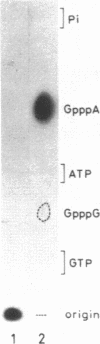
Abstract
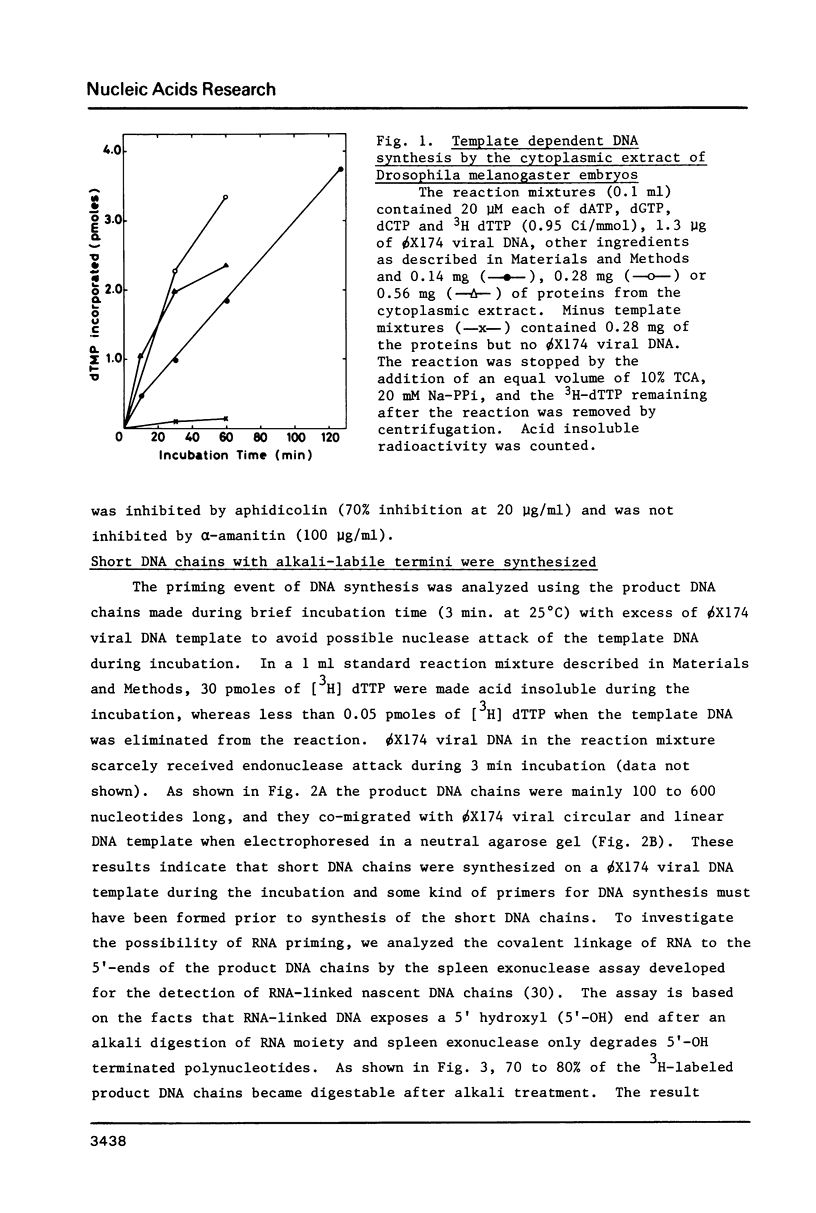
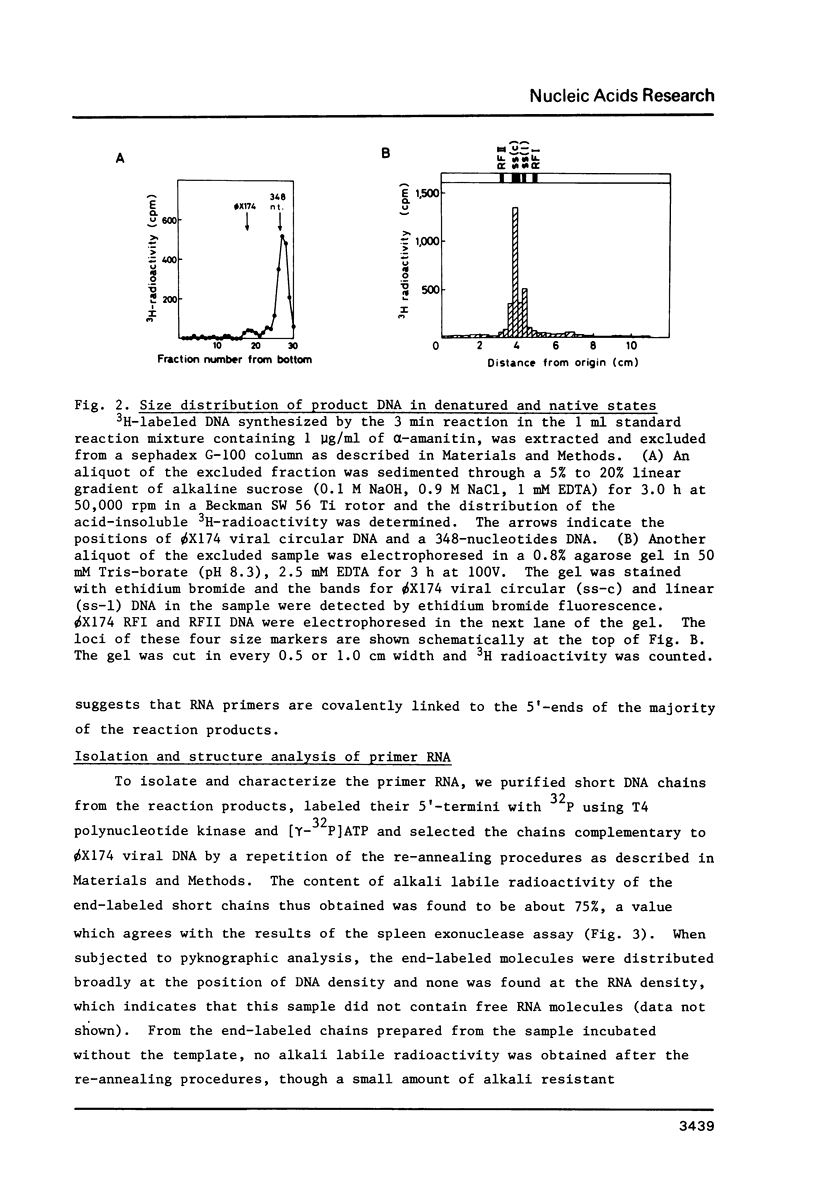
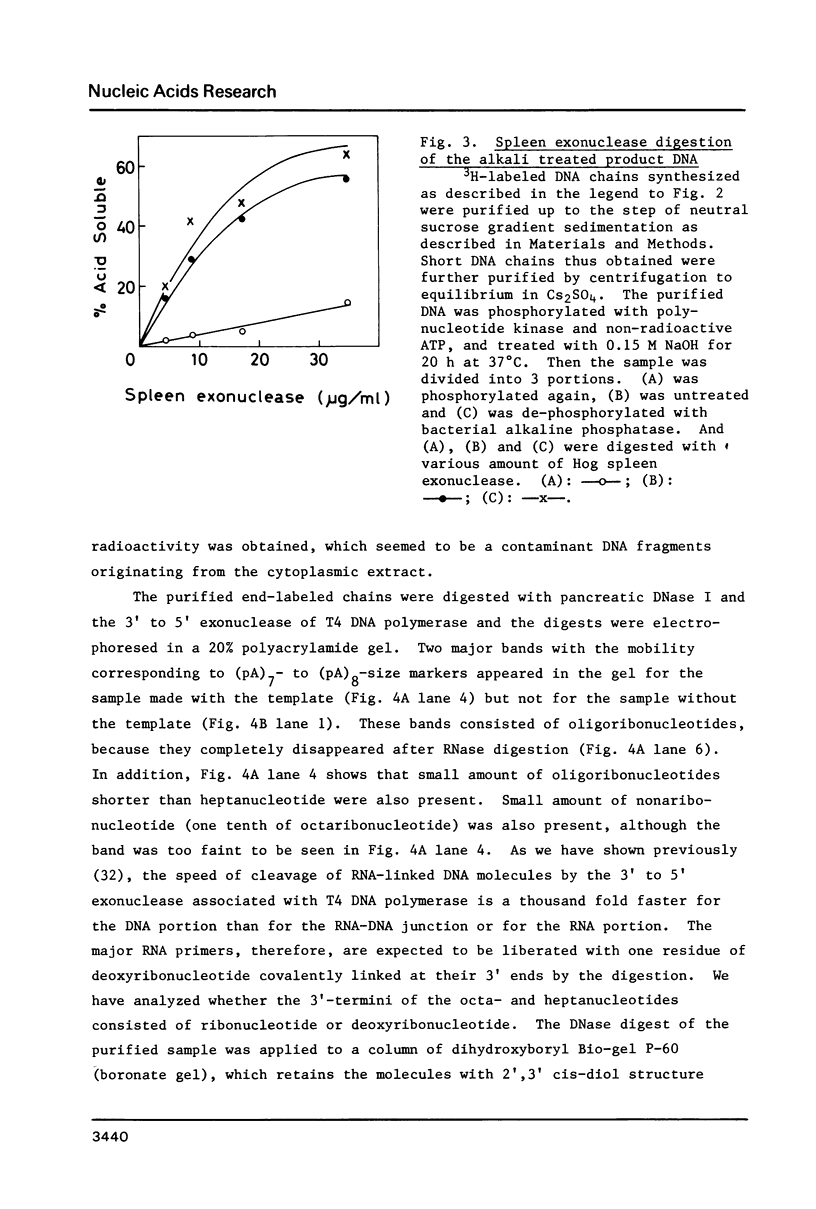
A cytoplasmic extract of Drosophila melanogaster early embryos supported DNA synthesis which was dependent on an added single stranded DNA template, phi X174 viral DNA. The product DNA made during early reaction was about 100 to 600 nucleotides in length and complementary to the added template. After alkali treatment, 70 to 80 per cent of the product DNA chains exposed 5'-hydroxyl ends, suggesting covalent linkage of primer RNA at their 5'-ends. Post-labeling of 5'-ends of the product DNA with polynucleotide kinase and [gamma-32P]ATP revealed that oligoribonucleotides, mainly hexa- and heptanucleotides, were covalently linked to the 5'-ends of the majority of the DNA chains. The nucleotide sequence of the linked RNA was mainly 5'(p)ppApA(prN)4-5, where tri- (or di-) phosphate terminus was detected by the acceptor activity for the cap structure with guanylyltransferase and [alpha-32P]GTP. The structure of this primer RNA was comparable to that of the octaribonucleotide primer isolated from the nuclei of Drosophila early embryos.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. Synthesis by the DNA primase of Drosophila melanogaster of a primer with a unique chain length. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4585–4588. doi: 10.1073/pnas.79.15.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. Synthesis and distribution of initiator RNA. J Biol Chem. 1978 Oct 25;253(20):7469–7475. [PubMed] [Google Scholar]
- Fujiyama A., Kohara Y., Okazaki T. Initiation sites for discontinuous DNA synthesis of bacteriophage T7. Proc Natl Acad Sci U S A. 1981 Feb;78(2):903–907. doi: 10.1073/pnas.78.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Hillenbrand G., Morelli G., Lanka E., Scherzinger E. Bacteriophage T7 DNA primase: a multifunctional enzyme involved in DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):449–459. doi: 10.1101/sqb.1979.043.01.051. [DOI] [PubMed] [Google Scholar]
