Abstract
Glycolate and 2-phosphoglycolate (PG) are 2-carbon monocarboxylic acids with ill-defined metabolic roles. Their concentrati ons have not yet been described in tissues apart from body fluids and erythrocytes. We describe the use of ion chromatography coupled with mass spectrometry (IC-MS) to quantify levels of glycolate and PG in tissue. Sample preparation and analysis can be performed within an hour. Low concentrations of glycolate (12 – 48 nmoles/g) and PG (4 – 17 nmoles/g) were detected in all tissues. The availability of this IC-MS assay will facilitate investigations of the origin, function, and metabolism of glycolate and PG in tissues.
Keywords: glycolate, phosphoglycolate, mass spectrometry, ion chromatography
An understanding of the origin, metabolism and biological function of glycolate and PG is lacking as suitable methods for determining their concentrations are not available. All cells most likely contain glycolate as a result of the metabolism of glyoxal by the glyoxalase system and the reduction of glyoxylate by glyoxylate reductase as these activities are present in all cells. Glyoxal arises from glucose autooxidation, the breakdown of glycation products and lipid peroxidation [1; 2; 3]. Liver and kidney hydroxyproline metabolism is likely to be the major source of glyoxylate synthesis [4].
The origin of 2-phosphoglycolate (PG) in mammalian cells is not known with certainty. In plants and some bacteria, it is synthesized during photorespiration following the oxygenation of ribulose-1,5,-bisphosphate [5; 6; 7]. In all cells PG is generated as a result of DNA repair of 3'-PG ends that are generated following strand breaks [8; 9]. The amount of PG formed during these processes has yet to be determined. Erythrocytes have been reported to contain a low concentration (2 – 5 μM) of PG [10], although earlier research was unable to identify the presence of PG in these cells [11; 12]. The method used to measure PG was not direct and relied on its ability to activate the 2,3-diphosphoglycerate (DPG) phosphatase activity of bisphosphoglycerate mutase (BPGM). The reliability of this procedure at such low concentrations of 2-PG has been questioned [13]. The biological role of PG, if any, is not known. It has attracted attention because low levels of PG stimulate the activity of 2,3-diphosphoglycerate (DPG) phosphatase and it is the most potent modifier yet identified [14]. This activity is potentially important as the concentration of DPG influences the binding of oxygen to hemoglobin. PG is dephosphorylated by phosphoglycolate phosphatase, an apparent ubiquitous enzyme in cells of eukaryotes and prokaryotes, and in plants this activity is required to maintain a functional Calvin cycle [8; 12; 15; 16].
In this report, we show that IC-MS reliably measures the concentration of both PG and glycolate and have used this method to measure their concentrations in a variety of tissues. This method does not require extensive sample preparation, and total assay time, including tissue preparation, can be completed in an hour.
Materials and Methods
Materials
Reagent grade chemicals were obtained from either Sigma-Aldrich Chemicals (St Louis, MO) or Fisher Scientific (Pittsburgh, PA). Sodium glycolate, 97%, and 2-phosphoglycolic acid as a tri-(monocyclohexylammonium) salt, 98%, were purchased from Sigma-Aldrich.
Tissue Preparation
Freeze clamped tissue was extracted with 10% ice cold trichloroacetic acid (TCA). This preparation was vortexed continuously for 1 minute at 4°C, frozen at -80°C for 10 minutes to promote protein precipitation, thawed and vortexed again continuously for 1 minute at 4°C. Precipitate was removed by centrifugation and TCA in the supernatant removed by vigorously vortexing with 3 volumes of 1,1,2-trichlorotrifluoroethane (FREON)-trioctylamine (3:1, vol/vol; Aldrich, Milwaukee, WI), centrifuging at 4°C to promote phase separation, and collecting the upper aqueous layer for analysis. Samples were typically further diluted two to ten fold in water prior to IC-MS analysis.
