Abstract
Introduction
Calcium ions are vital in many biological processes and qualify as an almost ubiquitous intracellular second messenger. This indicates the multiplicity of the effects associated with drug actions aimed at interfering with calcium ions. To examine the cellular process involved in the induction of infertility in males by calcium antagonist (CA) even in the presence of normal semen parameters, we studied the effects of different CA namely; nifedipine, verapamil and diltiazem on oxidative balance and acrosome reaction in the sperm.
Material and methods
For this purpose, lipid peroxidation, antioxidants such as superoxide dismutase, catalase and reduced glutathione, and acrosomal reaction were determined in sperm samples of rats.
Results
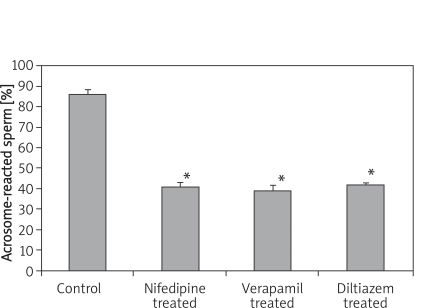
Calcium antagonist causes significant oxidative stress in the epididymal sperm with increased malondialdehyde level and a concomitant decrease in antioxidant activities of catalase and superoxide dismutase. The percentage value of acrosomal-reacted sperm in the nifedipine, verapamil and diltiazem-treated rats were 41 ±2.45, 39 ±2.92 and 42 ±1.22 respectively, compared with the control group value of 86 ±2.92.
Conclusions
It appears CA oxidatively modify the sperm resulting in functional inhibition of acrosomal reaction. Suppression of the sperm acrosomal reaction is known to have serious adverse implications for fertilization.
Keywords: calcium antagonist, acrosomal reaction, lipid peroxidation, reactive oxygen species, sperm
Introduction
One of the most enduring needs of reproductive biology is to understand the fundamental cellular mechanisms that control the fertilizing potential of human spermatozoa [1]. It is known that defective sperm function remains the single largest defined cause of male infertility [2]; moreover, it is also clear that male infertility has a multi-factorial origin including genetics, environment, diseases and iatrogenic [3, 4]. Clinical reports have suggested the induction of infertility in men taking calcium antagonists (CA) for the control of hypertension and migraine, even with normozoospermic semen parameters [5, 6].
Given this, an experiment involving calcium antagonist treatment was developed to characterize the possible mechanism and factors involved in CA-induced male infertility. Specifically, antioxidants play a crucial role in maintaining cell homeostasis, and when these defences are impaired or overwhelmed, oxidative stress products, namely reactive oxygen species (ROS), may induce enzymatic inactivation and peroxidation of cell constituents. Moreover, the role of ROS in mammalian sperm capacitation and the acrosomal reaction is well documented [7-9]. The acrosomal reaction is a compulsory prerequisite for fertilization as it confers on the mammalian sperm the capacity to bind and fuse with the oocyte [10]. Acrosome-reacted sperm must however be primed through the capacitation process [11].
Unexpectedly, sperm cells are intrinsically vulnerable to oxidative stress despite the critical role of redox-regulated processes in the control of mammalian sperm function [12]. The sperm membrane is known to be very rich in polyunsaturated fatty acids (PUFA), which renders it vulnerable to oxidative damage by chain reactions [13]. The high content of PUFA provides the sperm cells with the structural fluidity required to engage in the membrane fusion event (capacitation) associated with fertilization [1]. Although sperm cells possess cytoplasmic antioxidant enzymes such as glutathione and superoxide dismutase, the amount is relatively small, thereby limiting the degree of protection conferred on the spermatozoa [14]. The present study was therefore undertaken to evaluate sperm oxidative balance and the acrosomal reaction process in both in vivo and in vitro experimental models. The rationale of this study is based on the vulnerability of mammalian sperm to oxidative stress which finds expression in many cases of male infertility [15, 16] and the reported infertility caused by the therapeutic use of CA [5, 6, 17]. We have previously demonstrated that CA decreases sperm count, motility and epididymal weight, which appears not to occur through inhibition of the pituitary-gonadal axis [17].