- Kaufmann G. Characterization of initiator RNA from replicating simian virus 40 DNA synthesized in isolated nuclei. J Mol Biol. 1981 Mar 25;147(1):25–39. doi: 10.1016/0022-2836(81)90077-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Falk H. H. An oligoribonucleotide polymerase from SV40-infected cells with properties of a primase. Nucleic Acids Res. 1982 Apr 10;10(7):2309–2321. doi: 10.1093/nar/10.7.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozu T., Yagura T., Seno T. De novo DNA synthesis by a novel mouse DNA polymerase associated with primase activity. Nature. 1982 Jul 8;298(5870):180–182. doi: 10.1038/298180a0. [DOI] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., Ogawa T., Hirose S., Okazaki T., Okazaki R. Mechanism of DNA chain growth. XV. RNA-linked nascent DNA pieces in Escherichia coli strains assayed with spleen exonuclease. J Mol Biol. 1975 Aug 25;96(4):653–664. doi: 10.1016/0022-2836(75)90144-8. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Okazaki T. Structure of the RNA portion of the RNA-linked DNA pieces in bacteriophage T4-infected Escherichia coli cells. J Mol Biol. 1979 Dec 25;135(4):841–861. doi: 10.1016/0022-2836(79)90515-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Méchali M., Harland R. M. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982 Aug;30(1):93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Hirose S., Okazaki T., Okazaki R. Mechanism of DNA chain growth XVI. Analyses of RNA-linked DNA pieces in Escherichia coli with polynucleotide kinase. J Mol Biol. 1977 May 5;112(1):121–140. doi: 10.1016/s0022-2836(77)80160-5. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Okazaki T. RNA-linked nascent DNA pieces in phage T7-infected Escherichia coli. III. Detection of intact primer RNA. Nucleic Acids Res. 1979 Nov 24;7(6):1621–1633. doi: 10.1093/nar/7.6.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Ueda K., Hayaishi O. Purification of ADP-ribosylated nuclear proteins by covalent chromatography on dihydroxyboryl polyacrylamide beads and their characterization. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1111–1115. doi: 10.1073/pnas.75.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Gupta R. C., Randerath E. 3H and 32P derivative methods for base composition and sequence analysis of RNA. Methods Enzymol. 1980;65(1):638–680. doi: 10.1016/s0076-6879(80)65065-4. [DOI] [PubMed] [Google Scholar]
- Reichard P., Eliasson R. Synthesis and function of polyoma initiator RNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):271–277. doi: 10.1101/sqb.1979.043.01.033. [DOI] [PubMed] [Google Scholar]
- Reichard P., Eliasson R., Söderman G. Initiator RNA in discontinuous polyoma DNA synthesis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4901–4905. doi: 10.1073/pnas.71.12.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel H. D., König H., Stahl H., Knippers R. Circular single stranded phage M13-DNA as a template for DNA synthesis in protein extracts from Xenopus laevis eggs: evidence for a eukaryotic DNA priming activity. Nucleic Acids Res. 1982 Sep 25;10(18):5621–5635. doi: 10.1093/nar/10.18.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano L. J., Richardson C. C. Characterization of the ribonucleic acid primers and the deoxyribonucleic acid product synthesized by the DNA polymerase and gene 4 protein of bacteriophage T7. J Biol Chem. 1979 Oct 25;254(20):10483–10489. [PubMed] [Google Scholar]
- Seki T., Okazaki T. RNA-linked nascent DNA pieces in phage T7-infected Escherchia coli. II. Primary structure of the RNA portion. Nucleic Acids Res. 1979 Nov 24;7(6):1603–1619. doi: 10.1093/nar/7.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda M., Nelson E. M., Bayne M. L., Benbow R. M. DNA primase activity associated with DNA polymerase alpha from Xenopus laevis ovaries. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7209–7213. doi: 10.1073/pnas.79.23.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Mukai J., Akune S. Mode of action and base specificity of a nuclease from the silkworm. J Biochem. 1968 Jan;63(1):14–19. doi: 10.1093/oxfordjournals.jbchem.a128742. [DOI] [PubMed] [Google Scholar]
