Abstract
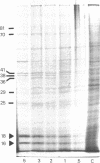
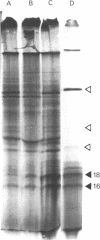
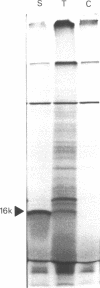
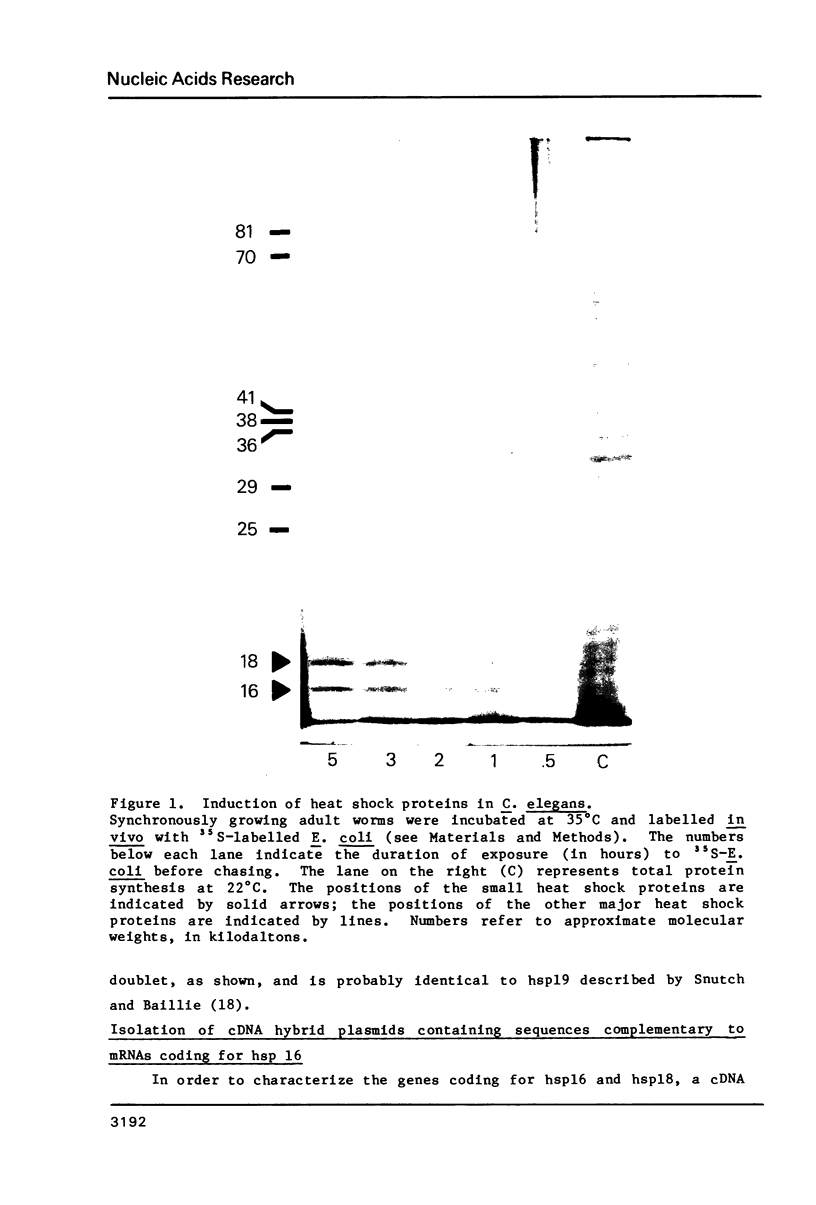
The nucleotide sequences of two different cDNAs, CEHS48 and CEHS41, coding for the 16,000 dalton heat shock proteins (hsps) of Caenorhabditis elegans have been determined. CEHS48 codes for a polypeptide of 135 amino acids, approximately 15 fewer than the complete protein while CEHS41 is missing approximately 46 amino acids. From nucleotide 113 to the TAA termination signal the extent of homology between the sequences is 91%. Toward the 5' ends, the homology drops to 20% and results in completely divergent amino acid sequences. The 3' noncoding regions are only 30% homologous. Only CEHS48 contains a poly(A) signal and a poly(A) tail, suggesting that CEHS41 has an incomplete 3' end. The region from amino acid 43 to amino acid 115 shows extensive homology with corresponding regions in the four small hsps of Drosophila melanogaster and in mammalian alpha-crystallin. Two-dimensional gel analysis of in vitro synthesized hsp16 reveals the existence of five distinct components of identical molecular weights, but with different isoelectric points.