Material and methods
Animals
Thirty-two male albino rats of the Sprague-Dawley strain weighing approximately 180-220 g, obtained from the Central Animal House of the College of Medicine of the University of Lagos, were used in the study. These animals were housed in clear polypropylene cages lined with wood chip beddings in a well-ventilated and photoperiod-controlled (12 h light : 12 h dark) animal house for at least 2 weeks prior to use in experimental protocols. Rats were fed on a commercial standard pellet diet (Livestock Feeds, Lagos, Nigeria) and water ad libitum. Generally, the research was conducted in accordance with the internationally accepted (Helsinki) guidelines for laboratory animal use and care.
Drugs, doses and design
Nifedipine, verapamil and diltiazem (Sigma Chemical Company) were used in this study. Dosing formulations for nifedipine, verapamil and diltiazem were prepared as earlier reported by our group [17]. Twenty-four male rats were divided into four equal groups and treated as follows: group 1 (control group) received distilled water which was the vehicle of the drug; group 2 (nifedipine group) rats were given nifedipine (0.571 mg/kg bw) orally; group 3 (verapamil group) rats were given verapamil (3.40 mg/kg bw); group 4 (diltiazem group) rats were given diltiazem (2.57 mg/kg bw). Treatment was done intra-gastrically via oral cannula for 30 days. The remaining animals were used in the in vitro study.
Sperm suspension and media
Sperm specimens were collected by (teasing) perfusion of the caudal epididymis of treated rats through the distal end of the vas deferens into the pre-warmed sperm capacitation medium (SCM) containing 110 mM NaCl, 2.7 mM KCl, 2.4 mM CaCl2, 0.49 mM MgCl2, 0.32 mM NaH2PO4, 24.9 mM NaHCO3, 5 mM glucose, 6.26 mM lactate, 0.125 mM pyruvate, 12 mg/ml BSA. The cells were incubated in this medium for 4 h at 37°C with 5% CO2. These conditions are known to induce capacitation and the acrosomal reaction [18-21].
In-vitro sperm exposure to CA was done by releasing the epididymal caudal contents from 2 mature male rats into 2 ml of the medium of normal saline. In the control group, the suspension specimen was incubated in 200 ml of SCM, while the sperm suspension in the experimental groups according to prior studies [18, 19] was incubated in 200 ml droplets containing nifedipine, verapamil and diltiazem at 25, 50, 100, 200 nmol/l.
Assessment of acrosomal reaction
Sperm acrosomal status was assessed with Coomassie brilliant blue staining techniques [22]. Briefly, sperm concentration was determined using a haemocytometer. About 100 µl of sperm sample was transferred to an Eppendorf tube containing 1 ml of medium. After 1 h of incubation at 37°C, the highly motile and thus potentially fertile sperm that appeared in the upper two thirds were transferred to fresh Eppendorf tubes, and sperm numbers were adjusted with the medium (SCM) to a concentration of 2 x 106 sperm/ml. Aliquots of sperm were removed from each group for assessment of sperm acrosomal status by using Coomassie brilliant blue staining techniques. Sperm samples were air-dried on glass slides, fixed with 5% paraformaldehyde in phosphate buffered saline (PBS) for 15 min and washed once with PBS. Slides were then stained with aqueous 0.25% Coomassie brilliant blue in 10% glacial acetic acid and 25% methanol. After that, they were rinsed with water and covered with coverslips under mounting media. The acrosome region is stained in the acrosome-intact sperm but unstained in the acrosome-reacted sperm. A minimum of 100 sperm cells/sample was assessed for the presence or absence of acrosome reaction per slide and the percentage of acrosome-reacted sperm was determined. All tests were repeated at least 3 times.
