Abstract
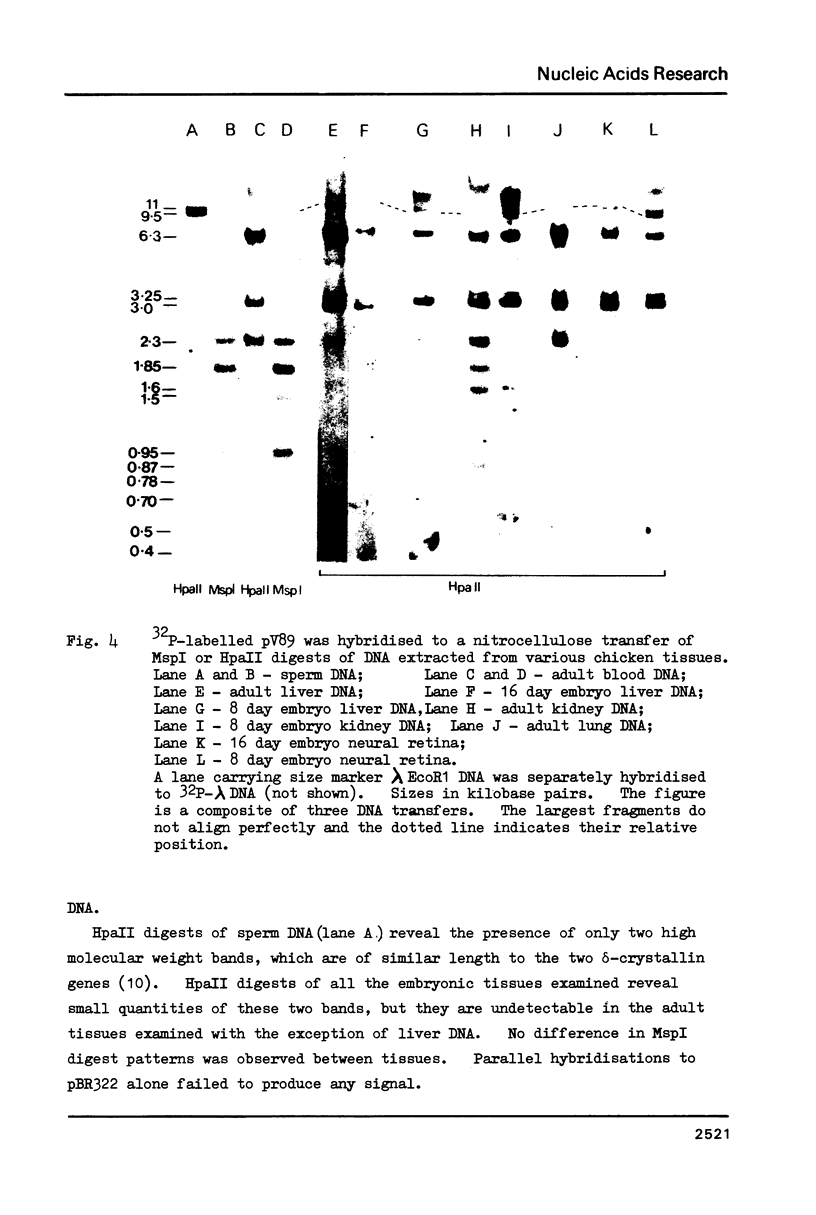
RNA sequences coding for the most abundant chicken lens proteins, delta-crystallin, were detected at very low levels in day old post hatch chick lung, heart, kidney and liver, and in 6 day embryo headless bodies. The pattern of cytosine methylation within the CCGG sequences of the delta-crystallin genes was also examined and shown to vary in several non-lens tissues, from several stages of development. Embryonic neural retina, which expresses a higher level of delta-crystallin RNA than the above tissues, is no less methylated in the sites studied than the tissues which have no association with the eye, and is actually more heavily methylated than the kidney. Thus no obvious correlation was found between undermethylation and gene expression.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Piatigorsky J. Molecular cloning and partial characterization of delta-crystallin cDNA sequences in a bacterial plasmid. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3299–3303. doi: 10.1073/pnas.76.7.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Bird A. P., Taggart M. H., Smith B. A. Methylated and unmethylated DNA compartments in the sea urchin genome. Cell. 1979 Aug;17(4):889–901. doi: 10.1016/0092-8674(79)90329-5. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Clayton R. M., Campbell J. C., Truman D. E. A re-examination of the organ specificity of lens antigens. Exp Eye Res. 1968 Jan;7(1):11–29. doi: 10.1016/s0014-4835(68)80022-3. [DOI] [PubMed] [Google Scholar]
- Clayton R. M., Thomson I., de Pomerai D. I. Relationship between crystallin mRNA expression in retina cells and their capacity to re-differentiate into lens cells. Nature. 1979 Dec 6;282(5739):628–629. doi: 10.1038/282628a0. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Genis-Galves J. M., Maisel H., Castro J. Changes in chick lens proteins with aging. Exp Eye Res. 1968 Oct;7(4):593–602. doi: 10.1016/s0014-4835(68)80014-4. [DOI] [PubMed] [Google Scholar]
- Haigh L. S., Owens B. B., Hellewell O. S., Ingram V. M. DNA methylation in chicken alpha-globin gene expression. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5332–5336. doi: 10.1073/pnas.79.17.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelle B. L., Phillips J. A., 3rd, Seeburg P. H. Relative levels of methylation in human growth hormone and chorionic somatomammotropin genes in expressing and non-expressing tissues. Nucleic Acids Res. 1982 Jun 11;10(11):3459–3474. doi: 10.1093/nar/10.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. F., Clayton R. M., Williamson R., Thomson I., Truman D. E., de Pomerai D. I. Sequence complexity and tissue distribution of chick lens crystallin mRNAs. Dev Biol. 1978 Aug;65(2):383–395. doi: 10.1016/0012-1606(78)90034-9. [DOI] [PubMed] [Google Scholar]