Full text
PDF




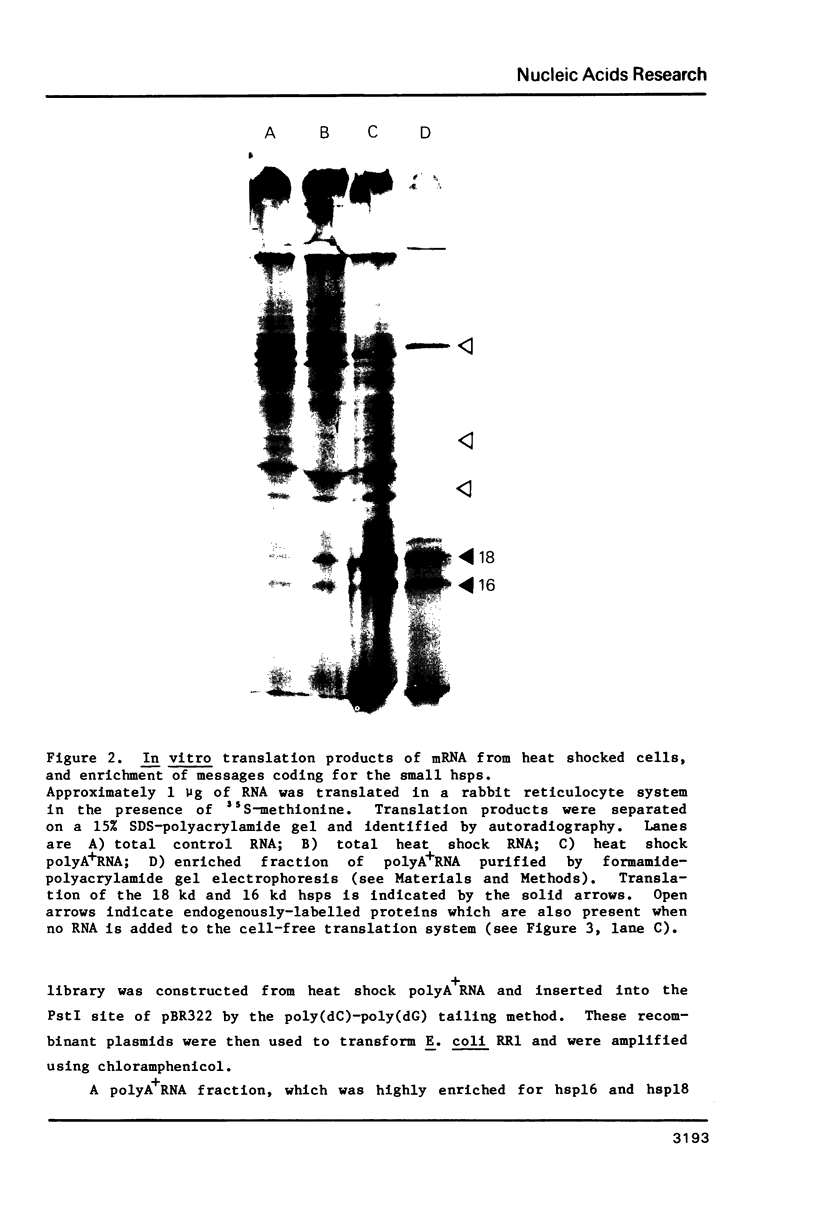



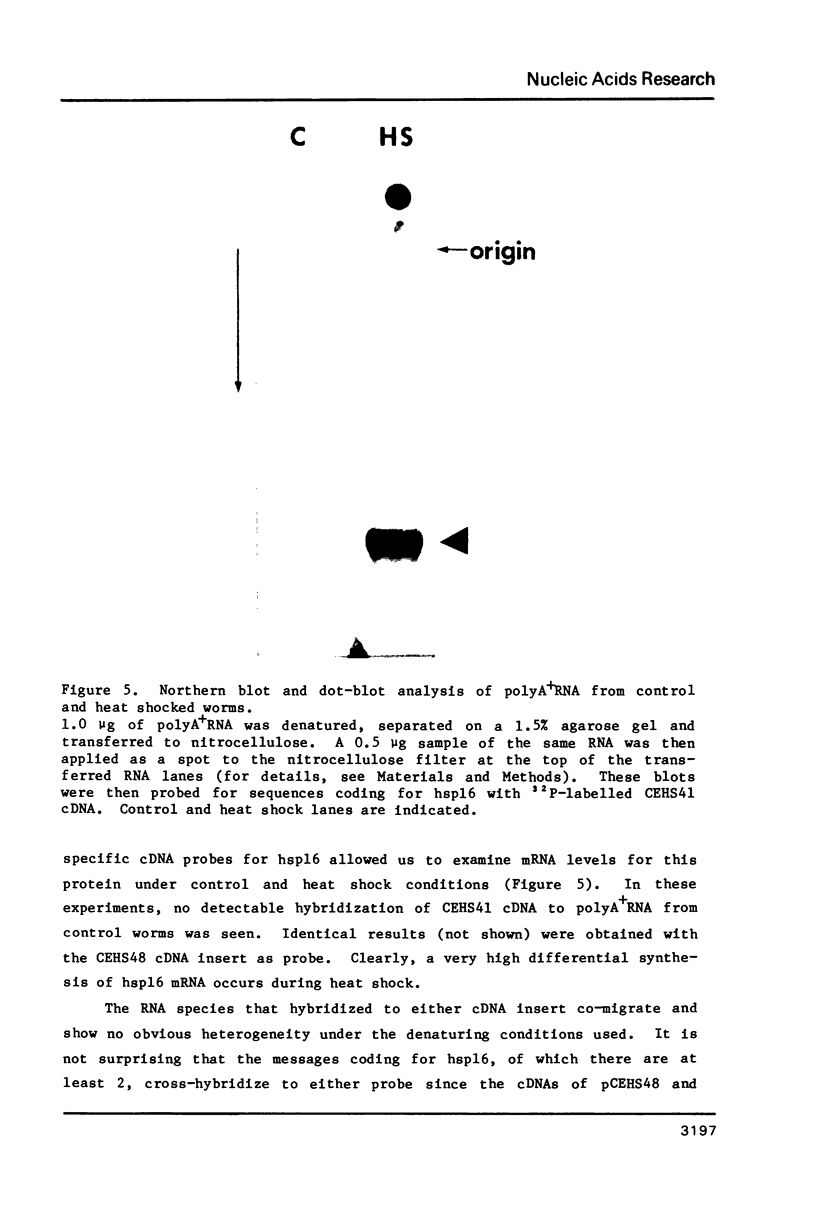








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Ahmad-Zadeh C. Immunofluorescence localization of a small heat shock protein (hsp 23) in salivary gland cells of Drosophila melanogaster. Mol Gen Genet. 1981;184(1):73–79. doi: 10.1007/BF00271198. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Atkinson B. G., Pollock M. Effect of heat shock on gene expression in human epidermoid carcinoma cells (strain KB) and in primary cultures of mammalian and avian cells. Can J Biochem. 1982 Mar;60(3):316–327. doi: 10.1139/o82-038. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H., Berns T., Zweers A., Hoenders H., Benedetti E. L. The state of aggregation of -crystallin detected after large-scale preparation by zonal centrifugation. Eur J Biochem. 1972 Jan 21;24(3):401–406. doi: 10.1111/j.1432-1033.1972.tb19699.x. [DOI] [PubMed] [Google Scholar]
- Bouche G., Amalric F., Caizergues-Ferrer M., Zalta J. P. Effects of heat shock on gene expression and subcellular protein distribution in Chinese hamster ovary cells. Nucleic Acids Res. 1979 Dec 11;7(7):1739–1747. doi: 10.1093/nar/7.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Smith A. E. Biosynthesis of 35 S-L-methionine of very high specific activity. Anal Biochem. 1972 May;47(1):310–312. doi: 10.1016/0003-2697(72)90308-9. [DOI] [PubMed] [Google Scholar]
- Buzin C. H., Petersen N. S. A comparison of the multiple Drosophila heat shock proteins in cell lines and larval salivary glands by two-dimensional gel electrophoresis. J Mol Biol. 1982 Jun 25;158(2):181–201. doi: 10.1016/0022-2836(82)90428-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Corces V., Holmgren R., Freund R., Morimoto R., Meselson M. Four heat shock proteins of Drosophila melanogaster coded within a 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5390–5393. doi: 10.1073/pnas.77.9.