Oxidative studies
Sperm cells from the caudal epididymis were finely minced with scissors in normal saline in a Petri dish to liquefy and provide migration of all spermatozoa from the epididymal tissue to the fluid. Thereafter, the epididymal tissue-spermatozoa mixture was filtered via a strainer to separate the sperm cells (supernatant) from the tissue particles. The sperm suspension was centrifuged at 200 x g for 10 min. The pellet was re-suspended in normal saline and gently homogenized (10 strokes) and used for the biochemical assays [23]. As a marker of lipid peroxidation, the level of malondialdehyde (MDA) in the sperm cells was measured by the method of Uchiyama and Mihara [24] as thiobarbituric acid reactive substances (TBARS). The development of a pink complex with absorption maximum at 535 nm is taken as an index of lipid peroxidation. The activity of the superoxide dismutase (SOD) enzyme in the homogenate was determined according to the method described by Sun and Zigman [25]. The reaction was carried out in 0.05 M sodium carbonate buffer, pH 10.3, and was initiated by the addition of epinephrine in 0.005 N HCl. Catalase (CAT) activity was determined by measuring the exponential disappearance of H2O2 at 240 nm and expressed in units/mg of protein as described by Aebi [26]. The reduced glutathione (GSH) content of the sperm homogenate was determined using the method described by Van Dooran et al. [27]. The GSH determination method is based on the reaction of Ellman’s reagent 5,5’ dithiobis-2-nitrobenzoic acid (DNTB) with the thiol group of GSH at pH 8.0 to produce 5-thiol-2-nitrobenzoate, which is yellow at 412 nm. Absorbance was recorded using Agilent UV-Visible Spectrophotometer in all measurements.
Data analysis
All the values are expressed as mean ± standard error of mean (SEM) and analysed using the GraphPad Instat Version 3.05 (GraphPad Software, San Diego California, USA) using the one-way ANOVA followed by Student-Newman-Keuls post-hoc test. Differences were considered significant when p < 0.05 was accepted as significant.
Results
Oxidative activities
The MDA level, SOD, CAT and GSH activities of all the groups are given in Table I. Lipid peroxidation in epididymal sperm assayed by malondialdehyde (MDA) levels in the nifedipine, verapamil and diltiazem treated animals were significantly increased (p < 0.05) compared with the control group. However, there were significant decreases (p < 0.05) in the activity of the SOD enzyme following CA treatment compared with the control rats. Additionally, treatment with nifedipine, verapamil and diltiazem produced significant decreases (p < 0.05) in the CAT enzyme activities of the epididymal sperm of drug-treated rats compared with controls. In contrast, the activity of GSH enzyme was not significantly different (p > 0.05) in any of the CA-treated groups compared with the control group.
Table I.
Effect of 30-day treatment with nifedipine, verapamil and diltiazem on lipid peroxidation and antioxidant activities in sperm cells
| Control | Nifedipine | Verapamil | Diltiazem | |
|---|---|---|---|---|
| MDA [nmol/ml] | 7.8 ±0.39 | 10.3 ±0.20* | 9.9 ±0.32* | 10.7 ±0.28* |
| SOD [µmol/min] | 3.8 ±0.18 | 2.7 ±0.29* | 2.7 ±0.14* | 3.1 ±0.22* |
| CAT [nmol/min] | 11.8 ±1.02 | 7.8 ±0.58* | 8.2 ±0.58* | 7.2 ±0.37* |
| GSH [nmol/ml] | 41.3 ±1.5 | 36.9 ±1.4 | 37.2 ±2.8 | 35.5 ±2.3 |
Values are expressed as mean ± SEM (n = 6);
p < 0.05, significantly different compared with control group; MDA – malondialdehyde, SOD – superoxide dismutase, CAT – catalase, GSH – reduced glutathione
Acrosomal reaction
The 30-day treatment with CA caused a suppressed sperm acrosomal reaction in all the groups. The reduction in the epididymal sperm acrosomal reaction was significantly different (p < 0.05) in all the CA-treated groups compared with the control group. As shown in Figure 1, the percentage value of acrosomal-reacted sperm in the nifedipine-treated, verapamil-treated and diltiazem-treated rats were 41 ±2.45, 39 ±2.92 and 42 ±1.22 respectively, compared with the control group value of 86 ±2.92.