- Steinschneider A., Fraenkel-Conrat H. Studies of nucleotide sequences in tobacco mosaic virus ribonucleic acid. IV. Use of aniline in stepwise degradation. Biochemistry. 1966 Aug;5(8):2735–2743. doi: 10.1021/bi00872a034. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Template recognition sequence for RNA primer synthesis by gene 4 protein of bacteriophage T7. Proc Natl Acad Sci U S A. 1981 Jan;78(1):205–209. doi: 10.1073/pnas.78.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. DNA primase activity from human lymphocytes. Synthesis of oligoribonucleotides that prime DNA synthesis. J Biol Chem. 1982 Jul 10;257(13):7280–7283. [PubMed] [Google Scholar]
- Tseng B. Y., Erickson J. M., Goulian M. Initiator RNA of nascent DNA from animal cells. J Mol Biol. 1979 Apr 25;129(4):531–545. doi: 10.1016/0022-2836(79)90467-4. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. Initiator RNA of discontinuous DNA synthesis in human lymphocytes. Cell. 1977 Oct;12(2):483–489. doi: 10.1016/0092-8674(77)90124-6. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Modification of the 5' end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-)methyltransferase complex from vaccinia virus. J Biol Chem. 1980 Feb 10;255(3):903–908. [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Wu R., Taylor E. Nucleotide sequence analysis of DNA. II. Complete nucleotide sequence of the cohesive ends of bacteriophage lambda DNA. J Mol Biol. 1971 May 14;57(3):491–511. doi: 10.1016/0022-2836(71)90105-7. [DOI] [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA polymerase accompanied by a novel RNA polymerase activity: purification and partial characterization. J Biochem. 1982 Feb;91(2):607–618. doi: 10.1093/oxfordjournals.jbchem.a133732. [DOI] [PubMed] [Google Scholar]
- Zalokar M. Autoradiographic study of protein and RNA formation during early development of Drosophila eggs. Dev Biol. 1976 Apr;49(2):425–437. doi: 10.1016/0012-1606(76)90185-8. [DOI] [PubMed] [Google Scholar]