Abstract
The prophylactic antiemetic effect of 3 dosages of promethazine injected into cats 1 h before administration of xylazine was compared with that of a saline solution. Prior treatment with 2 and 4 mg/kg of promethazine significantly reduced the frequency of emetic episodes. Promethazine may be used as a prophylactic antiemetic in cats treated with xylazine.
Résumé
Efficacité anti-émétique de la prométhazine sur les vomissements induits par la xylazine chez les chats. L’effet anti-émétique prophylactique de 3 doses de prométhazine injectées chez les chats 1 heure avant l’administration de la xylazine a été comparé avec celui d’une solution saline. Un traitement préalable avec 2 et 4 mg/kg de prométhazine a significativement réduit la fréquence d’épisodes émétiques. La prométhazine peut être utilisée comme un anti-émétique prophylactique chez les chats traités avec de la xylazine.
(Traduit par Isabelle Vallières)
Xylazine hydrochloride, an α2-adrenoceptor agonist, possessing analgesic, sedative, and muscle relaxant properties, has been widely used in veterinary practice following its introduction in 1962 (1). Xylazine frequently induces emesis in cats, thereby running the risk of aspiration pneumonia (2). This effect is mediated by α2-adrenoreceptors placed in the chemoreceptor trigger zone (CTZ) of the area postrema in cats (3). Alpha2-adrenoreceptor antagonists such as yohimbine, tolazoline, or phentolamine inhibit xylazine-induced emesis in cats but also prevent its sedative effects (4). The area postrema is very rich in biogenic amines, including histamine which stimulates the medullary CTZ, producing nausea and vomiting (5). Promethazine is a first-generation H1 receptor antagonist of the phenothiazine chemical class that competitively blocks histamine H1 receptors without blocking the secretion of histamine. It is also a very weak dopamine antagonist (6). Promethazine appears to have an antiemetic effect due to its antagonism of central histamine receptors. Since α2-adrenoreceptors, histamine, and dopamine receptors exist in the CTZ of the area postrema in cats, we hypothesized that prophylactic administration of promethazine may prevent vomiting in cats treated with xylazine HCl.
Eight healthy adult cats (4 of each gender) weighing between 1.4 and 2.8 kg (median, 2 kg) were vaccinated with feline rhinotracheitis-calici-panleukopenia vaccine (Fort Dodge Animal Health, Fort Dodge, Iowa, USA) and a rabies vaccine (Rabisin-R; Mérial, Lyon, France), prior to the study. The cats were housed separately in single cages placed in a well-ventilated room with temperature controlled at 22 ± 2°C and were fed a commercial cat food. Water was available ad libitum. The protocol for the study was approved by the institutional animal care committee, Faculty of Veterinary Medicine, University of Tabriz, Iran.
The prophylactic antiemetic effect of 3 doses of promethazine HCl [1, 2, and 4 mg/kg body weight (BW), IM] (Promethazine; Tehran Chemie, Tehran, Iran), selected on the basis of a preliminary study, was evaluated against saline (0.9% NaCl) solution (0.1 mL/kg BW, IM) as a control treatment. Control and drug treatments were injected 1 h before administration of xylazine (0.66 mg/kg BW, IM) (Xylazine, 2%; Alfasan, Woerden, The Netherlands). Saline was administered to the cats on day 0, and promethazine at 1, 2, and 4 mg/kg was administered on days 7, 14, and 21, respectively. All the cats were subjected to the same procedures, and food was withheld on the night preceding each treatment. Promethazine was diluted in saline solution to achieve an injection volume of 0.2 mL. Immediately after each injection of promethazine, the cats were provided with 150 g of commercial cat food. One hour later, xylazine was administered to each cat (0.66 mg/kg, BW, IM). A 2% solution of xylazine was diluted with saline solution to achieve the final injection volume of 0.2 mL.
Emesis was scored as an all-or-none response, and separate episodes of emesis were recorded when the interval between bouts of vomiting exceeded 5 s. During a 30-minute observation period after injection of xylazine, the time to onset of emesis and the number of episodes of emesis were noted. Productive emesis (food or bile) was recorded in this study and retching was excluded from the study. A sedative response was recorded when a cat assumed sternal or lateral recumbency and was unable to stand on its own. Time until onset of sedation after administration of xylazine was recorded.
