Abstract
Talpid moles across all northern continents exhibit a remarkably large, sickle-like radial sesamoid bone anterior to their five digits, always coupled with a smaller tibial sesamoid bone. A possible developmental mechanism behind this phenomenon was revealed using molecular markers during limb development in the Iberian mole (Talpa occidentalis) and a shrew (Cryptotis parva), as shrews represent the closest relatives of moles but do not show these conspicuous elements. The mole's radial sesamoid develops later than true digits, as shown by Sox9, and extends into the digit area, developing in relation to an Msx2-domain at the anterior border of the digital plate. Fgf8 expression, marking the apical ectodermal ridge, is comparable in both species. Developmental peculiarities facilitated the inclusion of the mole's radial sesamoid into the digit series; talpid moles circumvent the almost universal pentadactyly constraint by recruiting wrist sesamoids into their digital region using a novel developmental pathway and timing.
Keywords: Talpidae, os falciforme, autopodial development
1. Introduction
The constancy of pentadactyly among living land vertebrates has been linked to developmental constraints or strong pleiotropic gene interactions [1]. However, several species of the earliest tetrapods from the Devonian show more than five digits [2], and syndromes involving polydactyly in humans and other mammals are frequent, suggesting that a latent developmental programme for additional digits may exist in extant taxa. Many groups of vertebrates present accessory pre-axial structures in their limbs: tibial and radial skeletal elements anterior to the first phalanges that can assume positions similar to those ‘extra-digits’ of the earliest tetrapods [3,4].
A prominent example of such pre-axial elements is the massive radial sesamoid (Os radiale externum, prepollex, ‘os falciforme’) in the hands of moles [5]. It appears in several genera of talpids in all northern continents, and, as reported here, its presence is coupled with that of a distinctive tibial sesamoid (figures 1 and 2c). Their functional role is to increase the autopodial area and to brace the animal when digging. Although not segmented, these pre-axial elements in at least the most derived moles can be moved independently like a digit, becoming abducted to widen the autopod [7]. Tendinous insertions to the radial sesamoid in Talpa come from Musculus abductor pollicis longus and M. palmaris longus [8]. While sesamoids in association with the former are taxonomically far spread [5], the characteristics of the mole's radial sesamoid and its distal position are remarkable.
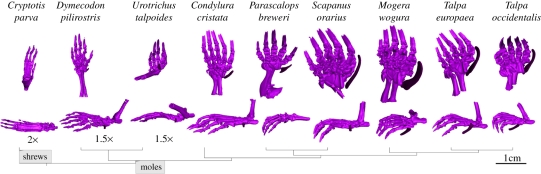
Figure 1.
Microtomography scan-images of autopodia of talpid species and of C. parva, demonstrating the distribution and proportions of the pre-axial sesamoids (highlighted); phylogenetic relationships are based on Sánchez-Villagra et al. [6].
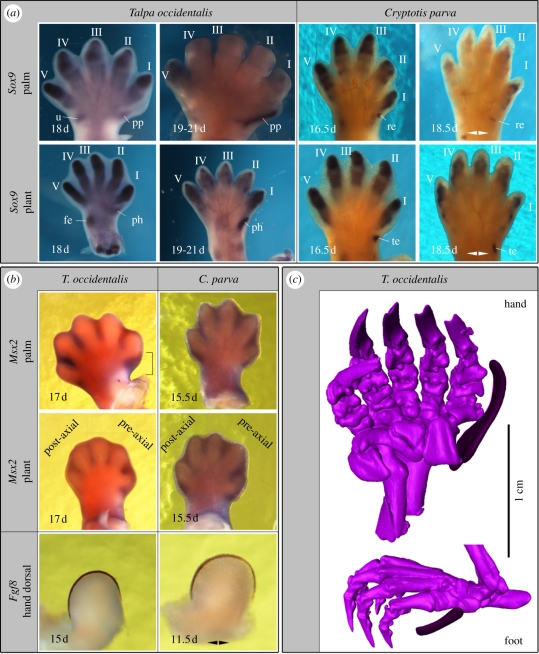
Figure 2.