Figure 1.
Epididymal sperm capacitation of rats following 30-day treatment with nifedipine, vera - pamil and diltiazem
Values are expressed as mean ± SEM (n = 6), *p < 0.05, significantly different compared with control group
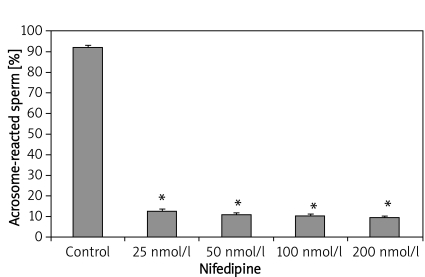
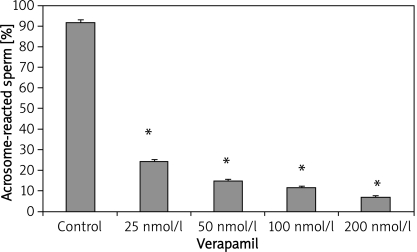
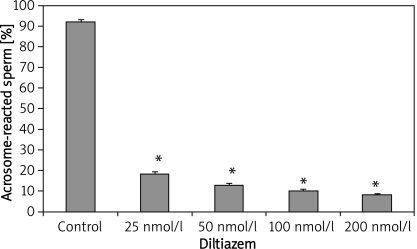
In-vitro exposure of epididymal sperm to varying concentration of nifedipine also resulted in a significant decrease in acrosomal-reacted sperm (Figure 2). The percentage of acrosome-reacted sperm in the control group was 92 ±1.22, while the CA-treated group exhibited reduced acrosome-reacted sperm levels of 12.6 ±1.08, 11 ±0.89, 10.6 ±0.68 and 9.8 ±0.58 with nifedipine concentration of 25 nmol/l, 50 nmol/l, 100 nmol/l and 200 nmol/l respectively. Similarly, sperm samples incubated with verapamil also exhibited significant suppression of the acrosomal reaction, as shown in Figure 3. The percentage values of acrosome-reacted sperm were 24.2 ±1.02, 14.8 ±0.66, 11.6 ±0.51 and 7.0 ±0.54 with verapamil concentration of 25 nmol/l, 50 nmol/l, 100 nmol/l and 200 nmol/l respectively, compared with the control group (93 ±1.47). Figure 4 shows that incubation of sperm cells with varying concentration of diltiazem also demonstrated significant inhibition of acrosome reaction. The percentage values of acrosome-reacted sperm in the control group was 93 ±1.47, while the treated groups had values of 18.4 ±1.08, 13.2 ±0.66, 10.2 ±0.73 and 8.4 ±0.51 with diltiazem concentration of 25 nmol/l, 50 nmol/l, 100 nmol/l and 200 nmol/l respectively.
Figure 2.
Epididymal sperm capacitation of rats following in-vitro exposure to nifedipine
Values are expressed as mean ± SEM (n = 6), *p < 0.05, significantly different compared with control group
Figure 3.
Epididymal sperm capacitation of rats following in-vitro exposure to verapamil
Values are expressed as mean ± SEM (n = 6), *p < 0.05, significantly different compared with control group
Figure 4.
Epididymal sperm capacitation of rats following in-vitro, exposure to diltiazem
Values are expressed as mean ± SEM (n = 6), *p < 0.05, significantly different compared with control group
Discussion
It is well known that antioxidants confer a protective cover for sperm survival and function. Nevertheless, there is no definite knowledge on the role of CA in the regulation of sperm antioxidants. We have previously shown that CA decreases sperm count, motility and epididymal weight, which appear not to occur through the inhibition of the pituitary-gonadal axis [17]. In this study, we addressed two questions concerning the effect of CA on rat sperm both in vivo and in vitro by focusing our attention on oxidative balance and the acrosomal reaction.
