Abstract
A wide spectrum of outcomes in the cognitive effects of aging is routinely observed in studies of the elderly. Individual differences in neurocognitive aging are also a characteristic of other species, such as rodents and non-human primates. In particular, investigations at behavioral, brain systems, cellular and molecular levels of analysis have provided much information on the basis for individual differences in neurocognitive aging among healthy outbred rats. These findings are likely to be relevant to an understanding of the effects of aging on the brain, apart from neurodegenerative conditions, such as Alzheimer’s disease, which do not naturally occur in rodents. Here we review and integrate those findings in a model supporting the concept that certain features of cognitive decline are caused by distributed alterations in the medial temporal lobe, which alter the information processing functions of the hippocampal formation. An additional emerging concept from this research is that preserved abilities at older ages may depend on adaptive changes in the hippocampal system that distinguish successful aging.
Key words: spatial memory, hippocampal formation, successful aging, rats, cognitive impairment
Average life span increased by more than 30 years during the 20th century. Today, the fastest growing segment of the population in the industrialized countries of the world is in the bracket over 85 years of age, focusing attention on neurocognitive aging in health and disease. The aging mind is subject to widely different outcomes. Significantly, aging is the main risk factor for Alzheimer’s disease (AD), the most common form of dementia. Aging is also associated with a range of milder cognitive difficulties affecting the acquisition and use of new information and the ability to plan, initiate, and execute many of life’s activities on a daily basis (Hedden and Gabrieli 2005). At the same time, community based studies of the elderly (Berkman et al. 1993) and well-controlled laboratory assessments in combination with modern brain imaging methods (Cabeza et al. 2002; Rosen et al. 2002) have stimulated a growing interest in high-performing older individuals. What adaptive or compensatory resources, biologically-based or rooted in life-style, sustain the functions of the mind in some of the elderly? Here we consider lessons learned from research on neurocognitive aging in an animal model—one that is informative about the mind/brain consequences of aging independent of neurodegenerative disease, such as AD (Gallagher and Rapp 1997). It is a model, nonetheless, in which widely different outcomes in cognitive abilities occur at older ages, despite the fact that the study population is maintained under “standard” laboratory conditions throughout life (Gallagher et al. 1993).
The question that impelled our research program from its inception was how to account for differences in cognitive abilities at older ages and how that understanding could translate into better outcomes that might not otherwise occur. Although this article will focus on research in a single study population of outbred aged male Long-Evans rats used in this work for over 15 years, correspondence with findings in other strains of rodents will be noted. How the lessons learned in this research inform our understanding of neurocognitive aging in humans will also be considered.
To characterize the cognitive abilities of older rats we use a version of the Morris water maze task, in which subjects navigate to a hidden escape platform using a configuration of spatial information surrounding the maze. This apparatus is widely used in behavioral neuroscience with many procedural variations. We optimized a standard protocol for sensitivity and reliability in detecting individual differences in cognitive aging. Key parameters in this regard are sparse training (only 2–3 trials per day) and interpolated probes to obtain multiple assessments for “place” memory rather than reliance on a single probe at the end of training, as is customary in many protocols (Gallagher et al. 1993). The overall performance of aged rats, in the range of 24–28 months, is less proficient than that of young adults (4–8 months), but, most notably, the range of aged rat performance far exceeds that at younger ages (Figure 1). Moreover, those individual differences in the standard assessment exhibit test–retest reliability over a span of many weeks (Colombo et al. 1997; Gallagher and Burwell 1989). In addition to previously published data on retest for a new escape location in the same environment (shown in Figure 1), Figure 2 shows results of a re-assessment in an entirely novel spatial environment, and Figure 3 also shows corresponding individual differences in a different motivational setting (e.g., food-reinforced radial maze) that depends on memory for spatial locations spanning a delay (previously unpublished data). Each shows the reliability of individual differences in the performance of aged rats within this cognitive domain. Although the study population is composed of male rats, a similar distribution of individual differences at older ages is also a feature of female rats in the outbred Long-Evans strain (Gallagher, unpublished data, and see Stemmelin et al. 2000).
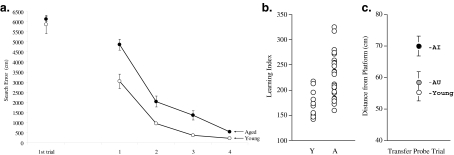
Figure 1.
Individual differences for cognitive aging in healthy rats. a Training trial performance for a group of young (6 months) and aged (28 months) male Long-Evans rats. Data points on the far left indicate performance on the first training trial, where no age difference was observed. The protocol consisted of three trials/day, with the last trial every other day consisting of a probe without the platform available for 30 s to monitor the rat’s spatial bias in searching for the platform location. b The learning index scores for individual rats in the young and aged groups obtained from probe trial performance to reflect search accuracy over the course of training. Note that the range of scores for aged rats encompasses and exceeds the range for the young, in the direction of impairment (higher index scores). c The retest scores from a probe trial after training 2 weeks later, with the platform in a new spatial location. In the re-test, data are presented for young rats and subgroups of aged rats based on the original learning index; aged rats that fell within the range of young performance are indicated as aged-unimpaired (AU), while aged rats that fell outside the range of young performance are designated impaired (AI). Aged rats that were impaired on the initial assessment were also impaired in the retest, while unimpaired aged rats again performed on a par with young. Note that the “search error” measure for training trials represents deviation from a direct path to the platform from the starting position, and that the learning index is calculated from probe trial search in X–Y coordinates (see Gallagher et al. 1993 for details). Data are reproduced from Colombo et al. 1997
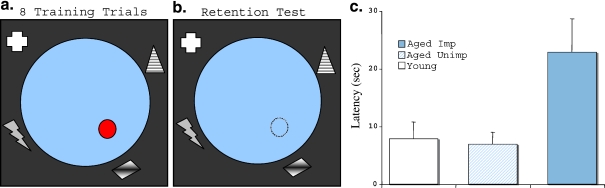
Figure 2.