(a) Sox9, (b) Msx2 and Fgf8 expression and (c) microtomography scan images (radial and tibial sesamoids highlighted) of right autopodia of mole and shrew embryos; gestational ages in days post coitum, double arrows indicate mirrored images of left autopodia; brackets highlight stronger anterior Msx2 expression in mole; roman numbers label digits; fe, fibulare; re, radial epiphysis; pp, radial sesamoid; te, tibiale; ph, tibial sesamoid; u, ulna.
In this study, we examined the early development of autopodial structures of the Iberian mole, Talpa occidentalis, and the North American least shrew Cryptotis parva, as shrews represent the closest relatives of moles but lack such conspicuous pre-axial sesamoids (figure 1).
2. Material and methods
(a). Embryo collection
Talpa occidentalis embryos were collected in Granada, Spain, and their gestational age was determined [9]; C. parva embryos were obtained from a captive breeding colony at ATSU. Specimens were fixed in 4 per cent paraformaldehyde, dehydrated through a methanol series and stored at −20°C.
(b). In situ hybridization
Digoxigenin-labelled antisense RNA probes were synthesized from plasmids containing PCR products of the major part of the coding sequences of Sox9 of T. occidentalis and Msx2 and Fgf8 of the mouse (Mus musculus), using cDNA retro-transcribed from embryonic mRNA of each species as a template (GenBank accession numbers: HQ260700, HQ260699 and HQ260698). Whole-mount in situ hybridizations and histological preparations were performed [10].
3. Results
The pre-axial elements of talpids are sesamoid bones [5,11] and have a discrete cartilaginous phase of development, as is typical for such elements [5] and are thus preceded by prechondral mesenchymal condensations.
In 17 d mole specimens (n = 2), there is strong asymmetric anterior Msx2 expression in the region that will become occupied by the distal radial sesamoid (figure 2a,b). Such a strong anterior expression domain could not be seen in the 17 d mole foot. The expression of Sox9, serving as an early marker for limb chondrification [12] marks the prospective domains of chondral autopodial elements: In 18 d T. occidentalis, Sox9 expression becomes apparent in a rod-like manner pre-axial to the region of digit I, after Sox9 expression in the digits had reached its peak (figure 2a). This pre-axial expression persists in later embryos when Sox9 transcription has already faded in the phalanges, with the domain extending well into the autopod. Both temporal persistence and the spatial situation of Sox9 expression in these domains do not exactly match with other basipodial, metapodial or acropodial elements; the expression pattern of this gene in the autopods of the shrew is comparable with those of the moles but there are no signs of condensations in the pre-axial regions of either the hand or the foot comparable to those seen in the mole. Instead, there are proximal, radial and tibial condensations that do not extend into the digit area (figure 2a).
Fgf8, which marks the apical ectodermal ridge (AER), shows a similar pattern in moles and shrews (figure 2b), with no evidence for an anterior extension of the AER in the former.
4. Discussion
Considering the modularity exhibited in the region of the first digit according to the expression of HoxD genes, it is not surprising that the plasticity around the pentadactyl pattern should originate at late stages in that region of the autopod; whereas changes to digits 2–5 seem interdependent, changes to digit 1 are independent and the thumb exhibits wrist-like characteristics [13,14].
The uncoupling between digit identity and position has been one of the major findings in research addressing digit identity at the dinosaur–bird transition [15]. For this, several approaches integrating experimental and comparative embryology have been paramount [16]. In the mole, the anatomical and developmental comparisons show important differences between the pre-axial elements and true digits. The timing of onset of skeletal differentiation is later than in the digits. The mole shows no conspicuous anterior extension of the AER, but there is a prominent domain of Msx2 expression that hints at the recruitment of autopodial developmental mechanisms normally involved in digit-patterning in the region anterior of the thumb.