Treatment with CA in this study significantly inhibited the sperm acrosomal reaction. Sperm cells must necessarily undergo a fundamental process known as capacitation before acquiring their ability to undergo the acrosomal reaction. Capacitated sperm readily and rapidly undergo the acrosome reaction in order to fertilize the oocyte on making contact with the zona pellucida [1]. The reduction in the number of acrosomal-reacted sperm in the present study therefore suggests that the priming of sperm cells, which is a fundamental cellular process [10, 28] that controls the fertilizing potential of sperm cells, is compromised. The inhibition of this process by CA in our study therefore implies a decreasing chance that sperm will encounter the egg in the oviductal lumen for fertilization. It is therefore plausible to suggest that CA-induced infertility partly derives from the suppression of the acrosomal reaction in treated rats.
The observed inhibition of the acrosomal reaction in CA-treated rats could also be a result of negative changes in the ionic environment and fluxes, especially of calcium ions, through the mammalian sperm membrane. Mammalian sperm possess several calcium ion pathways including one that is similar to the voltage-sensitive calcium channel found in a variety of somatic cells [29, 30]. Trans-membrane fluxes of calcium ions have been shown to be critically important for sperm-motility initiation and regulation [31, 32]. In particular, changes in intracellular calcium have been associated with sperm movement, capacitation, the acrosome reaction and fertilization [33, 34]. In addition, calcium ions have been shown to be increased during capacitation as a result of reduced calcium influx due to the activation of unidentified channels [10, 29]. The results from this study may be suggestive of a direct mechanism in which nifedipine, verapamil and diltiazem blocked the sperm calcium ion channels, thereby reducing calcium ion influx and subsequently inhibiting the capacitation process. This position is supported by previous studies in hamsters [22], mouse [35] and humans [36, 37], where a plasma calcium ion channel inhibitor (verapamil) and a voltage-dependent calcium ion channel inhibitor (nifedipine) significantly reduced capacitation and the acrosomal reaction.
Another potential reason for the inhibited capacitation process could be the increased oxidative stress caused by CA treatment. A minimal amount of ROS is required for normal sperm-oocyte fusion. However, if there is an abnormal increase in the production of ROS, sperm pathology such as ATP depletion results, leading to insufficient axonemal phosphorylation, lipid peroxidation and loss of motility. The lipid peroxidation process results in loss of membrane architecture and reduction in the activity of membrane enzymes and ion channels [1, 38]. As a result of high ROS levels, spermatozoa are unable to initiate the necessary biochemical reactions associated with the capacitation process [39, 40]. Reactive oxide species are generally neutralized by a protective antioxidant defence system consisting of enzymes such as catalase, superoxide dismutase and reduced glutathione, among others [41]. These antioxidant enzymes and free radical scavengers provide protection from peroxidative damage to the sperm cell, either by binding the transition metal ions, thereby preventing them from initiating a chain reaction, or by deactivating a damaging free radical, leading to the formation of non-radical end-products [42]. However, the generation of excessive amount of ROS in semen can overwhelm the antioxidant defence system of the sperm, leading to oxidative stress [8]. We have shown that the vulnerability of sperm to oxidative stress involving loss of capacitation in this study is associated with peroxidative damage and low scavenging antioxidant defence, as reflected by measurement of MDA, SOD and CAT. Therefore, CA appears to disrupt the fine balance between the production and scavenging of ROS, and thereby impairs normal sperm functions and acquisition of fertilizing ability.
In conclusion, calcium antagonists such as nifedipine, verapamil and diltiazem suppress the sperm capacitation process and/or inhibit the acrosomal reaction. Though the exact biochemical mechanism is not known, it may be related to the influence of CA to increase oxidative stress and cause peroxidative damage to sperm cells, thereby compromising the plasma membrane and resulting in changes in the calcium ionic fluxes in the sperm and seminal fluid.