All data were reported as mean ± standard error of the mean (Sx̄). Data for the time until onset of sedation, latency of emesis, and frequency of emesis after treatment with promethazine were analyzed, using the Wilcoxon signed-rank test. A two-sided test was used and a value of P < 0.05 was considered significant.
Treatment with promethazine at 1, 2, and 4 mg/kg did not significantly alter the time until onset of the first emetic episode in cats sedated with xylazine hydrochloride. Time until onset of the first emetic episode (mean ± Sx̄ ) was 4.17 ± 0.48 min when cats were administered the control saline solution prior to administration of xylazine. When cats were administered doses of 1, 2, or 4 mg of promethazine/kg BW prior to administration of xylazine, times until first emetic episode were 4.86 ± 0.77, 5.17 ± 0.60, and 3.86 ± 0.51 min, respectively.
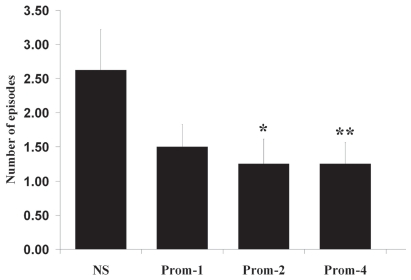
Number of episodes of emesis was 2.63 ± 0.60 for the saline treatment, and 1.50 ± 0.33, 1.25 ± 0.37, and 1.25 ± 0.31 for promethazine at dosages of 1, 2, and 4 mg/kg BW, respectively. Prior treatment with promethazine at dosages of 2 and 4 mg/kg BW significantly reduced the number of episodes of emesis induced by xylazine (Figure 1). Time until onset of sedation was 12.25 ± 1.45 min for the saline treatment and 11.33 ± 2.17, 14.38 ± 1.53, and 10.57 ± 1.74 minutes for promethazine at dosages of 1, 2, and 4 mg/kg BW, respectively. Prior treatment with promethazine did not significantly alter time to onset of sedation after administration of xylazine.
Figure 1.
Effect of 3 doses of promethazine (Prom-1, 1 mg/kg BW; Prom-2, 2 mg/kg BW; and Prom-4, 4 mg/kg BW) compared with normal saline (NS) on the number of episodes of emesis in 8 healthy adult cats sedated with xylazine hydrochloride (0.66 mg/kg BW, IM). Results are presented as means ± standard error of the mean. *P = 0.036, **P = 0.008, compared with control treatment (normal saline).
This study showed that prior intramuscular treatment with 2 and 4 mg/kg of promethazine significantly reduced the frequency of emesis with no significant effect on the time to the first emetic episode after xylazine injection. Phenothiazine agents such as promethazine have been widely used as antiemetics in patients with morning sickness (7), motion sickness (8), or following surgery (post-operative nausea and vomiting) (9). The prominent mechanism of action is antagonism of central histamine and dopamine receptors (6) but the effect of promethazine on xylazine-induced emesis in cats has not been studied previously. We have shown that metoclopramide significantly reduces the frequency of emetic episodes induced by xylazine with no effect on the time until onset of the first emetic episode (10). Others have shown that prior treatment with dexamethasone prevents xylazine-induced emesis in cats through activation of the glucocorticoid receptors in the bilateral nucleus tractus solitarii (NTS) in the medulla oblongata (11). In this respect, maropitant, a potent neurokinin 1 receptor antagonist, reduced the mean number of emetic events induced by xylazine in cats. Maropitant has a low affinity for adrenergic receptors including the α2-adrenergic receptor (12).
In line with these findings, the present study showed that prior treatment with promethazine is effective in reducing the frequency of xylazine-induced emesis in cats, but the mechanisms involved in this effect are not clear. It is well-known that the medulla oblongata has substantial neuronal activity in regulation of the emetic reflex (13) and NTS is richly supplied with many kinds of vomiting-related neurotransmitters and neuro-modulators, such as opioid, gamma-amino butyric acid (GABA), adrenaline, noradrenaline, dopamine, serotonin, histamine, and substance P (14). Also, some authors have hypothesized that the bilateral NTS may be the common final pathway that leads to the vomiting center (15). It is assumed that promethazine, a histamine and dopamine receptor antagonist (like metoclopramide, dexamethasone, and maropitant), doesn’t inhibit α2-adrenoreceptors for its antiemetic action and produces its antiemetic action on xylazine-induced emesis via inhibiting histamine and dopamine receptors in the bilateral NTS in this nervous pathway. The mechanism of this effect remains to be studied in detail; however, our results showed that promethazine in any of the dosages did not alter the time to onset of sedation of cats injected with xylazine HCl.