Retest in a novel spatial environment. Behaviorally characterized young and aged rats were tested after 1 month in an entirely novel spatial water maze environment (new maze in different physical location and surrounding cues, etc.). Rats received a single session of training (eight trials, 8 min intertrial interval), (a), using a visible platform that remained in a constant location with respect to extra-maze cues. During training trials all rats, irrespective of age or cognitive status, rapidly navigated to the escape platform (data not shown). Those data, similar to those for performance in other cue training protocols with a visible platform, show that the performance demands of the task are unaffected by aging. After a delay of 1 h, the rats were tested with the platform removed (b). Young rats and aged rats, originally characterized as “unimpaired” in the standardized protocol, swam directly to the location where the escape platform had been positioned in the earlier training session. In contrast, the previously characterized “impaired” aged rats did not navigate as accurately in the retention test (c), p<0.005). This study further demonstrates the reliability of differences in the aged population in a spatial memory assessment
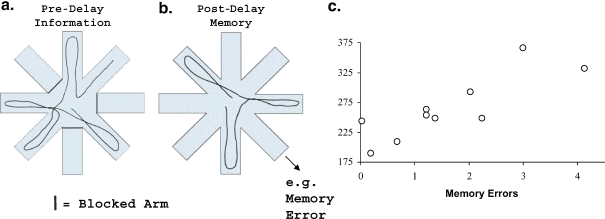
Figure 3.
Spatial memory in a radial-maze assessment. Data are shown for aged rats behaviorally characterized in the water maze and, 8 weeks later, tested on an eight-arm radial maze. The radial maze protocol was similar to that described in Chappell et al. (1998). After training both young and aged rats in the win-shift version of the radial maze task (each arm rewarded only once in a trial), we increased the memory demand of the task by imposing a brief delay during the trial. At the beginning of each trial a subset of arms was blocked; the identity and configuration of the blocked arms were varied across trials. Rats were allowed to obtain food on the arms to which access was permitted (Pre-Delay Information; a). Rats were then removed from the maze for a delay interval, during which time the barriers on the maze were removed, thus allowing access to all eight arms. Rats were then placed back onto the center platform and allowed to obtain the remaining food rewards (Post-Delay Memory; b). A memory error occurred after the delay when a rat returned to one of the arms that had been visited prior to the delay. Each rat’s performance was averaged across four consecutive trials, with a pre-delay information set of five open arms and delay interval of 60 s. Aged rats committed more memory errors than did young rats (p<0.025; on average, young rats committed 0.17 errors, whereas aged rats committed an average of 1.52 errors). Correlational analysis (Pearson’s r) was used to examine the relationship between performance of aged rats (N =10) in the radial maze and in the Morris water maze (learning index scores). The aged rats exhibited a wide range of performance on the radial maze (c), such that individual differences in this task were correlated with the prior water maze assessment (r=0.82, for aged rats; p<0.01)
The development of this model for cognitive aging opened the way for a powerful approach to the investigation of consequential neurobiological effects of aging at brain system, circuit, cellular and molecular levels of analysis, all in the same study population of behaviorally characterized aged rats. It is important to note that the viability of this approach depends on the exclusion of sources of disability or illness in older animals that could influence performance in behavioral measures independent of critical cognitive functions. Beyond the widely accepted criterion of using pathogen-free aged subjects, rats included in the research program are fit and healthy, as determined by sensorimotor tests for disability, clinical tests for adverse physiological conditions (e.g., impaired renal function) and necropsies when they are killed (e.g., to exclude subjects with pituitary or testicular tumor). Of these well-screened healthy rats at an advanced chronological age (24–28 months) approximately 50% qualify as cognitively impaired by falling outside the range of young performance in the standard protocol (Gallagher et al. 1993). Among the “unimpaired” members of the aged cohort are subjects that perform on a par with young adults (Figures 1 and 2). Of interest in this setting are brain measures that are closely coupled to cognitive status as markers and potential mediators of neurocognitive aging.
The cognitive model in aged rodents is geared to detecting the integrity of the medial temporal lobe (MTL) system, with particular sensitivity to the hippocampal formation. Because characteristic features of cognitive decline in human aging are linked to MTL structures (Grady and Craik, 2000; Uttl and Graf 1993; Wilkniss et al. 1997), the systems under study are relevant to the condition of mammalian brain aging, apart from diseases, such as AD, that do not occur naturally in rodents. The circuitry of the medial temporal lobe system is schematically shown in Figure 4. Extensive analysis of components of this system supports the understanding of neurocognitive aging primarily as a functional disorder rather than a frank neurodegenerative condition in the brain.