Ion chromatography / mass spectrometry
Glycolate and PG were quantified in tissue by ion chromatography coupled with negative electrospray mass spectrometry (IC-MS) (Dionex Corporation, Sunnyvale, CA). The IC portion of the IC-MS consisted of an ED50 conductivity detector, a GS50 gradient pump, an AS50 refrigerated autosampler, an EG50 potassium hydroxide gradient generator, a continuously regenerated anion trap column, an AS50 thermal compartment containing the columns controlled at a temperature of 30°C, and an 2mm ASRS 300 suppressor operating with external water at 0.5ml/min to provide superior suppressed conductivity detection. The columns used for the analysis of glycolate and PG are described in Table 1. The MS is a Thermo-Finnigan MSQ ELMO single quadrupole mass spectrometer that is specifically designed for the analysis of low molecular weight ions (less than Mw 1000). Acetonitrile (50%), delivered by an auxillary pump at 0.3 ml/min, was mixed with column eluent via a zero dead volume mixing Tee (Upchurch Scientific, Oak Harbor, WA) to improve electrospray ionization. A nitrogen generator (18LA, Peak Scientific) was used to deliver nitrogen nebulizer gas at 80psi. Glycolate and PG were determined by selected-ion monitoring (SIM) at the following mass/charge ratios, glycolate (SIM75) and PG (SIM155). Detailed running conditions for glycolate and PG analysis are provided in Table 1.
Table 1.
IC-MS conditions used to measure glycolate and phosphoglycolate in tissue.
| Glycolate | 2-Phosphoglycolate | |
|---|---|---|
| Column | AS15, 2 × 250 mm, with AG15 guard | AS11-HC, 2 × 250 mm with AG11 guard |
| Eluent | 3 mM KOH | 32 mM KOH |
| IC Flow rate (ml/min) | 0.3 | 0.3 |
| Injection Volume (μL) | 10 | 10 |
| Cone Voltage (V) | 30 | 40 |
| Needle Voltage (kV) | 1.5 | 3.0 |
| Probe Temperature (°C) | 450 | 450 |
Results and Discussion
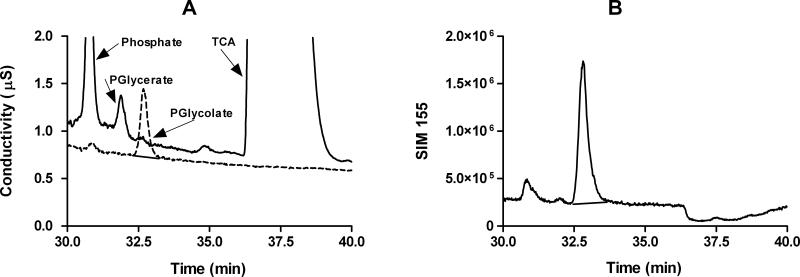
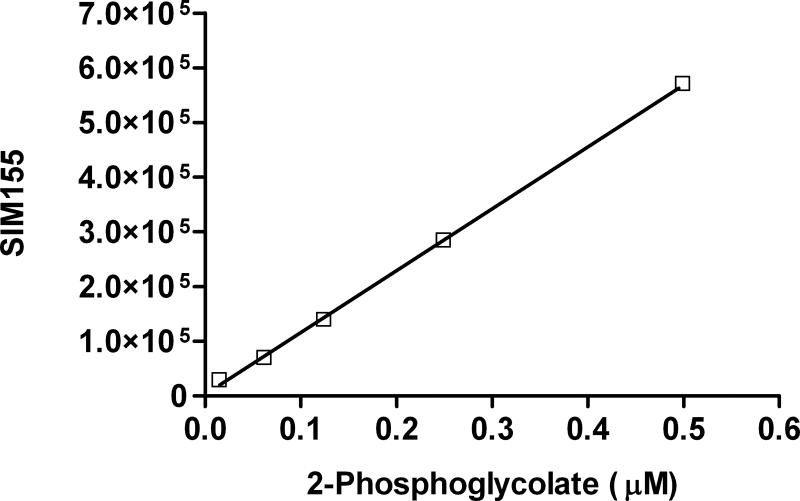
On an AS11-HC column, PG does not co-elute with other anions in tissue extracts (Fig. 1). SIM responses at various PG concentrations are shown in Fig.2. Limit of detection (LOD), defined as mean blank signal plus 3XSD blank signal, and limit of quantification (LOQ), defined as mean blank signal plus 10XSD blank signal, for PG are 6 nM and 25 nM, respectively. Recovery of PG in the tissues shown in Table 2 spiked with PG at a concentration equivalent to LOQ ranged from 97 – 102 %. The determination of the phosphoglycolate concentration in tissues by an independent method would help substantiate the validity of this method, but none are currently available. The incubation of PG in 32mM potassium hydroxide for 1 hour prior to IC showed no change in PG signal, or presence of glycolate or phosphate, compared to a non-incubated preparation, indicating PG is not degraded in the presence of the potassium hydroxide eluent.
