM containing high glucose (H) or low glucose (L) for 24 h. IPMK was immunoprecipitated, and Western blots were performed. (D) Relative quantifications of bound AMPKα2 and IPMK levels are shown (*Student t test, P < 0.005). (E) Schematic representation of a model explaining how IPMK interacts with AMPK in response to glucose signals. In low glucose, tyrosine phosphorylation of IPMK is decreased, and, subsequently, AMPK can interact with and be phosphorylated by LKB1. In high glucose, increased phosphorylation of Y174 in IPMK interferes with the action of LKB1 to decrease phosphorylation of AMPK. Alternatively, IPMK may promote dephosphorylation of AMPK.
We investigated whether tyrosine 174 phosphorylation mediates the regulation by IPMK of AMPK (Fig. 4C). In WT preparations, glucose deprivation markedly decreases IPMK-AMPK binding. By contrast, we observe no change in binding using IPMK-Y174F.
Discussion
Our study establishes that IPMK is a physiologic regulator of AMPK function. Thus, glucose signaling strikingly augments phosphorylation of IPMK at tyrosine 174, which facilitates the binding of IPMK to AMPK that, in turn, inhibits phosphorylation of AMPK and its catalytic activity (Fig. 4E).
Mechanisms whereby tyrosine phosphorylation of IPMK impacts its binding to AMPK are unclear. Src homology (SH)2 domains of proteins typically bind phosphotyrosines, but AMPK lacks SH2 domains. Our mapping studies indicate that exon 4 of IPMK determines binding to AMPK. Because tyrosine 174 occurs within exon 4, tyrosine phosphorylation might influence nearby clusters of amino acids that are responsible for binding.
Although our data establishes that tyrosine phosphorylation of IPMK regulates its binding to AMPK, the impact of the phosphorylation upon hypothalamic AMPK activity is not known. Conceivably, one could ascertain whether virally administered constructs for WT or Y174F-IPMK differentially rescue hypothalamic AMPK activity of IPMK knockout mice. In intact mice, feeding regulates total protein levels of IPMK which, in turn, influences AMPK. Whether refeeding also alters tyrosine phosphorylation of hypothalamic IPMK is unknown. Evidence for IPMK regulating AMPK includes the use of a dominant-negative construct that blocks IPMK-AMPK binding and abrogates the influence of glucose upon AMPK activity. We have developed evidence in intact mice showing that IPMK regulates phospho-AMPK, which determines feeding behavior (5). Thus, genetic depletion of hypothalamic IPMK markedly diminishes the effect of feeding upon hypothalamic phospho-AMPK.
Besides IPMK, there exist other upstream regulators of AMPK. LKB1 (liver kinase B1) phosphorylates and activates AMPK in a fashion that appears to be constitutive under normal physiological conditions (17, 18). AMP stimulates AMPK by binding to the γ regulatory subunit, which exposes the site of LKB phosphorylation (19, 20). Calmodulin kinase-kinase (CAMKK)2 also phosphorylates AMPK in a calcium-dependent fashion (21, 22). Accordingly, AMPK function can be influenced by diverse signaling mechanisms that alter intracellular calcium. Conceivably, the impact of IPMK on AMPK interfaces with one or more of these other phosphorylating systems. As LKB1 is the proximal determinant of AMPK phosphorylation, we speculate that tyrosine phosphorylation of IPMK leads to its altering the sensitivity of AMPK to phosphorylation by LKB1 (Fig. 4E) or to dephosphorylation, perhaps by PP2C, which is known to dephosphorylate phospho-AMPK (23). Mechanisms whereby glucose treatment stimulates tyrosine phosphorylation of IPMK are unknown. Tyrosine kinases regulated by glucose include focal adhesion kinase (FAK), insulin receptor kinase, and Src kinase (24–26).