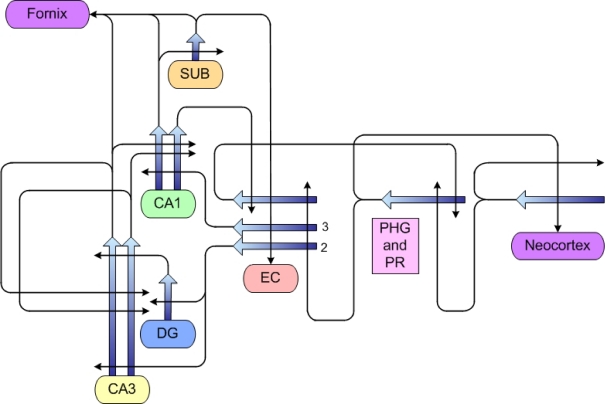
Figure 4.
Some of the connectional circuitry of the medial temporal lobe system. Studies in this research program have shown that aged rats, irrespective of cognitive status, have preserved numbers of neurons in both cortical (PHG, PR and EC) and hippocampal areas (DG, CA3, CA1). Our studies have also indicated no loss of connectional integrity, with the exception of input originating from layer II entorhinal cortical (EC) neurons. Specifically, aged rats with cognitive impairment have sparser input from that source terminating on both granule cells of the dentate gyrus (DG) and pyramidal neurons of the CA3 region (see text for further details and references). PHG parahippocampal/postrhinal cortex, PR perirhinal cortex, SUB subiculum
Structural features of neurocognitive aging in the hippocampal system
Brain aging in the medial temporal lobe in healthy rats, irrespective of cognitive outcome, is characterized by substantial preservation of structural integrity. Systematic investigations of neuron number using unbiased stereology revealed no loss of the principle neurons in the dentate gyrus, subregions of the hippocampus proper (CA1 and CA2/3) or cortical areas of the medial temporal lobe system (perirhinal, postrhinal and entorhinal) that are interconnected with the hippocampal formation. Importantly, no evidence for loss of neurons was found in these regions in rats with identified cognitive impairment relative to either young adults or unimpaired aged cohorts (Rapp and Gallagher 1996; Rapp et al. 2002). In addition to numbers, key morphological features of neurons are also preserved. Stereological techniques to measure dendritic spines throughout the dentate gyrus and hippocampal subfields revealed no evidence for a loss of spines associated with cognitive impairment, in any region; only a small increase in spine number was detected in aged brains in the molecular layer of the dentate and CA1 regions, which was equally present, across the spectrum of cognitive performance, in older rats compared with young ones (Calhoun et al. 2002).
Indeed, connectional integrity is clearly evident in much of the MTL circuitry. In quantitative electron microscopy (EM), no difference in total numbers of synapses in the CA1 region of the hippocampus was observed, either as a function of age or of cognitive status (Geinisman et al. 2004). Those EM data are consistent with earlier indicators of connectional integrity in CA1 in this study population of aged rats, which provided additional data on markers for inputs to CA1 of different origins (Smith et al. 2000). Thus, among the hippocampal subregions, the CA1 area is particularly notable in its apparent maintenance of input, both directly from the entorhinal cortex (layer III) and from earlier processing in the hippocampal system via CA3 Schaffer collaterals. Similarly, connectional integrity was found in the CA3 region for its inputs from the dentate gyrus (mossy fibers) and auto-associative connections (recurrent collaterals from the CA3), which form the majority of synapses onto the CA3 pyramidal cells (Smith et al. 2000).
In contrast to the substantial structural integrity described thus far, the layer II entorhinal input to the hippocampal formation becomes somewhat more sparse in older brains, as established in this model and also reported in other research (Barnes 1979; Geinisman et al. 1992; Smith et al. 2000). Further, we showed that loss of the layer II entorhinal input similarly affects connections onto both granule cells in the dentate gyrus and pyramidal neurons in the CA3 region selectively in aged rats with cognitive impairment (Smith et al. 2000).
Recent findings have demonstrated that elderly humans with memory impairments possess a remarkably similar pattern of connectional loss affecting the layer II entorhinal input to the hippocampus. Scheff and colleagues (2006) reported that the number of synapses in the outer molecular layer of dentate gyrus receiving layer II entorhinal input was reduced in autopsied aged brains from individuals diagnosed with mild cognitive impairment, in comparison with cognitively intact elderly subjects, and the extent of synapse reduction correlated with performance on an earlier test of delayed recall. Further, the use of diffusion tensor imaging, a new MRI technique to assess projection systems, has shown a significant decrease in volume of the perforant path, which comprises the entorhinal afferents to the dentate gyrus, of aged memory-impaired humans, compared with values obtained in young subjects (Kalus et al. 2005). This parallel with the rodent is noteworthy, because entorhinal cortex itself is recognized as a site of neuronal degeneration in individuals with Alzheimer’s disease, with substantial cell loss occurring in an early phase of that condition (Gomez-Isla et al. 1996; Killiany et al. 2002; Kordower et al. 2001). The data in older memory-impaired rodents, where synapse loss occurs with preserved numbers of neurons, suggests that, in the absence of frank cell loss, the maintenance of synaptic connections providing cortical input to the dentate can be a prominent feature of neurocognitive aging. How this loss factors into cognitive dysfunction will be considered along with additional features of neurocognitive aging in other components of the MTL system.