Figure 1.
Phosphoglycolate conductivity (A) and mass spectrometry single ion monitoring (B) chromatograms of red blood cells extracted with 10% TCA. Panel A shows conductivity chromatogram overlays of a 10 μM phosphoglycolate standard (dotted line), and TCA extracted red blood cells (solid line) diluted 30 fold prior to analysis. Separation of anions was done using an AS11-HC column. Panel B shows the SIM 155 response of phosphoglycolate in the TCA extracted red blood cells. The majority of TCA was removed prior to IC-MS using TOA/FREON.
Figure 2.
Phosphoglycolate SIM155 calibration curve showing a linear SIM response between 0.016 and 5 μM. Tissue levels of phosphoglycolate after TCA extraction and preparation fall within this concentration range. Each data point represents mean ± SD, n=4.
Table 2.
Glycolate and phosphoglycolate content of mouse tissues.
| Tissue | Glycolate (nmol/g wet wt) | Phosphoglycolate (nmol/g wet wt) |
|---|---|---|
| Liver | 48.3 ± 4.5 | 12.6 ± 3.5 |
| Kidney | 48.1 ± 5.6 | 4.3 ± 0.5 |
| Heart | 23.8 ± 1.9 | 16.9 ± 3.9 |
| Brain | 25.2 ± 2.5 | 5.4 ± 0.6 |
| Muscle | 12.3 ± 1.1 | 5.9 ± 0.5 |
| Erythrocytes* | 12.3 ± 2.2 | 10.0 ± 0.2 |
| Plasma* | 12.5 ± 2.9 | 0.17 ± 0.11 |
The plasma levels above are presented as nmol/ml. Erythrocyte concentration is presented as nmol/ml packed red cell lysate.