By phosphorylating a variety of targets, AMPK influences a wide range of signaling systems to mediate its pleiotropic cellular actions. AMPK acts upon the mTOR system in several ways. It phosphorylates the GTPase TSC2 to augment catalytic activity, which inhibits the ability of Rheb, a small G protein, to activate mTOR (27, 28). Via this mechanism, AMPK down-regulates mTOR signaling. AMPK also phosphorylates raptor, a key component of the mTORC1, rapamycin-sensitive system that stimulates protein translation (13). Phospho-raptor dissociates from mTOR so that its phosphorylation by AMPK also impairs mTOR signaling. By inhibiting AMPK activity, IPMK would be anticipated to increase mTOR signaling. Other actions of IPMK may also increase mTOR pathways. Thus, IPMK, via its PI3-kinase activity, stimulates Akt kinase activity, which leads to augmented mTOR signaling (10). In a kinase-independent fashion, IPMK binds to mTOR, stabilizing its binding interactions with raptor and also increasing mTOR signaling (11).
AMPK also plays a potentially important role in subtypes of autophagy. It phosphorylates and activates ULK1, unc-51 like kinase1 Atg13 and FIP200, enhances selectively the autophagic degradation of mitochondria (29). IPMK might impact autophagy via its influences upon AMPK.
IPMK may influence the regulation of feeding behavior by hypothalamic centers that are regulated by AMPK. In our experiments, selective depletion of hypothalamic IPMK prevents the influence of feeding alterations on phospho-AMPK. Whereas refeeding fasted animals virtually abolishes hypothalamic phospho-AMPK, this treatment dramatically increases IPMK protein levels within 2 h of refeeding. This finding implies a very rapid turnover rate for IPMK, which would be consistent with a role in the dynamic regulation of phospho-AMPK and other signaling systems.
Our findings may have clinical ramifications. By augmenting various catabolic systems, AMPK activation may be therapeutic in type 2 diabetes and related conditions. For instance, AMPK is a major stimulant to lipolysis and an inhibitor of fatty acid synthesis (1, 4). Moreover, AMPK increases glycolysis. Metformin, one of the principal antidiabetic drugs, acts, at least in part, by stimulating AMPK activity. The exact molecular mechanism of action of metformin is unknown, but its effects are lost in the absence of LKB1 (30). Conceivably, metformin and related drugs act via some link to IPMK.
Materials and Methods
Cells and cDNA Transfection.
GT1-7 cells (a generous gift from Dr. Pamela Mellon, University of California, San Diego, CA) and MEFs were maintained in a humid atmosphere of 95% air and 5% CO2 at 37 °C in DMEM (Invitrogen) supplemented with 10% FBS (Gemini Bio-Products), l-glutamine (2 mM; Invitrogen), and penicillin (100 units/mL)/streptomycin (100 μg/mL) (Invitrogen). Transfection of HEK293T cells (2 million cells per 100-mm plate) was performed by using Polyfect transfection reagent (Qiagen), according to the protocol of the manufacturer.
Cell Treatment with Glucose.
Cells were glucose-deprived in Kreb's Ringer bicarbonate buffer [118 mM NaCl/20 mM Hepes/12 mM NaHCO3/4.6 mM KCl/1 mM MgCl2/0.5 mM CaCl2/0.2% (wt/vol) BSA (pH 7.4)] for 2 h. Then, cells were incubate with 0, 5, or 25 mM glucose in Kreb's Ringer bicarbonate buffer for 30 min.
Immunoprecipitation.
Cells were lysed in 50 mM Tris at pH 7.4, 150 mM NaCl, 1% Triton X-100, 15% glycerol, phosphatase inhibitor mixture 2&3 (Sigma), and protease inhibitor mixture 1&2 (Roche). Total protein (500 μg) was incubated with 2 μg of antibodies as indicated for 16 h at 4 °C and precipitated with 50 μL of TrueBlot anti-rabbit Ig IP beads and TrueBlot anti-mouse Ig IP beads (eBioscience) for an additional 3 h. For endogenous interaction, rabbit IgG antibody or against IPMK antibody was added to the hypothalamic lysates (1 mg) and performed on immunoprecipitates as described above. Then, samples were washed five times with lysis buffer, and SDS-loading sample buffer was added. Samples were separated by SDS/PAGE and analyzed by immunoblotting as described elsewhere (13). For coimmunoprecipitates of Myc-IPMK blots, Mouse TrueBlot ULTRA: anti-mouse Ig HRP-conjugated secondary antibody (eBioscience) was used for detection with SuperSignal West Pico chemiluminescence reagent (Thermo Scientific).