Features of neurocognitive aging in the function of existing cells and synapses
While numbers of neurons are largely preserved throughout the medial temporal lobe in aged rats with cognitive impairment, critical cellular machinery necessary for information processing and plasticity is affected in a manner that closely relates to behavioral outcome in aging (see Burke and Barnes 2006; Wilson et al. 2006 for reviews). For example, a number of studies has shown that the responsiveness of post-synaptic signaling in hippocampal neurons through muscarinic cholinergic receptors is strongly correlated with the performance of aged rats (Chouinard et al. 1995; Nicolle et al. 1999; Rossi et al. 2005; Zhang et al. 2006). Although the post-synaptic density of muscarinic receptors remains stable in older animals, these sites are less able to stimulate downstream effector responses via phospholipase C (PLC)-mediated phosphoinositide (PI) turnover. Recent findings in our study population have further shown that muscarinic receptor activation of its G-protein, Gq, using time-resolved fluorometry GTP-Eu binding assay, is reduced in aged rats with cognitive deficits, relative to both unimpaired aged and young rats, a receptor coupling deficiency that is also correlated with severity of cognitive impairment across the aged spectrum (Zhang et al. 2006). Using other methods (and a different strain of aged rat, i.e., Fischer 344), Rossi et al. (2005) obtained similar evidence for a loss of coupling at cholinergic receptors in their aged rats that correlated with impaired cognitive function. In additional studies we observed a corresponding failure in cholinergic stimulation of PLC-dependent neural plasticity in the hippocampus as a feature of neurocognitive aging; this loss was associated with impairment but was not evident in aged rats with preserved cognitive function (Lee et al. 2005). Thus, the behavioral outcome in the model corresponds to functional measures of cellular signaling and plasticity. It is notable that a deficiency in the muscarinic post-synaptic signaling pathway occurs not only in experimental laboratory animals but also in human brain, for which evidence consistent with this mechanism has also been reported, both in aging and in AD (e.g., Crews et al. 1994; Jope et al. 1997), again providing a parallel that translates across aging species.
Importantly, studies of cellular function and plasticity in neurocognitive aging have revealed that changes do not similarly affect all components of the MTL system. A dramatic example of region-specific differences became evident in recordings from single neurons in different areas of the hippocampus (CA1 and CA3) while rats were exposed to a familiar environment and then allowed to explore a novel environment for the first time. “Place cells”, referring to the encoding of location-specific information by CA1 and CA3 pyramidal neurons, will rapidly form new spatial representations in a novel environment that distinctly differ from the representations in a familiar one (Guzowski et al. 2004; Leutgeb et al. 2005). In contrast to this observed “remapping” seen in young rats, place cells of aged rats tend to maintain the initially formed spatial representation, suggesting that, instead of creating a new representation, a previously stored representation is more likely to be retrieved (Ikonen et al. 2002; Tanila et al. 1997a, b; Wilson et al. 2003, 2004, 2005). The degree of such rigidity corresponded in aged rats to severity of cognitive impairment (Wilson et al. 2003). Notably, while this feature of neurocognitive aging is prominent in the CA3 region, where firing rates are also markedly elevated in the older rats, no difference in this experimental setting was observed in either information encoding or cell firing rates in the CA1 (Wilson et al. 2005).
Notwithstanding the substantial structural integrity of the CA1 region, and certain features of CA1 information encoding that seem quite unaffected in the brains of aged rats with cognitive impairment, a very large body of research has also documented effects of aging on the functional properties of CA1 neurons (see Burke and Barnes 2006; Foster 1999 for reviews). Much of this evidence comes from in vitro preparations in which the synaptic functions/plasticity of the CA1 are isolated. That work has demonstrated significant changes in the excitability of neuron response to input in the form of an augmented after-hyperpolization (AHP) and a reduction in plasticity, using submaximal stimulation protocols, changes that have also been shown to correlate with cognitive outcome in aging (e.g., Tombaugh et al. 2002, 2005). Our studies have similarly provided evidence for alterations affecting the function of connections in CA1. Notably, the EM analysis, while indicating preserved numbers of synapses (Geinisman et al. 2004), showed a striking difference in the area of the post-synaptic density (PSD) at perforated synapses. The aged rats with preserved cognitive ability had a distribution of PSD areas entirely overlapping with that of young rats, whereas that distribution for the impaired aged rats was substantially shifted to smaller sizes (Nicholson et al. 2004). Reduced PSD area implies less effective connections despite a preserved number of connections. These effects in the CA1 are likely to contribute to certain characteristics of CA1 encoding seen in older animals. For example, aged rats demonstrate less backwards expansion of their CA1 place fields than do young rats as they run repeatedly around a circular track (Shen et al. 1997), a well-studied phenomenon arising from experience-dependent plasticity (Mehta et al. 1997). Additionally, Barnes and colleagues (Barnes et al. 1997) characterized the spatial representations of CA1 hippocampal cells in young and aged rats first exploring one environment and then, following a tour of several new environments (without their cellular activity being recorded), being placed back into the original arena. In young rats the same spatial representations were consistently re-generated on repeated exposure to the initial environment, but aged rats often failed to show such reliable encoding. The failure to retrieve representations reliably may have been due to an inadequate initial strength of encoding. Although such features of CA1 neurons in aged rats were not explicitly studied in relation to cognitive status, the findings are consistent with in vitro evidence of individual differences affecting plasticity within the CA1 region.
A neural systems approach to models for neurocognitive aging
Considerable evidence indicates that individual differences in a specific functional domain during aging are not necessarily associated with other age-related behavioral changes that depend on different neural systems (Gage et al. 1989; Gallagher and Burwell, 1989; Glisky et al. 1995). Such evidence has been garnered in the study population that has been the focus of our work; reliable individual differences in a range of aging-sensitive behavioral measures, e.g., cued reaction time and diurnal behavior, have been dissociated from cognitive decline (Burwell and Gallagher, 1993; Burwell et al. 1992; Gallagher and Burwell, 1989). Even within the broad scope of cognitive abilities that are affected by aging, dissociations have been shown between functions that depend on different neural systems, e.g., medial temporal lobe and prefrontal cortex (Barense et al. 2002; Glisky et al. 1995; Schoenbaum et al. 2002; Zyzak et al. 1995). Such dissociations support the general concept of modeling the effects of aging on the brain in a system-specific manner.