The interdigital tissue is important in patterning species-specific autopodia, it has furthermore been shown to have chondrogenic potential and to regulate digit identity [17–20]. Msx2 marks the interdigital tissue where, in cases where the fingers are separated from one other (the mole hand is much webbed), apoptosis will happen, thus influencing further AER development. Msx expression may also repress Shh anteriorly, influencing pattering of the acropodium as a Shh gradient is involved in determining digit numbers. Generally, the Msx genes are thought to have several influences on limb development, they have been shown to be involved in the apoptotic programme (although not to be sufficient to initiate it), and they are involved in controlling bone development and differentiation including the suppression of ectopic cranial neural crest-derived bones [18,21–23] and are involved in digit number regulation [24]. Also, defects in likely upstream factors to Msx1/Msx2 such as bone morphogenetic proteins are known to cause malformations including syndactyly and polydactyly [25].
A singular aspect of the biology of moles is relevant to address their autopodial innovation. Females of several species, including T. occidentalis, have ovotestes, instead of normal ovaries, and masculinized genitalia, very uncommon specializations among mammals hinting at specific androgen exposure of the embryos [9]. Those species are those also specialized for exclusive or partial fossoriality and show the presence of well-differentiated pre-axial elements. These facts are relevant, as such steroids influence bone turnover, growth and transitions between tendinous tissue and cartilage [26]. Additionally, high maternal testosterone levels have been hypothesized to be one cause of polydactyly [27]: high levels of testosterone were found to be associated with the birth of males, and there was a significant excess of post-axial and pre-axial polydactylous male probands. Moles also develop a tibial sesamoid bone which, however, does not extend into the acropodial area as clearly as the radial sesamoid of the hand nor does it develop in association with a well-developed Msx2 expression domain as the radial sesamoid does. However, different sesamoid elements develop frequently coincidently [28]. Taking into account high incidences of certain sesamoids in conjunction with primary osteoarthritis [29], it was suggested that sesamoids tend to be linked in their appearance and that their presence may be owing to an increased tendency of endochondral ossification [28], perhaps manifested through genetic assimilation [30].
Pre-axial elements have evolved numerous times in tetrapod evolution; as shown by the mole case, the co-option of similar developmental mechanisms to those of true (anterior) digits in conjunction with changes in developmental timing may be one way to facilitate the recruitment of wrist skeletogenic material into the acropodial region.
Acknowledgements
Animal handling was in accordance with institutional guidelines.
We thank the Swiss National Fund (SNF31003A_133032/1), the Spanish ministry of Science (CGL2004-00863/BOS and CGL2008-00828/BOS), the Andalusian Government (CVI2057), the Smart Mix programme of the Netherlands Ministry of Economic Affairs and the Netherlands Scientific Organisation for support. Thanks to J. Hugi, L. Wilson, C. Kolb, D. Koyabu, J. Neenan, T. Scheyer, I. Werneburg, D. Lupiañez, F. Real and R. Dadhich for help, comments and discussion. C. Zollikofer and N. Morimoto, Zürich, kindly made available the micro-CT facilities and supervised their use.