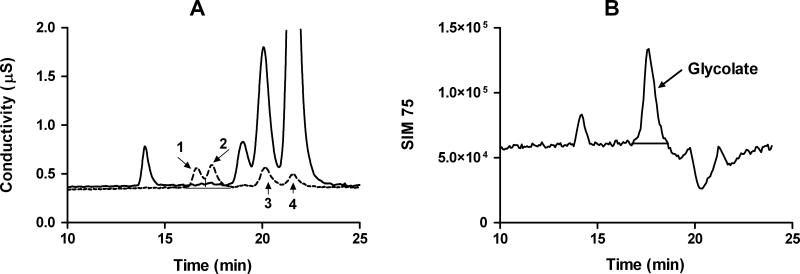
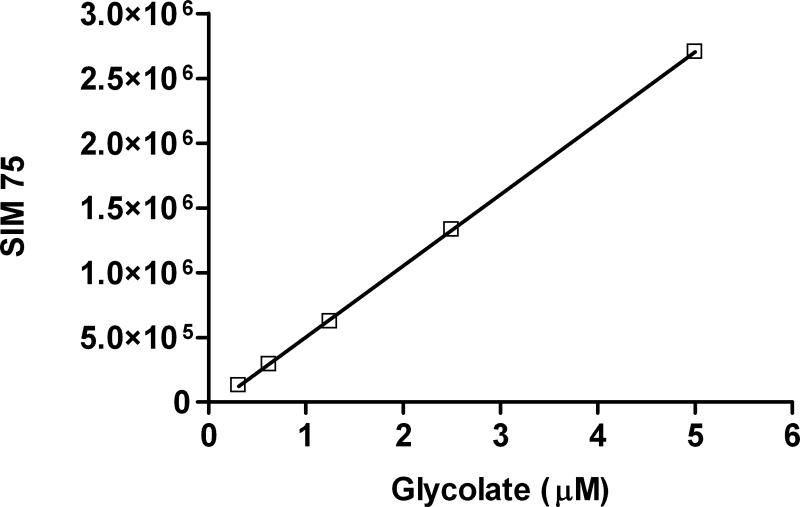
In many biological matrices, including plasma and tissue, the elution of lactate on an AS11HC column suppresses the glycolate peak which elutes just after it. On an AS15 column, glycolate elutes before lactate freeing it from lactate suppression (Fig.3). SIM responses at various glycolate concentrations are shown in Fig.4. LOD and LOQ for glycolate are 0.25 μM and 0.48 μM, respectively. Recovery of glycolate in the tissues shown in Table 2 spiked with glycolate at a concentration equivalent to LOQ ranged from 92 – 98%.
Figure 3.
Separation of glycolate (2) from other major anions, including, glycerate (1), lactate (3), and formate (4) in red blood cells extracted with 10% TCA using an AS15 column (Panel A), and quantification of glycolate by single ion monitoring in the TCA extracted red blood cells (Panel B). Panel A shows conductivity chromatogram overlays of a 10 μM standard (dotted line), and TCA extracted red blood cells (solid line). Formate seen in Panel A is a product of the TCA extraction process.
Figure 4.
Glycolate SIM75 calibration curve showing a linear SIM response between 0.31 and 5 μM. Tissue levels of glycolate after TCA extraction and preparation fall within this concentration range. Each data point represents mean ± SD, n=4.
The PG and glycolate levels in several mouse tissues are shown in Table 2. PG was found in all tissues examined with mean concentrations ranging from 4.3 – 16.9 nmoles/g wet wt. The PG levels in human red blood cells was also measured and found to be 10.0 ± 0.2 nmoles/ml, similar to levels measured in mouse red blood cells. PG was measurable in mouse and human plasma, but levels were < 0.25 nmoles/ml. Glycolate was detected in all tissues at mean concentrations ranging from 12.3 – 48.3 nmoles/g wet wt. Mouse plasma glycolate levels are similar to that measured in human plasma [17]. The results obtained assaying urinary glycolate by this method agree well with its determination by the more tedious procedure of converting it to glyoxylate and quantifying the glyoxylate by HPLC after derivatization with phenylhydrazine [18].
This IC-MS assay should be useful in identifying the origin of PG in cells and tissues and factors that influence its concentration. Direct phosphorylation of glycolate is one possible origin of PG, but high, non physiological concentrations of glycolate are required in vitro and whether such a reaction occurs in vivo has been questioned [13; 19; 20]. It is possible that it is derived in animal cells from the repair of DNA lesions, as Lindahl has estimated that up to 10,000 purine bases are lost from DNA in every human cell daily and subsequently repaired [21]. PG is often derived from 3'-termini created with single strand DNA breaks [22; 23]. Thus, the measurement of PG may prove to be an indicator of increased damage to DNA and its associated repair. It is possible that the concentration of intracellular PG is maintained at low levels by PG phosphatase to ensure that the activities of enzymes modified by PG, including BPGM, triose phosphate isomerase, pyruvate kinase, phosphoenolpyruvate carboxykinase, and enolase are not affected [14; 24; 25]. The phenotype of a KO mouse lacking PG phosphatase activity, perhaps under conditions accelerating DNA damage and repair and phosphoglycolate formation would be of interest. In erythrocytes, PG levels may represent that formed during hematopoiesis and retained in cells following enucleation.