At the same time, within the MTL, a number of alterations that are closely coupled to cognitive impairment are distributed throughout the components of this system. Any one specific alteration in isolation may be insufficient to cause the cognitive impairment of older animals. For example, in young rats the entire removal of the hippocampal cholinergic system, using a selective immunotoxin, fails to reproduce the deficits observed in older animals on a range of hippocampal-dependent tasks, including the precise protocol used in our work to characterize neurocognitive aging (Baxter et al. 1995; Baxter and Gallagher 1996; Chappell et al. 1998). Similarly, despite a diminished entorhinal input, CA1 and CA3 “place fields” of aged rats with behavioral impairments are not degraded with respect to field size, coherence, and other parameters that characterize spatial information content (Rosenzweig et al. 2003; Wilson et al. 2003). Instead, alterations in encoding are revealed in the experience-dependent plasticity of those cells, effects that are manifest in distinct settings across different hippocampal subregions (Barnes et al. 1997; Mizumori and Kalyani 1997; Shen et al. 1997; Wilson et al. 2005).
Rather than the cause of cognitive impairment being attributed to isolated features of neurobiological aging or global cellular failure, the wealth of information that has accumulated from detailed study of the MTL system might be better understood from a neural systems and information processing perspective. For example, a recent model that incorporates findings in our study population proposes that already stored information becomes the dominant pattern of hippocampal processing, to the detriment of new information being encoded in the brains of impaired aged rats. (Wilson et al. 2006) This result is not merely due to the failure of an information storage device at a specific site, such as potentiation of CA1 synapses, but is based on local changes in the computational functions of each component of the hippocampal system. Indeed, a number of factors affecting the dentate gyrus and CA3 in the aged brain are consistent with a condition that alters the balance between processes of pattern separation and pattern completion, which are thought to play a critical role in episodic memory, allowing experiences that share common elements to be distinguished. By this view, circuit specific alterations in concert determine the cognitive outcome in the affected aging brain. Such models of neurocognitive aging are potentially powerful in drawing on a vast amount of knowledge already gained, as well as the rapid pace of progress in neuroscience research; the hippocampal system is the focus of much work, bridging from molecular and cellular mechanisms at distributed sites of plasticity to empirical studies of experience-dependent encoding within circuit elements and computational models that link these different levels of analysis to behavioral analysis of cognitive functions.
Preserved function: differential and adaptive aging
Individual differences in the study population have provided a valuable background for examining the basis of neurocognitive aging in older animals. To the extent that cognitive decline is not invariably tied to chronological age, behavioral characterization allows a more sensitive approach to detect neurobiological and neurophysiological alterations that might be obscured by simply comparing old subjects with young. Individual differences also provide a setting of interest for examining the basis for a better outcome in aged subjects with preserved cognitive function. The basis for such individual differences, often referred to as “successful aging”, is a topic of high interest in current research on aging in humans.
One account for the preservation of cognitive abilities at advanced ages is differential aging, specifically referring to individual differences in the rate or magnitude of aging. Such differences could be due to biological endowment, i.e., differences in expression of aging mechanisms or greater inherent protective mechanisms. Alternatively, rate or impact of aging might be modified by experience, as suggested by associations between specific life exposures, such as education and occupational status, and aging outcomes. Firm evidence for this hypothesis from either source, however, is difficult to obtain in studies of human aging (see Salthouse 2006 for recent discussion).
A substantial proportion of outbred rats at older ages (24–28 months) perform on a par with young adults (4–9 months of age). The cognitive status of unimpaired animals has proven to be highly reliable for those individuals, both on retest after 1 month and even at a 3 month retest after the original assessment (Colombo et al. 1997; Gallagher and Burwell 1989). The absence of significant neurobiological alterations that characterize rats that age without cognitive impairment could indicate a diminished impact or delayed rate of aging among some animals in this outbred model. Two examples noted earlier in this review, loss of connectional integrity for the inputs from layer II entorhinal neurons and the muscarinic coupling deficiency, are not evident in rats with preserved cognition (Nicolle et al. 1999; Smith et al. 2000). Such individual differences are notable, given the relative similarity in environmental conditions during rearing and over the lifespan in this study population.
Although biological differences in aging might account for the absence of certain changes in neurobiology and cognition in a subpopulation of older rats, it is important to note that rather dramatic and invariant indicators of aging do occur in the hippocampal system, equally affecting the brains of unimpaired and impaired rats. Here we refer, in particular, to the decline in neurogenesis of granule cells in the dentate gyrus. A substantial reduction in neurogenesis in the dentate occurs around 1 year of age in the rodent (Bizon and Gallagher 2003; Kuhn et al. 1996; Nacher et al. 2003). Although some strains appear to exhibit little further decline at later ages (Nacher et al. 2003; Rao et al. 2005) our studies in the Long-Evans strain have shown a further significant drop occurring between 12 and 24 months (Bizon and Gallagher 2003). Remarkably, at least in Long-Evans rats, there is no behavioral phenotype of substantially reduced neurogenesis at any age, and, by 2 years of age, similar large decreases are seen in both aged rats that exhibit cognitive impairment and aged cohorts with preserved cognitive abilities (Bizon and Gallagher 2003; Bizon et al. 2004; Merrill et al. 2003). Indeed, our studies show modestly greater reduction in unimpaired than in impaired aged rats (Bizon et al. 2004; but, in a different rat strain, see Drapeau et al. 2003). A second feature, which appears to coincide at middle-age with reduced neurogenesis (and is thought to have direct bearing on that change, see Howell et al. 2003), is a reduction in the number of neuropeptide Y (NPY)-positive neurons that are normally abundant in the hilus (Hattiangady et al. 2005). Similar to the profound drop in proliferation of new cells, the reduction of NPY-containing hilar interneurons is equally evident in both impaired and unimpaired aged Long-Evans rats at 2 years of age (Cadiacio et al. 2003).