References
- 1.Aitken RJ. The biology of fertilization. Adv Exp Med Biol. 1997;424:291–9. doi: 10.1007/978-1-4615-5913-9_51. [DOI] [PubMed] [Google Scholar]
- 2.Hull MG. Infertility. Practitioner. 1985;229:943–5. [PubMed] [Google Scholar]
- 3.Chadley AC. Genetic contribution to male infertility. Hum Reprod. 1998;13(Suppl 3):76–83. doi: 10.1093/humrep/13.suppl_3.76. [DOI] [PubMed] [Google Scholar]
- 4.Nudell DM, Monoski NM, Lipshultz LI. Common medications and drugs: how they affect male fertility. Urol Cli N Am. 2002;29:965–73. doi: 10.1016/s0094-0143(02)00079-4. [DOI] [PubMed] [Google Scholar]
- 5.Benoff S, Cooper GW, Hurley L, et al. The effect of calcium ion channel blockers on sperm fertilization potential. Fertil Steril. 1994;62:606–17. [PubMed] [Google Scholar]
- 6.Hershlag A, Cooper GW, Benoff S. Pregnancy following discontinuation of a calcium channel blocker in the male partner. Hum Reprod. 1995;10:599–606. doi: 10.1093/oxfordjournals.humrep.a135996. [DOI] [PubMed] [Google Scholar]
- 7.Purohit SB, Laloraya M, Kumar GP. Acrosome reaction inducers impose alterations in repulsive strain and hydration barrier in human sperm membranes. Biochem Mol Biol Int. 1998;45:227–35. doi: 10.1080/15216549800202592. [DOI] [PubMed] [Google Scholar]
- 8.de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997;2:48–54. doi: 10.1530/ror.0.0020048. [DOI] [PubMed] [Google Scholar]
- 9.Tremellon K. Oxidative stress and male infertility - a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 10.Darszon A, Beltra´n C, Felix R, Nishigaki T, Treviño CL. Ion transport in sperm signaling. Dev Biol. 2001;240:1–14. doi: 10.1006/dbio.2001.0387. [DOI] [PubMed] [Google Scholar]
- 11.Yanagimachi R. Mammalian fertilization. In: Knobill E, Neill JD, editors. The physiology of reproduction. NewYork: Raven Press; 2004. pp. 189–317. [Google Scholar]
- 12.Aitken RJ, Ryan AL, Baker MA, McLaughlin EA. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radic Biol Med. 2004;36:994–1010. doi: 10.1016/j.freeradbiomed.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–46. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- 14.Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl. 1993;16:183–8. doi: 10.1111/j.1365-2605.1993.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharma JK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–50. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–85. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 17.Morakinyo AO, Iranloye BO, Adegoke OA. Antireproductive effect of calcium channel blockers on male rats. Reprod Med Biol. 2009;8:97–102. doi: 10.1007/s12522-009-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser LR, Mclntyre K. Calcium channel antagonists modulate the acrosome reaction but not capacitation in mouse spermatozoa. J Reprod Fertil. 1989;86:223–33. doi: 10.1530/jrf.0.0860223. [DOI] [PubMed] [Google Scholar]
- 19.Li MW, Chen DY. Relationship between plasma membrane Ca2+-ATPase activity and acrosome reaction in guinea pig sperm. Sci China C Life Sci. 1996;39:418–26. [PubMed] [Google Scholar]
- 20.Rivlin J, Mendel J, Rubnistein S, Etkovitz N, Breibart H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol Reprod. 2004;70:518–22. doi: 10.1095/biolreprod.103.020487. [DOI] [PubMed] [Google Scholar]
- 21.Ain R, Devi KU, Shivaji S, Seshagirl PB. Pentoxifylline-stimulated capacitation and acrosome reaction in hamster spermatozoa: involvement of intracellular signaling molecules. Mol Hum Reprod. 1999;5:618–26. doi: 10.1093/molehr/5.7.618. [DOI] [PubMed] [Google Scholar]
- 22.Feng HL, Han YB, Hershlag A, Zheng LJ. Impact of Ca2+ flux inhibitors on acrosome reaction of hamster spermatozoa. J Androl. 2007;28:561–4. doi: 10.2164/jandrol.106.001958. [DOI] [PubMed] [Google Scholar]
