Abstract
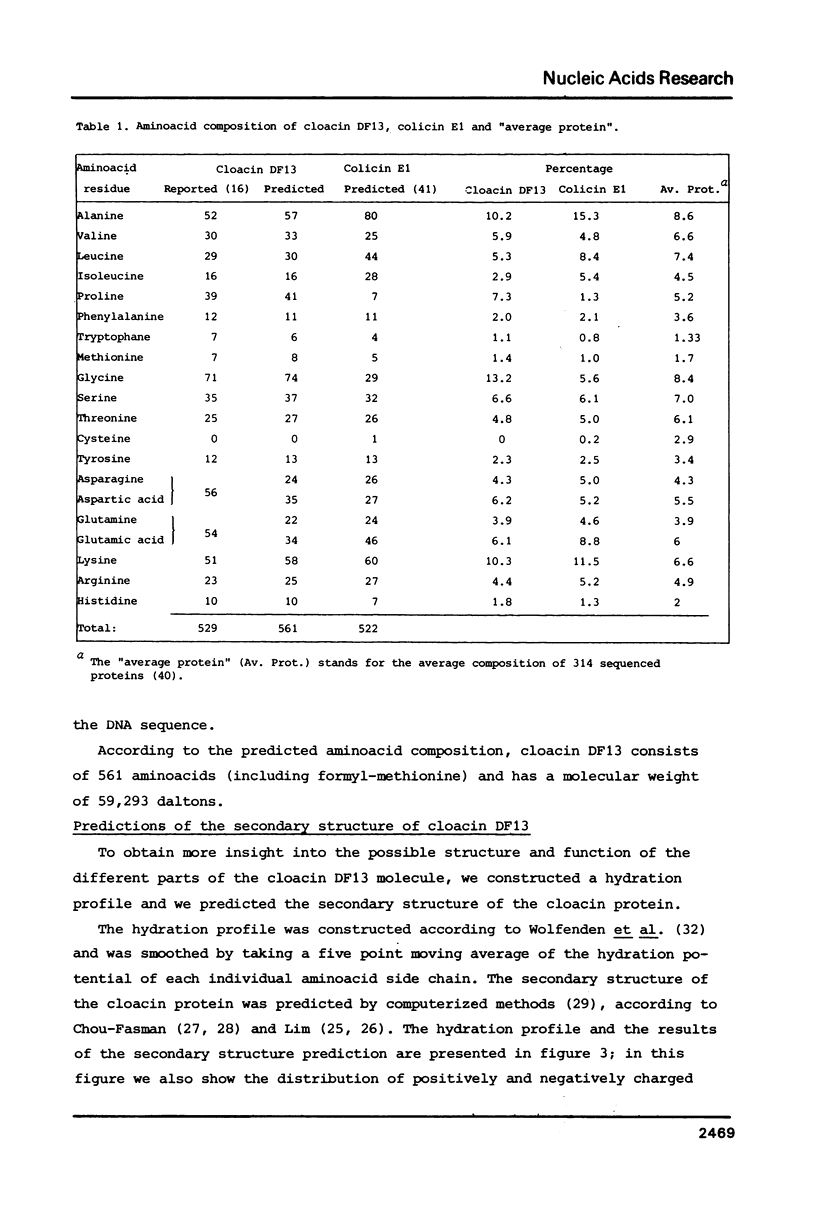
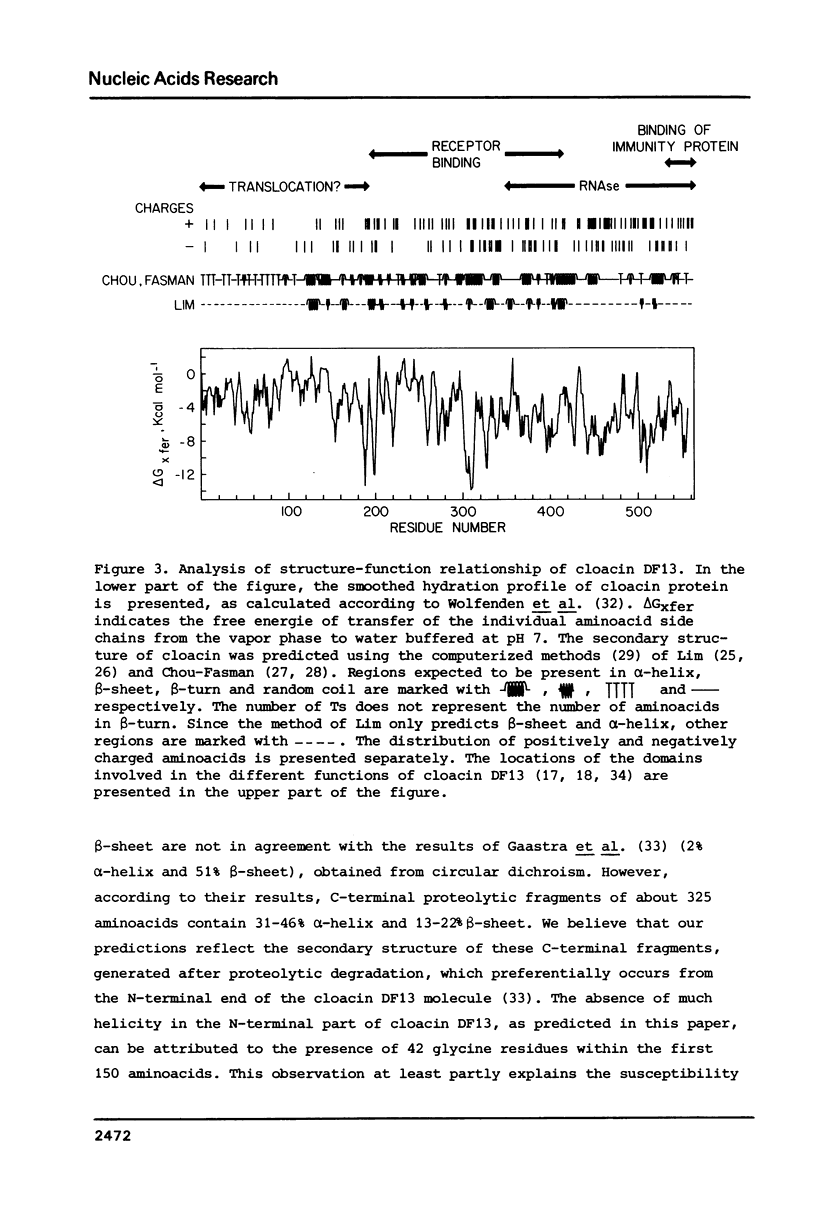
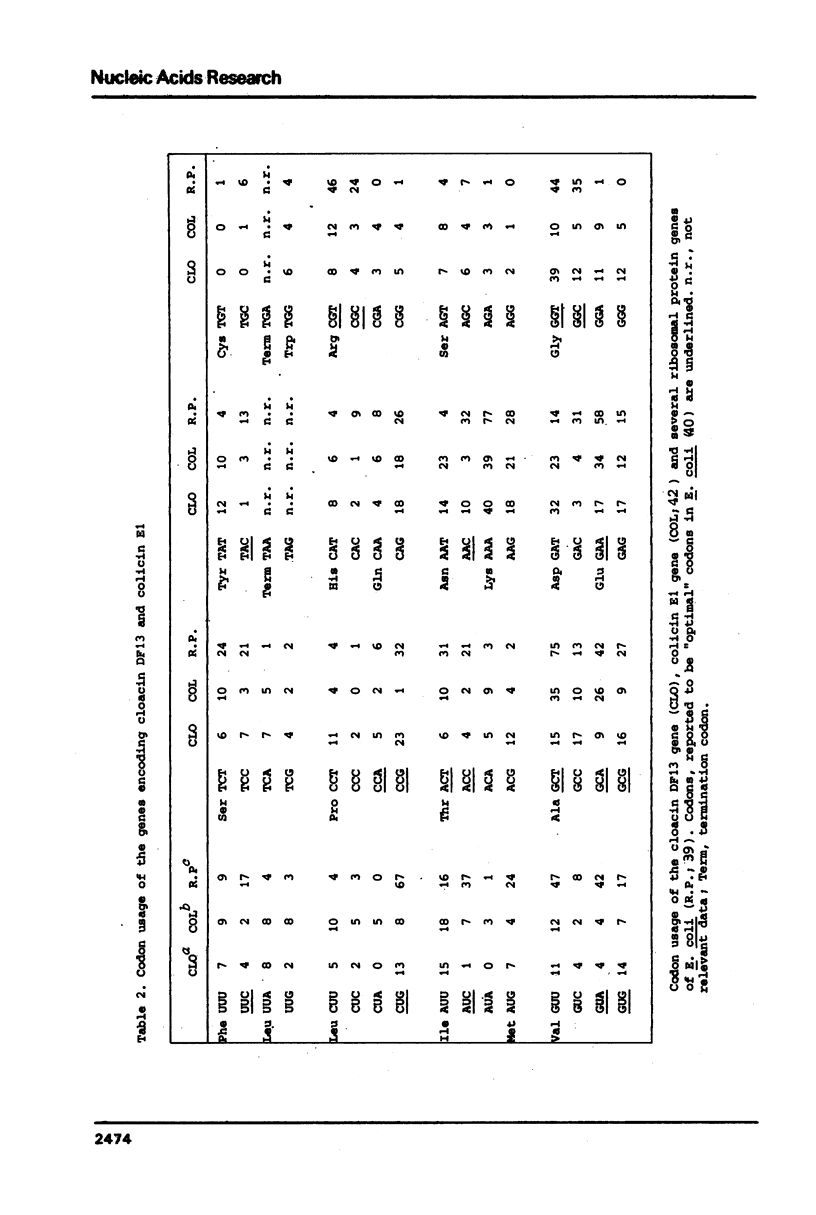
In this paper we present the complete nucleotide sequence of the bacteriocin gene of plasmid Clo DF13. According to the predicted aminoacid sequence the bacteriocin, cloacin DF13, consists of 561 aminoacids and has a molecular weight of 59,293 D. To obtain insight into the structure and function of specific parts of the cloacin molecule, we constructed a hydration profile and we predicted the secondary structure of the protein. According to our predictions, the N-terminus of cloacin DF13 (corresponding to the first 150-180 aminoacids) is relatively hydrophobic and is rich in glycine residues. The data obtained support previous findings that the N-terminal part of cloacin DF13 is involved in translocation of this protein across the cell membrane. The C-terminal part of the cloacin protein is rich in positively charged aminoacids; this might reflect the RNase activity located within this domain. A comparison of the bacteriocin genes and corresponding proteins of Clo DF13 and Col E1 did not reveal any homology at the level of either the nucleotide or the aminoacid sequence. The codon usage of both genes, however, exhibits striking similarities. The sequence data obtained during this study enabled us to present the nucleotide sequence of the entire cloacin operon. The structure of this operon and the regulation of expression of the genes, located within this operon, is discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli P. M., Overbeeke N., Veltkamp E., van Embden J. D., Nijkamp H. J. Genetic map of the bacteriocinogenic plasmid CLO DF13 derived by insertion of the transposon Tn901. Mol Gen Genet. 1978 Mar 20;160(1):1–11. doi: 10.1007/BF00275113. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Klaasen-Boor P. Purification and characterization of a complex between cloacin and its immunity protein isolated from Enterobacter cloacae (Clo DF13). Dissociation and reconstitution of the complex. Eur J Biochem. 1977 Feb 15;73(1):107–114. doi: 10.1111/j.1432-1033.1977.tb11296.x. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K. Mode of action of colicin E2, colicin E3 and cloacin DF13. Zentralbl Bakteriol Orig A. 1979 Jun;244(1):121–134. [PubMed] [Google Scholar]
- Ebina Y., Kishi F., Miki T., Kagamiyama H., Nakazawa T., Nakazawa A. The nucleotide sequence surrounding the promoter region of colicin E1 gene. Gene. 1981 Nov;15(2-3):119–126. doi: 10.1016/0378-1119(81)90121-9. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Kishi F., Nakazawa A. Direct participation of lexA protein in repression of colicin E1 synthesis. J Bacteriol. 1982 Jun;150(3):1479–1481. doi: 10.1128/jb.150.3.1479-1481.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., Koopmans G., de Graaf F. K. Circular dichroism and structure- function relationships in cloacin DF13- immunity protein complex. Biochem Biophys Res Commun. 1978 Jan 13;80(1):97–103. doi: 10.1016/0006-291x(78)91109-9. [DOI] [PubMed] [Google Scholar]
- Gaastra W., Oudega B., de Graaf F. K. The use of mutants in the study of structure-function relationships in cloacin DF13. Biochim Biophys Acta. 1978 May 3;540(2):301–312. doi: 10.1016/0304-4165(78)90143-5. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. I. Localisation of the gene and identification and subcellular localisation of the gene H product. Mol Gen Genet. 1981;183(2):318–325. doi: 10.1007/BF00270635. [DOI] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. II. Functions and regulation of synthesis of the gene H product. Mol Gen Genet. 1981;183(2):326–332. doi: 10.1007/BF00270636. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Kool A. J., Pols C., Nijkamp H. J. Bacteriocinogenic Clo DF13 minicells of Escherichia coli synthesize a protein that accounts for immunity to bacteriocin Clo DF13: purification and characterization of the immunity protein. Antimicrob Agents Chemother. 1975 Jul;8(1):67–75. doi: 10.1128/aac.8.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H., Sekiya T., Rosenberg M., Egan J., Landy A. A rho-dependent termination site in the gene coding for tyrosine tRNA su3 of Escherichia coli. Nature. 1978 Mar 30;272(5652):423–428. doi: 10.1038/272423a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra J. A., Hofsteenge J., Beintema J. J. Invariant features of the structure of pancreatic ribonuclease. A test of different predictive models. J Mol Biol. 1977 Jan 15;109(2):185–193. doi: 10.1016/s0022-2836(77)80028-4. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Algorithms for prediction of alpha-helical and beta-structural regions in globular proteins. J Mol Biol. 1974 Oct 5;88(4):873–894. doi: 10.1016/0022-2836(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol. 1974 Oct 5;88(4):857–872. doi: 10.1016/0022-2836(74)90404-5. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Maat J., Smith A. J. A method for sequencing restriction fragments with dideoxynucleoside triphosphates. Nucleic Acids Res. 1978 Dec;5(12):4537–4545. doi: 10.1093/nar/5.12.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Imahori K. Assignment of the functional loci in colicin E2 and E3 molecules by the characterization of their proteolytic fragments. Biochemistry. 1980 Feb 19;19(4):652–659. doi: 10.1021/bi00545a008. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Imahori K. Assignment of the functional loci in the colicin E1 molecule by characterization of its proteolytic fragments. J Biol Chem. 1982 Jun 10;257(11):6446–6451. [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Imahori K. Comparative studies on the structures of colicins E2 and E3. FEBS Lett. 1979 Apr 15;100(2):249–252. doi: 10.1016/0014-5793(79)80344-0. [DOI] [PubMed] [Google Scholar]