While reduced neurogenesis has been tied to hippocampal-dependent learning in young adults and it has been suggested that age-dependent loss of neurogenesis might play a role in cognitive decline, there is clearly a mismatch between behavioral outcome and loss of neurogenesis at older ages. An age-related reduction in neurogenesis would not appear to be sufficient to cause cognitive decline in the absence of other features that distinguish older rats with impairment from those with preserved cognitive ability. Further, those data might argue against a simple rate of aging account, because aged rats with preserved cognitive function clearly differ from young rats with respect to neurogenesis. At a minimum, it appears that there is substantial heterogeneity in the effects of aging on different components within the MTL.
As distinct from the notion that good outcomes are due to less aging per se, other recent evidence on the basis for successful aging in humans has supported the notion that adaptive/compensatory adjustments may account, at least in part, for sustained function in high-performing individuals. Notably, older individuals with preserved cognitive function show activations in functional neuroimaging that differ from those of young adults, suggesting that preserved function at older ages is not simply a relative absence of aging (due to altered rate or selective impact), but that adaptive/compensatory adjustments contribute to maintenance of cognitive ability (Cabeza et al. 2002; Rosen et al. 2002). In the current context, the notion of compensatory/adaptive aging in the MTL system is potentially compatible with findings in older rats that perform on a par with young adults. Notably, certain neurobiological markers in the hippocampal system, as further described below, distinguish unimpaired aged rats from both younger adults and their impaired aged cohorts. Perhaps then, aged rats with preserved cognitive function do not simply age less but, rather, exhibit an adaptive form of aging within the MTL system. While this is highly speculative at this time, perhaps those invariant effects of aging, such as reduced neurogenesis, which occur relatively early in the aging process within this system, may even provide a background that drives such adaptive adjustments in the aged brain.
Evidence for distinctive features of older rats with preserved cognitive function appeared early in our research program. For example, an examination of glutamate receptor subtypes using autoradiography showed a decrease in kainate binding in the mossy fiber projection from the dentate granule cells to the CA3 region, which was entirely attributable to reduced kainate binding among the aged rats with preserved cognitive function (Gallagher and Nicolle 1993, see Figure 3 in that report). Neurobiological alterations that are distinctive features of unimpaired aged rats are also evident across hippocampal subregions, including an apparent switch in plasticity mechanisms at CA1 synapses (Lee et al. 2005). In those studies of long-term depression (LTD) in behaviorally characterized aged rats, N-methyl-D-aspartate receptor (NMDAR)-mediated LTD was unrelated to individual differences in cognitive impairment, but NMDAR-independent LTD was selectively increased in magnitude in unimpaired aged rats relative to both young and impaired aged cohorts.
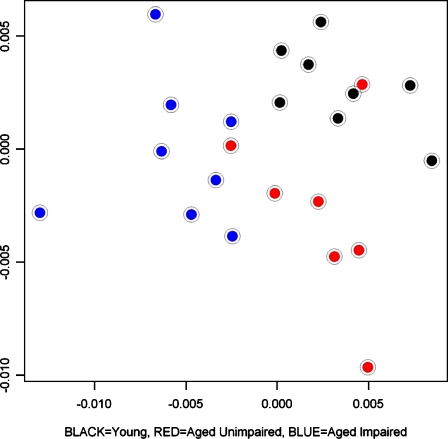
Further data suggestive of an adaptive aging hypothesis comes from broad molecular screening (Figure 5; unpublished data). Here, we illustrate findings from microarray profiling in behaviorally characterized young and aged rats in the CA3 microdissected subregion of the hippocampal formation. The plot in that figure shows each rat’s gene expression profile in multidimensional scaling, where proximity–distance in the two-dimensional plot represents similarity–difference in the multidimensional profile encompassing the full gene array. The profiles of the aged rats with cognitive impairment (blue dots) are entirely segregated from those of young rats (black dots). The profiles of the aged rats that perform on a par with young rats (red dots represent aged unimpaired rats) are largely segregated from those of each of the other groups (young and aged impaired). Such findings could be consistent with a profile that distinguishes adaptive aging. If that is the case, aged unimpaired rats in this study population would provide a useful tool for studies of biological factors affecting better outcomes at older ages.
Figure 5.