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., McCarthy B. J. Four Drosophila heat shock genes at 67B: characterization of recombinant plasmids. Nucleic Acids Res. 1980 Oct 10;8(19):4441–4457. doi: 10.1093/nar/8.19.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Deng G., Wu R. An improved procedure for utilizing terminal transferase to add homopolymers to the 3' termini of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4173–4188. doi: 10.1093/nar/9.16.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Klass M. R., Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D., Lin L. Heat shock response in a cellular slime mold, Polysphondylium pallidum. Dev Biol. 1980 Sep;79(1):238–242. doi: 10.1016/0012-1606(80)90087-1. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Leung D. W., Dull T. J., Gross M., Lawn R. M., McCandliss R., Seeburg P. H., Ullrich A., Yelverton E., Gray P. W. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981 Mar 5;290(5801):20–26. doi: 10.1038/290020a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J. J., Schultz G. A., Iatrou K., Gedamu L. Expression of a set of fish genes following heat or metal ion exposure. J Biol Chem. 1982 Oct 25;257(20):12000–12005. [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch F., Török I., Tissières A. Extensive regions of homology in front of the two hsp70 heat shock variant genes in Drosophila melanogaster. J Mol Biol. 1981 May 25;148(3):219–230. doi: 10.1016/0022-2836(81)90536-2. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel D. A., Maizel J. V., Jr, Leder P. The evolution and sequence comparison of two recently diverged mouse chromosomal beta--globin genes. Cell. 1979 Nov;18(3):865–873. doi: 10.1016/0092-8674(79)90138-7. [DOI] [PubMed] [Google Scholar]