[3H]Inositol Labeling of Cells.
Cells were seeded at a density of 2 × 106 cells per 10-cm dish and then labeled with 200 μCi (1 Ci = 37 GBq) [3H]inositol (Perkin–Elmer Life Sciences) for 4 d. Soluble inositol phosphates were extracted from labeled cells as described previously (10). Inositol incorporated into lipids were measured by extracting the remaining cell pellet with 0.1 M NaOH and 0.1% Triton X-100 overnight at room temperature with shaking and counting a fraction of the solubilized material in a liquid scintillation counter. The [3H]-labeled inositol phosphates were resolved by HPLC as described above. Soluble inositol phosphate levels were normalized against total lipid inositol content for each cell line.
Supplementary Material
Acknowledgments
This work was funded by US Public Health Service Grant DA-00266 and Research Scientist Award DA-00074 (to S.H.S.), The Jane Coffin Childs Memorial Fellowship (to S.K.), and the American Federation of Ageing Research (S.F.K.), and National Institutes of Health Grant DK084336 (to S.F.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119751109/-/DCSupplemental.
References
- 1.Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase—development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–1070. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- 5.Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 6.Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SC, Majerus PW. Inositol polyphosphate multikinase regulates inositol 1,4,5,6-tetrakisphosphate. Biochem Biophys Res Commun. 2006;339:209–216. doi: 10.1016/j.bbrc.2005.10.201. [DOI] [PubMed] [Google Scholar]
- 8.Resnick Saiardi AC, Saiardi A. Inositol polyphosphate multikinase: Metabolic architect of nuclear inositides. Front Biosci. 2008;13:856–866. doi: 10.2741/2726. [DOI] [PubMed] [Google Scholar]
- 9.Tsui MM, York JD. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv Enzyme Regul. 2010;50:324–337. doi: 10.1016/j.advenzreg.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maag D, et al. Inositol polyphosphate multikinase is a physiologic PI3-kinase that activates Akt/PKB. Proc Natl Acad Sci USA. 2011;108:1391–1396. doi: 10.1073/pnas.1017831108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, et al. Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase. Cell Metab. 2011;13:215–221. doi: 10.1016/j.cmet.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: Evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 15.Mori H, et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9:362–374. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wetsel WC, Mellon PL, Weiner RI, Negro-Vilar A. Metabolism of pro-luteinizing hormone-releasing hormone in immortalized hypothalamic neurons. Endocrinology. 1991;129:1584–1595. doi: 10.1210/endo-129-3-1584. [DOI] [PubMed] [Google Scholar]
- 17.Hawley SA, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Adams J, et al. Intrasteric control of AMPK via the gamma1 subunit AMP allosteric regulatory site. Protein Sci. 2004;13:155–165. doi: 10.1110/ps.03340004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346(Pt 3):659–669. [PMC free article] [PubMed] [Google Scholar]
- 21.Hawley SA, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Woods A, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 24.Hauguel-de-Mouzon S, Mrejen C, Alengrin F, Van OE. Glucose-induced stimulation of human insulin-receptor mRNA and tyrosine kinase activity in cultured cells. Biochem J. 1995;305(Pt 1):119–124. doi: 10.1042/bj3050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol. 2008;295:C1647–C1657. doi: 10.1152/ajpcell.00322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SH, Lee YJ, Park SW, Kim HS, Han HJ. Caveolin-1 and integrin β1 regulate embryonic stem cell proliferation via p38 MAPK and FAK in high glucose. J Cell Physiol. 2011;226:1850–1859. doi: 10.1002/jcp.22510. [DOI] [PubMed] [Google Scholar]
- 27.Garami A, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan DF, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.