- Oudega B., Stegehuis F., van Tiel-Menkveld G. J., de Graaf F. K. Protein H encoded by plasmid CloDF13 is involved in excretion of cloacin DF13. J Bacteriol. 1982 Jun;150(3):1115–1121. doi: 10.1128/jb.150.3.1115-1121.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., de Graaf F. K. Enzymatic properties of cloacin DF13 and kinetics of ribosome inactivation. Biochim Biophys Acta. 1976 Mar 17;425(3):296–304. doi: 10.1016/0005-2787(76)90256-2. [DOI] [PubMed] [Google Scholar]
- Oudega B., van der Molen J., de Graaf F. K. In vitro binding of cloacin DF13 to its purified outer membrane receptor protein and effect of peptidoglycan on bacteriocin-receptor interaction. J Bacteriol. 1979 Dec;140(3):964–970. doi: 10.1128/jb.140.3.964-970.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rossi J., Egan J., Hudson L., Landy A. The tyrT locus: termination and processing of a complex transcript. Cell. 1981 Nov;26(3 Pt 1):305–314. doi: 10.1016/0092-8674(81)90199-9. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Staden R. Further procedures for sequence analysis by computer. Nucleic Acids Res. 1978 Mar;5(3):1013–1016. doi: 10.1093/nar/5.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje A. R., Spelt C. E., Veltkamp E., Nijkamp H. J. Identification of mutations affecting replication control of plasmid Clo DF13. Nature. 1981 Mar 19;290(5803):264–267. doi: 10.1038/290264a0. [DOI] [PubMed] [Google Scholar]
- Tieze G. A., Stouthamer A. H., Jansz H. S., Zandberg J., van Bruggen E. F. A bacteriocinogenic factor of Enterobacter cloacae. Mol Gen Genet. 1969;106(1):48–65. [PubMed] [Google Scholar]
- Veltkamp E., Stuitje A. R. Replication and structure of the bacteriocinogenic plasmids Clo DF13 and CoI E1. Plasmid. 1981 Jan;5(1):76–99. doi: 10.1016/0147-619x(81)90078-0. [DOI] [PubMed] [Google Scholar]