Multidimensional scaling plot of gene profiles in the CA3 region for young rats (black dots, N=7), aged unimpaired rats (red dots, N=7) and aged impaired rats (blue dots, N=8). In order to assess global similarity between CA3 RNA expression profiles, we calculated a Pearson’s correlation coefficient (r) for all pair-wise sample comparisons. This was done using gene expression metrics derived from G+C content-robust microchip average (GC-RMA) analysis (Qin et al. 2006) of Affymetrix microarrays (230-2.0 chips). A multidimensional scaling algorithm (MDS, using a distance metric of 1-r for each pair-wise comparison) was used to represent each CA3 RNA sample in two-dimentional space. Hence, each point in this plot represents the Affymetrix microarray data from a single CA3 RNA sample corresponding to an individual rat, and proximity reflects global similarity of gene expression profiles across samples/rats. The plot reveals a fairly clear separation of the three groups of animals studied, indicative of greater similarity in expression profiles within each group, and dissimilarity across the groups. The gene profiles of the aged impaired rats (blue dots) are entirely segregated from those of the young rats (black dots). Although the aged unimpaired rats performed on a par with young rats, there is little overlap between their gene expression profiles (red dots) and those of either young rats or aged cohorts with impaired cognition
Conclusion
Individual differences in healthy aged rodents are clearly tied to the condition of relevant circuitry in the brain, providing evidence on the basis for neurocognitive aging. Because the MTL system in humans is also essential for normal memory of the facts and events of daily life, findings in the animal model may be informative about the basis of neurocognitive aging in elderly individuals. This review further suggests that an integration of findings from research on neurocognitive aging in laboratory animals could have applications in the preclinical search for effective interventions and therapies to treat cognitive impairment late in life. Assessments of cognitive function in animal models are typically used as key indicators of therapeutic potential. Behavioral changes under drug treatment, however, can occur for many reasons beyond the desired effect on specific cognitive function. Models that integrate findings across multiple levels of analysis in a neurocognitive system, such as information encoding monitored by recording single neuron activity during behavioral performance, or broad molecular screening of cellular components within the system, not only will aid in the understanding of the contribution of component changes in the brain to overall performance but also can offer multi-level readouts of a targeted intervention. Such data in an animal model, in conjunction with the results of behavioral cognitive assessments, could increase confidence that candidate therapies will be relevant to conditions affecting hippocampal function in clinical settings. A similar approach would be informative in assessing the effects of other interventions, such as dietary restriction or exposure to environmental enrichment, on the trajectory of and outcome in aging. Such efforts are needed to ensure that mind span, referring to the protection and preservation of optimal cognitive function, will match the expected longevity of added decades of life that has become commonplace.
Acknowledgments
Supported by the National Institute on Aging. All co-authors, listed alphabetically, made substantial contributions to this article and the work upon which it is based.
Contributor Information
Michela Gallagher, Email: michela@jhu.edu.
Carlo Colantuoni, Email: ccolantu@jhsph.edu.
Howard Eichenbaum, Email: hbe@bu.edu.
Rebecca P. Haberman, Email: rahabs@jhu.edu
Peter R. Rapp, Email: peter.rapp@mssm.edu
Heikki Tanila, Email: Heikki.Tanila@uku.fi.
Iain A. Wilson, Email: Iain.Wilson@ed.ac.uk
References
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Intact spatial learning in both young and aged rats following selective removal of hippocampal cholinergic input. Behav Neurosci. 1996;3:460–467. doi: 10.1037/0735-7044.110.3.460. [DOI] [PubMed] [Google Scholar]
- Baxter M, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells; effects on learning and memory in rats. Behav Neurosci. 1995;109:714–722. doi: 10.1037/0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Seeman TE, Albert M, et al. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on successful aging. J Clin Epidemiol. 1993;46:1129–1140. doi: 10.1016/0895-4356(93)90112-E. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity and the aging brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Gallagher M. A longitudinal study of reaction time performance in Long-Evans rats. Neurobiol Aging. 1993;14:57–64. doi: 10.1016/0197-4580(93)90023-5. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Whealin J, Gallagher M. Effects of aging on the diurnal pattern of water intake in rats. Behav Neural Biol. 1992;58:196–203. doi: 10.1016/0163-1047(92)90468-J. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, et al. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cadiacio CL, Milner TA, Gallagher M, et al. Hilar neuropeptide Y interneuron loss in the aged rat hippocampal formation. Exp Neurol. 2003;183:147–158. doi: 10.1016/S0014-4886(03)00126-2. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Yi S, Gallagher M, et al (2002) No reduction in the number of spinophilin-positive dendritic spines in cognitively-impaired aged rats. Poster presented at the 32nd annual meeting of the Society for Neuroscience, 2–7 November 2002. No 889.19
- Chappell J, McMahan R, Chiba A, Gallagher M. A re-examination of the role of basal forebrain cholinergic neurons in spatial working memory. Neuropharmacology. 1998;37:481–488. doi: 10.1016/S0028-3908(98)00032-X. [DOI] [PubMed] [Google Scholar]