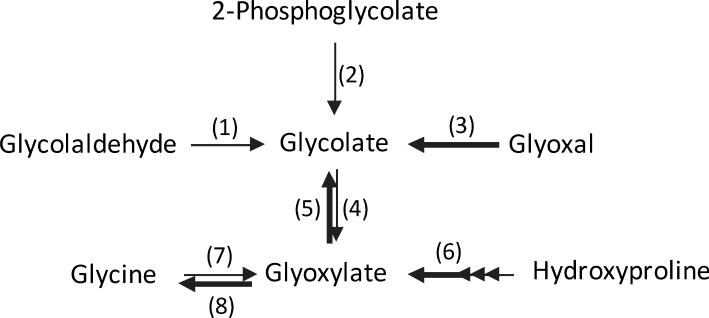
A biological role for glycolate has not yet been identified. Known metabolic reactions involving glycolate are illustrated in Fig. 5. A major source of glycolate in most cells appears to be the detoxification of glyoxal by the glyoxalase system [2]. A small amount of glyoxal is produced in cells from the peroxidation that occurs as a result of normal metabolism [3; 4; 5]. Some glycolate may also be produced by the reduction of glyoxylate by glyoxylate reductase (GRHPR), particularly in tissues that metabolize hydroxyproline such as the liver and kidney [1]. Our results with cultured hepatocytes have shown that there is a metabolic pathway from fructose to glycolate that is believed to arise from glycolaldehyde production, but in vivo studies with human studies indicated that ingesting fructose in amounts up to 21% of the calories did not alter glycolate excretion, suggesting this pathway is minor [26].
Figure 5.
Pathways associated with glycolate production. (1) aldehyde dehydrogenase, (2) phosphoglycolate phosphatase, (3) glyoxalase system, (4) glycolate oxidase, (5) glyoxylate reductase, (6) 4-hydroxy-2-oxoglutarate aldolase, (7) alanine:glyoxylate aminotransferase, (7) D-amino acid oxidase. The thicker arrows denote reactions believed to have the largest fluxes.
The measurement of glycolate has clinical significance as its measurement in a 24 hour urine collection, together with that of oxalate, is a reliable non-invasive method for determining whether patients have the rare monogenetic disease, primary hyperoxaluria type 1 [27]. These patients have a deficiency in peroxisomal alanine:glyoxylate transferase activity in the liver and cannot convert glyoxylate to glycine. As a result, glyoxylate is converted to excessive amounts of glycolate and oxalate [1].
Acknowledgements
This work was supported by NIH grant RO1 DK54468.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dudda A, Spiteller G, Kobelt F. Lipid oxidation products in ischemic porcine heart tissue. Chem Phys Lipids. 1996;82:39–51. doi: 10.1016/0009-3084(96)02557-1. [DOI] [PubMed] [Google Scholar]
- 2.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344(Pt 1):109–16. [PMC free article] [PubMed] [Google Scholar]
- 3.Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–9. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- 4.Phang JM, Hu CA, Valle D. Disorders of proline and hydroxyproline metabolism. In: Scriver CR, Beaudet AL, Sly WS, Vallee D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 1821–1838. [Google Scholar]
- 5.Andrews TJ, Lorimer GH, Tolbert NE. Ribulose diphosphate oxygenase. I. Synthesis of phosphoglycolate by fraction-1 protein of leaves. Biochemistry. 1973;12:11–8. doi: 10.1021/bi00725a003. [DOI] [PubMed] [Google Scholar]
- 6.Bowes G, Ogren WL, Hageman RH. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971;45:716–22. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- 7.Lorimer GH, Andrews TJ, Tolbert NE. Ribulose diphosphate oxygenase. II. Further proof of reaction products and mechanism of action. Biochemistry. 1973;12:18–23. doi: 10.1021/bi00725a004. [DOI] [PubMed] [Google Scholar]