- Kothary R. K., Candido E. P. Induction of a novel set of polypeptides by heat shock or sodium arsenite in cultured cells of rainbow trout, Salmo gairdnerii. Can J Biochem. 1982 Mar;60(3):347–355. doi: 10.1139/o82-041. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roccheri M. C., Di Bernardo M. G., Giudice G. Synthesis of heat-shock proteins in developing sea urchins. Dev Biol. 1981 Apr 15;83(1):173–177. doi: 10.1016/s0012-1606(81)80020-6. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Talaat M., Hamdy H., el-Bagoury I. M., Abdel-Rahman Y. M., Makarem F., el-Shewy T. M. The effect of short- and long-acting barbiturates on the oxygen uptake of the intact lens and its homogenate. Exp Eye Res. 1974 Apr;18(4):381–382. doi: 10.1016/0014-4835(74)90115-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Goldschmidt-Clermont M., Southgate R., Tissières A., Levis R., Gehring W. A DNA segment isolated from chromosomal site 67B in D. melanogaster contains four closely linked heat-shock genes. Cell. 1981 Jan;23(1):261–270. doi: 10.1016/0092-8674(81)90290-7. [DOI] [PubMed] [Google Scholar]
- Wadsworth S. C., Craig E. A., McCarthy B. J. Genes for three Drosophila heat-shock-induced proteins at a single locus. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2134–2137. doi: 10.1073/pnas.77.4.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. Appearance of heat shock proteins during the induction of multiple flagella in Naegleria gruberi. J Biol Chem. 1980 Apr 10;255(7):2629–2632. [PubMed] [Google Scholar]
- Wang C., Gomer R. H., Lazarides E. Heat shock proteins are methylated in avian and mammalian cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3531–3535. doi: 10.1073/pnas.78.6.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. T., Mattson T., Selzer G., Van Houwe G., Bolle A., Epstein R. Bacteriophage T4 gene transcription studied by hybridization to cloned restriction fragments. J Mol Biol. 1980 Apr 15;138(3):423–445. doi: 10.1016/s0022-2836(80)80011-8. [DOI] [PubMed] [Google Scholar]
- van der Ouderaa F. J., de Jong W. W., Bloemendal H. The amino-acid sequence of the alphaA2 chain of bovine alpha-crystallin. Eur J Biochem. 1973 Nov 1;39(1):207–222. doi: 10.1111/j.1432-1033.1973.tb03119.x. [DOI] [PubMed] [Google Scholar]