- 23.Chitra KC, Sujatha R, Latchoumycandane C, Mathur PP. Effect of lindane on antioxidant enzymes in epididymis and epididymal sperm of adult rats. Asian J Androl. 2001;3:205–8. [PubMed] [Google Scholar]
- 24.Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituris acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 25.Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978;247:81–9. doi: 10.1016/0003-2697(78)90010-6. [DOI] [PubMed] [Google Scholar]
- 26.Aebi H. Catalase in vitro. Methods of enzymology. 1984;8:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 27.Van Dooran R, Liejdekker CM, Handerson PT. Synergistic effects of phorone on the hepatotoxicity of bromo-benzene and paracetamol in mice. Toxicology. 1978;11:225–33. doi: 10.1016/s0300-483x(78)91389-6. [DOI] [PubMed] [Google Scholar]
- 28.Purohit SB, Laloraya M, Kumar GP. Role of ions and ion channels in capacitation and acrosome reaction of spermatozoa. Asian J Androl. 1999;1:95–107. [PubMed] [Google Scholar]
- 29.Jagannathan S, Publicover SJ, Barratt CL. Voltage-operated calcium channels in male germ cells. Reprod. 2002;123:203–15. doi: 10.1530/rep.0.1230203. [DOI] [PubMed] [Google Scholar]
- 30.Hille B. Ionic channels of excitable membranes. Sunderland, MA: Sinauer Associates Inc; 1990. [Google Scholar]
- 31.Krasznai Z, Marian T, Izumi H, et al. Membrane hyperpolarization removes inactivation of Ca2+ channel leading to Ca2+ influx and subsequent initiation of sperm motility in the common carp. Proc Natl Acad Sci. 1993;97:2052–7. doi: 10.1073/pnas.040558097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vines CA, Yoshida K, Griffin FJ, et al. Motility in herring sperm is regulated by reverse sodium-calcium exchange. Proc Natl Acad Sci. 2002;99:2026–31. doi: 10.1073/pnas.042700899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahamd K, Bracho GE, Wolf DP, Tash JS. Regulation of human sperm motility and hyperactivation components by calcium, calmodulin and proteins phosphatises. Arch Androl. 1995;35:187–208. doi: 10.3109/01485019508987871. [DOI] [PubMed] [Google Scholar]
- 34.Ho HC, Suarez SS. An inosotil 1,4,5-triphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65:1606–15. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- 35.Feng HL, Sandlow JI, Sandra A. The c-kit receptor and its possible role signalling transduction pathway in mouse spermatozoa. Mol Reprod Dev. 1998;48:317–26. doi: 10.1002/(SICI)1098-2795(199803)49:3<317::AID-MRD12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 36.Kirkman-Brown JC, Barratt CL, Publicover SJ. Slow calcium oscillations in human spermatozoa. Biochem J. 2003;378:827–32. doi: 10.1042/BJ20031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandelli A, Miranda PV, Tezon JG. Voltage-dependent calcium channels and Gi regulatory protein mediate the human sperm acrosomal exocytosis induced by N-acetylglucosaminyl/mannosyl neoglycoproteins. J Androl. 1996;17:522–9. [PubMed] [Google Scholar]
- 38.Griveau JF, Le-Lannou D. Reactive oxygen species and human spermatozoa. Int J Androl. 1997;20:61–9. doi: 10.1046/j.1365-2605.1997.00044.x. [DOI] [PubMed] [Google Scholar]
- 39.Fedder J, Ellerman-Erickson S. Effects of cytokines on sperm motility and ionophore stimulated acrosome reaction. Arch Androl. 1995;35:173–85. doi: 10.3109/01485019508987870. [DOI] [PubMed] [Google Scholar]
- 40.Kodama H, Kuribayashi Y, Gagnon C. Effect of sperm lipid peroxidation on fertilization. J Androl. 1996;17:151–7. [PubMed] [Google Scholar]
- 41.Sikka SC. Role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J Androl. 2004;25:5–18. doi: 10.1002/j.1939-4640.2004.tb02751.x. [DOI] [PubMed] [Google Scholar]
- 42.Sies H. Strategies of antioxidants defense. Eur J Biochem. 1993;215:213–9. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]