- Wolfenden R., Andersson L., Cullis P. M., Southgate C. C. Affinities of amino acid side chains for solvent water. Biochemistry. 1981 Feb 17;20(4):849–855. doi: 10.1021/bi00507a030. [DOI] [PubMed] [Google Scholar]
- Yamada M., Ebina Y., Miyata T., Nakazawa T., Nakazawa A. Nucleotide sequence of the structural gene for colicin E1 and predicted structure of the protein. Proc Natl Acad Sci U S A. 1982 May;79(9):2827–2831. doi: 10.1073/pnas.79.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Goedvolk-de Groot L. E., Stouthamer A. H. Purification of a bacteriocin produced by Enterobacter cloacae DF 13. Biochim Biophys Acta. 1970 Dec 22;221(3):566–575. doi: 10.1016/0005-2795(70)90228-x. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Stukart M. J., Boogerd F. C., Metselaar K. Limited proteolysis of cloacin DF13 and characterization of the cleavage products. Biochemistry. 1978 Mar 21;17(6):1137–1142. doi: 10.1021/bi00599a031. [DOI] [PubMed] [Google Scholar]
- van den Elzen P. J., Gaastra W., Spelt C. E., de Graaf F. K., Veltkamp E., Nijkamp H. J. Molecular structure of the immunity gene and immunity protein of the bacteriocinogenic plasmid Clo DF13. Nucleic Acids Res. 1980 Oct 10;8(19):4349–4363. doi: 10.1093/nar/8.19.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Konings R. N., Veltkamp E., Nijkamp H. J. Transcription of bacteriocinogenic plasmid CloDF13 in vivo and in vitro: structure of the cloacin immunity operon. J Bacteriol. 1980 Nov;144(2):579–591. doi: 10.1128/jb.144.2.579-591.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Maat J., Walters H. H., Veltkamp E., Nijkamp H. J. The nucleotide sequence of the bacteriocin promoters of plasmids Clo DF13 and Co1 E1: role of lexA repressor and cAMP in the regulation of promoter activity. Nucleic Acids Res. 1982 Mar 25;10(6):1913–1928. doi: 10.1093/nar/10.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]