In conclusion, the present study indicates that promethazine (2 and 4 mg/kg, IM) significantly reduces the frequency of emetic episodes induced by xylazine with no effect on the time until onset of the first emetic episode. Promethazine may be used as a prophylactic antiemetic in cats treated with xylazine HCl. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Greene SA. Pros and cons of using α-2 agonists in small animal anesthesia practice. Anesthesiology. 1999;14:10–14. doi: 10.1016/s1096-2867(99)80022-x. [DOI] [PubMed] [Google Scholar]
- 2.Greene SA, Thurmon JC. Xylazine: A review of its pharmacology and use in veterinary medicine. J Vet Pharmacol Ther. 1988;11:295–313. doi: 10.1111/j.1365-2885.1988.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 3.Hikasa Y, Akiba T, Iino Y, Matsukura M, Takase K, Ogasawara S. Central alpha-2 adrenoceptor subtypes involved in the emetic pathway in cats. Eur J Pharmacol. 1992;229:241–251. doi: 10.1016/0014-2999(92)90562-i. [DOI] [PubMed] [Google Scholar]
- 4.Hikasa Y, Takase K, Saito K, Ogasawara S. Antagonism of the emetic action of xylazine by alpha-2 adrenoceptor blocking agents. Eur J Pharmacol. 1986;130:229–235. doi: 10.1016/0014-2999(86)90272-4. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava KP, Dixit KS. Role of the chemoreceptor trigger zone in histamine-induced emesis. Br J Pharmacol. 1968;34:508–513. doi: 10.1111/j.1476-5381.1968.tb08479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCann DJ, Roth B. Toxicity, Antihistamine, eMed J. [Last accessed December 5, 2011]. Available from http://www.emedicine.com/EMERG/topic38.htm.
- 7.Magee LA, Mazzotta P, Koren G. Evidence-based view of safety and effectiveness of pharmacologic therapy for nausea and vomiting of pregnancy (NVP) Am J Obstet Gynecol. 2002;186:256–261. doi: 10.1067/mob.2002.122596. [DOI] [PubMed] [Google Scholar]
- 8.Wood CD, Graybiel A. Theory of antimotion sickness drug mechanisms. Aerospace Med. 1972;43:249–252. [PubMed] [Google Scholar]
- 9.Carlisle JB, Stevenson CA. Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2006;19:CD004125. doi: 10.1002/14651858.CD004125.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolahian S, Jarolmasjed SH. Effects of metoclopramide on emesis in cats sedated with xylazine hydrochloride. J Feline Med Surg. 2010;12:899–903. doi: 10.1016/j.jfms.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho CM, Ho ST, Wang JJ, Tsai SK, Chai CY. Dexamethasone has a central antiemetic mechanism in decerebrated cats. Anesth Analg. 2004;99:734–739. doi: 10.1213/01.ANE.0000130003.68288.C7. [DOI] [PubMed] [Google Scholar]
- 12.De la Puente-Redondo VA, Tingley FD, Schneider RP, Hickman MA. The neurokinin-1 antagonist activity of maropitant, an antiemetic drug for dogs, in a gerbil model. J Vet Pharmacol Ther. 2007;30:281–287. doi: 10.1111/j.1365-2885.2007.00847.x. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter DO. Neural mechanisms of emesis. Can J Physiol Pharmacol. 1990;68:230–236. doi: 10.1139/y90-036. [DOI] [PubMed] [Google Scholar]
- 14.Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15:301–320. doi: 10.1006/frne.1994.1012. [DOI] [PubMed] [Google Scholar]
- 15.Andrews PLR, Rapeport WG, Sanger GJ. Neuropharmacology of emesis induced by anti-cancer therapy. Trends Pharmacol Sci. 1988;9:334–341. doi: 10.1016/0165-6147(88)90106-x. [DOI] [PubMed] [Google Scholar]