- Chouinard ML, Gallagher M, Yasuda RP, et al. Hippocampal muscarinic receptor function in spatial learning-impaired aged rats. Neurobiol Aging. 1995;16:955–963. doi: 10.1016/0197-4580(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Wetsel WC, Gallagher M. Relationships between spatial memory and hippocampal subcellular concentrations of protein kinase C gamma in young and aged rats. Proc Natl Acad Sci U S A. 1997;94:14195–14199. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Kurian P, Freund G. Cholinergic and serotonergic stimulation of phosphoinositide hydrolysis is decreased in Alzheimer’s disease. Life Sci. 1994;55:1993–2002. doi: 10.1016/0024-3205(94)00379-3. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, et al. Spatial performances of aged rats in the water maze predicts levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Rev. 1999;30:236–249. doi: 10.1016/S0165-0173(99)00017-X. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol Aging. 1989;10:347–352. doi: 10.1016/0197-4580(89)90047-X. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell RD. Relationship of age-related decline across several behavioral domains. Neurobiol Aging. 1989;10:691–708. doi: 10.1016/0197-4580(89)90006-7. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037/0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Toledo-Morrell L, Morrell F, et al. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Ganeshina O, Yoshida R, et al. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Glisky E, Polster M, Routhieaux B. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. doi: 10.1037/0894-4105.9.2.229. [DOI] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Curr Opin Neurobiol. 2000;10:224–231. doi: 10.1016/S0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, et al. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Healthy and pathological processes in adult development: new evidence from neuroimaging of the aging brain. Curr Opin Neurol. 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48. [DOI] [PubMed] [Google Scholar]
- Howell OW, Scharfman HE, Herzog H, et al. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86:646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus. 2002;12:386–397. doi: 10.1002/hipo.1109. [DOI] [PubMed] [Google Scholar]
- Jope RS, Song L, Powers RE. Cholinergic activation of phosphoinositide signaling is impaired in Alzheimer’s disease brain. Neurobiol Aging. 1997;18:111–120. doi: 10.1016/S0197-4580(96)00205-9. [DOI] [PubMed] [Google Scholar]
- Kalus P, Slotboom J, Gallinat J, et al. Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. Neuroimage. 2005;30:713–720. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. doi: 10.1002/1531-8249(20010201)49:2<202::AID-ANA40>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, et al. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, et al. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Barnes CA, McNaughton BL. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc Natl Acad Sci U S A. 1997;94:8918–8921. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, et al. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Kalyani A. Age and experience-dependent representational reorganization during spatial learning. Neurobiol Aging. 1997;18:651–659. doi: 10.1016/S0197-4580(97)00150-4. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, et al. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24:273–284. doi: 10.1016/S0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, et al. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle MM, Colombo PJ, Gallagher M, et al. Metabotropic glutamate receptor-mediated hippocampal phosphoinositide turnover is blunted in spatial learning-impaired aged rats. J Neurosci. 1999;19:9604–9610. doi: 10.1523/JNEUROSCI.19-21-09604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin LX, Beyer RP, Hudson FN, Linford NJ, Morris DE, Kerr KF. Evaluation of methods for oligonucleotide array data via quantitative real-time PCR. BMC Bioinformatics. 2006;7:23. doi: 10.1186/1471-2105-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, et al. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Deroche PS, Mao Y, et al. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb Cortex. 2002;12:1171–1179. doi: 10.1093/cercor/12.11.1171. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O’Hara R, et al. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Redish AD, McNaughton BL, et al. Hippocampal map realignment and spatial learning. Nat Neurosci. 2003;6:609–615. doi: 10.1038/nn1053. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Mash DC, Toledo-Morrell L. Spatial memory in aged rats is related to PKC gamma-dependent G-protein coupling of the M1 receptor. Neurobiol Aging. 2005;26:53–68. doi: 10.1016/j.neurobiolaging.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mental exercise and mental aging. Perspectives on Psychol Sci. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, et al. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, et al. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiol Aging. 2002;23:555–564. doi: 10.1016/S0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA, McNaughton BL, et al. The effect of aging on experience-dependent plasticity of hippocampal place cells. J Neurosci. 1997;17:6769–6782. doi: 10.1523/JNEUROSCI.17-17-06769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, et al. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmelin J, Lazarus C, Cassel S, Kelche C, Cassel JC. Immunohistochemical and neurochemical correlates of learning deficits in aged rats. Neurosci. 2000;96:275–289. doi: 10.1016/S0306-4522(99)00561-8. [DOI] [PubMed] [Google Scholar]
- Tanila H, Shapiro M, Gallagher M, et al. Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci. 1997;17:5155–5166. doi: 10.1523/JNEUROSCI.17-13-05155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H, Sipila P, Shapiro M, et al. Brain aging: impaired coding of novel environmental cues. J Neurosci. 1997;17:5167–5174. doi: 10.1523/JNEUROSCI.17-13-05167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Chow AR, et al. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci. 2002;22:9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher rats. J Neurosci. 2005;25:2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttl B, Graf P. Episodic spatial memory in adulthood. Psychol Aging. 1993;8:257–273. doi: 10.1037/0882-7974.8.2.257. [DOI] [PubMed] [Google Scholar]
- Wilkniss SM, Jones MG, Korol DL, et al. Age-related differences in an ecologically based study of route learning. Psychol Aging. 1997;12:372–375. doi: 10.1037/0882-7974.12.2.372. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, et al. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging. 2003;24:297–305. doi: 10.1016/S0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gureviciene I, et al. Cognitive aging and the hippocampus: how old rats represent new environments. J Neurosci. 2004;24:3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, et al. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, et al (2006) Old memories persist in the aged hippocampus. Trends Neurosci (in press) doi:10.1016/j.tins.2006.10.002
- Zhang, HY, Watson ML, Gallagher M, et al (2006) Muscarinic receptor-mediated GTP-Eu binding in the hippocampus and prefrontal cortex is correlated with spatial memory impairment in aged rats. Neurobiol Aging (in press) doi:10.1016/j.neurobiolaging.2006.02.016 [DOI] [PubMed]
- Zyzak DR, Otto T, Eichenbaum H, et al. Cognitive decline associated with normal aging in rats: a neuropsychological approach. Learn Mem. 1995;2:1–16. doi: 10.1101/lm.2.1.1. [DOI] [PubMed] [Google Scholar]