References
- 1.Galis F., van Alphen J. J. M., Metz J. A. J. 2001. Why five fingers? Evolutionary constraints on digit numbers. Trends. Ecol. Evol. 16, 637–646 10.1016/S0169-5347(01)02289-3 (doi:10.1016/S0169-5347(01)02289-3) [DOI] [Google Scholar]
- 2.Coates M. I., Clack J. A. 1990. Polydactyly in the earliest known tetrapod limbs. Nature 347, 66–69 10.1038/347066a0 (doi:10.1038/347066a0) [DOI] [Google Scholar]
- 3.Endo H., Yamagiwa D., Hayashi Y., Koie H., Yamaya Y., Kimura J. 1999. Role of the giant panda's ‘pseudo-thumb’. Nature 397, 309–310 10.1038/16830 (doi:10.1038/16830) [DOI] [PubMed] [Google Scholar]
- 4.Fabrezi M. 2001. A survey of prepollex and prehallux variation in anuran limbs. Z. J. Linn. Soc. 131, 227–248 10.1111/j.1096-3642.2001.tb01316.x (doi:10.1111/j.1096-3642.2001.tb01316.x) [DOI] [Google Scholar]
- 5.Vickaryous M. K., Olson W. M. 2007. Sesamoids and ossicles in the appendicular skeleton. In Fins into limbs: evolution, development, and transformation (ed. Hall B. K.), pp. 323–341 Chicago, London: The University of Chicago Press [Google Scholar]
- 6.Sánchez-Villagra M. R., Horovitz I., Motokawa M. 2006. A comprehensive morphological analysis of talpid moles (Mammalia) phylogenetic relationships. Cladistics 22, 59–88 10.1111/j.1096-0031.2006.00087.x (doi:10.1111/j.1096-0031.2006.00087.x) [DOI] [PubMed] [Google Scholar]
- 7.Yalden D. 1966. The anatomy of mole locomotion. J. Zool. 149, 55–64 10.1111/j.1469-7998.1966.tb02983.x (doi:10.1111/j.1469-7998.1966.tb02983.x) [DOI] [Google Scholar]
- 8.Whidden H. P. 2000. Comparative myology of moles and the phylogeny of the Talpidae (Mammalia, Lipotyphla). Am. Mus. Nov. 3294, 1–53 (doi:10.1206/0003-0082(2000)3294<0001:CMOMAT>2.0.CO;2) [DOI] [Google Scholar]
- 9.Barrionuevo F. J., Zurita F., Burgos M., Jiménez R. 2004. Developmental stages and growth rate of the mole Talpa occidentalis (Insectivora, Mammalia). J. Mammal. 85, 120–125 10.1644/BPR-010 (doi:10.1644/BPR-010) [DOI] [Google Scholar]
- 10.Welten M. C. M., Verbeek F. J., Meijer A. H., Richardson M. K. 2005. Gene expression and digit homology in the chicken embryo wing. Evol. Dev. 7, 18–28 10.1111/j.1525-142X.2005.05003.x (doi:10.1111/j.1525-142X.2005.05003.x) [DOI] [PubMed] [Google Scholar]
- 11.Prochel J. 2006. Early skeletal development in Talpa europaea, the common European mole. Zool. Sci. 23, 427–434 10.2108/zsj.23.427 (doi:10.2108/zsj.23.427) [DOI] [PubMed] [Google Scholar]
- 12.Chimal-Monroy J., Rodriguez-Leon J., Montero J., Gañan Y., Macias D., Merino R., Hurle J. M. 2003. Analysis of the molecular cascade responsible for mesodermal limb chondrogenesis: Sox genes and BMP signaling. Dev. Biol. 257, 292–301 10.1016/S0012-1606(03)00066-6 (doi:10.1016/S0012-1606(03)00066-6) [DOI] [PubMed] [Google Scholar]
- 13.Wagner G. P., Vargas A. O. 2008. On the nature of thumbs. Genome Biol. 9, 213. 10.1186/gb-2008-9-3-213 (doi:10.1186/gb-2008-9-3-213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woltering J. M., Duboule D. 2010. The origin of digits: expression patterns versus regulatory mechanisms. Dev. Cell 18, 526–532 10.1016/j.devcel.2010.04.002 (doi:10.1016/j.devcel.2010.04.002) [DOI] [PubMed] [Google Scholar]
- 15.Tamura K., Nomura N., Seki R., Yonei-Tamura S., Yokoyama H. 2011. Embryological evidence identifies wing digits in birds as digits 1, 2 and 3. Science 331, 753–757 10.1126/science.1198229 (doi:10.1126/science.1198229) [DOI] [PubMed] [Google Scholar]