- Jones R. E., DeFeo D., Piatigorsky J. Transcription and site-specific hypomethylation of the delta-crystallin genes in the embryonic chicken lens. J Biol Chem. 1981 Aug 10;256(15):8172–8176. [PubMed] [Google Scholar]
- Kakidani H., Furutani Y., Takahashi H., Noda M., Morimoto Y., Hirose T., Asai M., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature. 1982 Jul 15;298(5871):245–249. doi: 10.1038/298245a0. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- McKeon C., Ohkubo H., Pastan I., de Crombrugghe B. Unusual methylation pattern of the alpha 2 (l) collagen gene. Cell. 1982 May;29(1):203–210. doi: 10.1016/0092-8674(82)90104-0. [DOI] [PubMed] [Google Scholar]
- Okada T. S. Cellular metaplasia or transdifferentiation as a model for retinal cell differentiation. Curr Top Dev Biol. 1980;16:349–380. [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Cassio D., Levilliers J., Sala-Trepat J., Weiss M. C. Undermethylation at the 5' end of the albumin gene is necessary but not sufficient for albumin production by rat hepatoma cells in culture. Cell. 1982 Oct;30(3):825–833. doi: 10.1016/0092-8674(82)90287-2. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Jackson J. F., McAllister L. B., Rothman B. S., Mayeri E., Axel R. A single gene encodes multiple neuropeptides mediating a stereotyped behavior. Cell. 1983 Jan;32(1):7–22. doi: 10.1016/0092-8674(83)90492-0. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Goldstein S. Interclonal variation in methylation patterns for expressed and non-expressed genes. Nucleic Acids Res. 1982 Jul 24;10(14):4293–4304. doi: 10.1093/nar/10.14.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Markham P. D., Baluda M. A. Reliability of the RNA-DNA filter hybridization for the detection of oncornavirus-specific DNA sequences. J Virol. 1974 Aug;14(2):225–230. doi: 10.1128/jvi.14.2.225-230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider T. W. The 5'-cytosine in CCGG1 is methylated in two eukaryotic DNAs and Msp I is sensitive to methylation at this site. Nucleic Acids Res. 1980 Sep 11;8(17):3829–3840. doi: 10.1093/nar/8.17.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomson I., Wilkinson C. E., Burns A. T., Truman D. E., Clayton R. M. Characterization of chick lens soluble proteins and the control of their synthesis. Exp Eye Res. 1978 Mar;26(3):351–362. doi: 10.1016/0014-4835(78)90081-7. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Wilks A. F., Cozens P. J., Mattaj I. W., Jost J. P. Estrogen induces a demethylation at the 5' end region of the chicken vitellogenin gene. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4252–4255. doi: 10.1073/pnas.79.14.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]