- 8.Pellicer MT, Nunez MF, Aguilar J, Badia J, Baldoma L. Role of 2-phosphoglycolate phosphatase of Escherichia coli in metabolism of the 2-phosphoglycolate formed in DNA repair. J Bacteriol. 2003;185:5815–21. doi: 10.1128/JB.185.19.5815-5821.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winters TA, Henner WD, Russell PS, McCullough A, Jorgensen TJ. Removal of 3'-phosphoglycolate from DNA strand-break damage in an oligonucleotide substrate by recombinant human apurinic/apyrimidinic endonuclease 1. Nucleic Acids Res. 1994;22:1866–73. doi: 10.1093/nar/22.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose ZB, Salon J. The identification of glycolate-2-P as a constituent of normal red blood cells. Biochem Biophys Res Commun. 1979;87:869–875. doi: 10.1016/0006-291x(79)92038-2. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett GR. Human red cell glycolytic intermediates. J Biol Chem. 1959;234:449–58. [PubMed] [Google Scholar]
- 12.Richardson KE, Tolbert NE. Phosphoglycolic acid phosphatase. J Biol Chem. 1961;236:1285–90. [PubMed] [Google Scholar]
- 13.Fujii S, Beutler E. Where does phosphoglycolate come from in red cells? Acta Haemat. 1985;73:26–30. doi: 10.1159/000206268. [DOI] [PubMed] [Google Scholar]
- 14.Rose ZB, Liebowitz J. 2,3-diphosphoglycerate phosphatase from human erythrocytes. General properties and activation by anions. J Biol Chem. 1970;245:3232–41. [PubMed] [Google Scholar]
- 15.Badwey JA. Phosphoglycolate phosphatase in human erythrocytes. J Biol Chem. 1977;252:2441–3. [PubMed] [Google Scholar]
- 16.Schwarte S, Bauwe H. Identification of the photorespiratory 2-phosphoglycolate phosphatase, PGLP1, in Arabidopsis. Plant Physiol. 2007;144:1580–6. doi: 10.1104/pp.107.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight J, Jiang J, Assimos DG, Holmes RP. Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int. 2006;70:1929–34. doi: 10.1038/sj.ki.5001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrarulo M, Pellegrino S, Bianco O, Marangella M, Linari F, Mentasti E. Derivatization and high-performance liquid chromatographic determination of urinary glycolic acid. J Chromatogr. 1989;465:87–93. doi: 10.1016/s0021-9673(01)83575-5. [DOI] [PubMed] [Google Scholar]
- 19.Fujii S. Glycolate kinase activity in human cells. Blood. 1985;65:480–483. [PubMed] [Google Scholar]
- 20.Sasaki H, Fujii S, Yoshizaki Y, Nakashima K, Kaneko T. Phosphoglycolate synthesis by human erythrocyte pyruvate kinase. Acta Haematol. 1987;77:83–6. doi: 10.1159/000205959. [DOI] [PubMed] [Google Scholar]
- 21.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–15. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 22.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–31. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 23.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–85. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 24.Nowak T, Mildvan AS. Stereoselective interactions of phosphoenolpyruvate analogues with phosphoenolpyruvate-utilizing enzymes. J Biol Chem. 1970;245:6057–64. [PubMed] [Google Scholar]
- 25.Wolfenden R. Transition state analogues for enzyme catalysis. Nature. 1969;223:704–5. doi: 10.1038/223704a0. [DOI] [PubMed] [Google Scholar]
- 26.Knight J, Assimos DG, Easter L, Holmes RP. Metabolism of fructose to oxalate and glycolate. Horm Metab Res. 2010;42:868–73. doi: 10.1055/s-0030-1265145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int. 2009;75:1264–71. doi: 10.1038/ki.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]