- 16.Young R. L., Wagner G. P. 2011. Why ontogenetic homology criteria can be misleading: lessons from digit identity transformations. J. Exp. Zool. (Mol. Dev. Evol.) 316B, 165–170 10.1002/jez.b.21396 (doi:10.1002/jez.b.21396) [DOI] [PubMed] [Google Scholar]
- 17.Gañan Y., Macias D., Basco R. D., Merino R., Hurle J. M. 1998. Morphological diversity of the avian foot is related with the pattern of msx gene expression in the developing autopod. Dev. Biol. 196, 33–41 10.1006/dbio.1997.8843 (doi:10.1006/dbio.1997.8843) [DOI] [PubMed] [Google Scholar]
- 18.Weatherbee S. D., Behringer R. R., Rasweiler J. J., IV, Niswander L. A. 2006. Interdigital webbing retention in bat wings illustrates genetic changes underlying amniote limb diversification. Proc. Natl Acad. Sci. USA 103, 15 103–15 107 10.1073/pnas.0604934103 (doi:10.1073/pnas.0604934103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gañan Y., Macias D., Hurle J. M. 1994. Pattern regulation in the chick autopodium at advanced stages of embryonic development. Dev. Dyn. 199, 64–72 10.1002/aja.1001990107 (doi:10.1002/aja.1001990107) [DOI] [PubMed] [Google Scholar]
- 20.Dahn R. D., Fallon J. F. 2000. Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science 289, 438–441 10.1126/science.289.5478.438 (doi:10.1126/science.289.5478.438) [DOI] [PubMed] [Google Scholar]
- 21.Lallemand Y., Nicola M., Ramos C., Bach A., Cloment C., Robert B. 2005. Analysis of Msx1; Msx2 double mutants reveals multiple roles for Msx genes in limb development. Development 132, 3003–3014 10.1242/dev.01877 (doi:10.1242/dev.01877) [DOI] [PubMed] [Google Scholar]
- 22.Lallemand Y., Bensoussan V., Saint Cloment C., Robert B. 2009. Msx genes are important apoptosis effectors downstream of the Shh/Gli3 pathway in the limb. Dev. Biol. 331, 189–198 10.1016/j.ydbio.2009.04.038 (doi:10.1016/j.ydbio.2009.04.038) [DOI] [PubMed] [Google Scholar]
- 23.Roybal P. G., Wu N. L., Sun J., Ting M., Schafer C. A., Maxson R. E. 2010. Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Dev. Biol. 343, 28–39 10.1016/j.ydbio.2010.04.007 (doi:10.1016/j.ydbio.2010.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bensoussan-Trigano V., Lallemand Y., Cloment C. S., Benoît R. 2011. Msx1 and Msx2 in limb mesenchyme modulate digit number and identity. Dev. Dyn. 240, 1190–1202 10.1002/dvdy.22619 (doi:10.1002/dvdy.22619) [DOI] [PubMed] [Google Scholar]
- 25.Montero J. A., Lorda-Diez C. I., Gañan Y., Macias D., Hurle J. M. 2008. Activin/TGFβ and BMP crosstalk determines digit chondrogenesis. Dev. Biol. 321, 343–356 10.1016/j.ydbio.2008.06.022 (doi:10.1016/j.ydbio.2008.06.022) [DOI] [PubMed] [Google Scholar]
- 26.Hall B. K. 2005. Bones and cartilage: developmental and evolutionary skeletal biology. Amsterdam, The Netherlands: Elsevier Academic Press [Google Scholar]
- 27.James W. H. 1998. Hypothesis: one cause of polydactyly. J. Theor. Biol. 192, 1–2 10.1006/jtbi.1997.0591 (doi:10.1006/jtbi.1997.0591) [DOI] [PubMed] [Google Scholar]
- 28.Sarin V., Erickson G., Giori N., Bergman A., Carter D. 1999. Coincident development of sesamoid bones and clues to their evolution. Anat. Rec. 257, 174–180 (doi:10.1002/(SICI)1097-0185(19991015)257:5<174::AID-AR6>3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 29.Pritchett J. 1984. The incidence of fabellae in osteoarthrosis of the knee. J. Bone Joint Surg. 66, 1379–1380 [PubMed] [Google Scholar]
- 30.Waddington C. H. 1953. Genetic assimilation of acquired character. Evolution 7, 118–126 10.2307/2405747 (doi:10.2307/2405747) [DOI] [Google Scholar]