Abstract
Background: Obesity is a serious chronic disease. Controlled-release phentermine/topiramate (PHEN/TPM CR), as an adjunct to lifestyle modification, has previously shown significant weight loss compared with placebo in a 56-wk study in overweight and obese subjects with ≥2 weight-related comorbidities.
Objective: This study evaluated the long-term efficacy and safety of PHEN/TPM CR in overweight and obese subjects with cardiometabolic disease.
Design: This was a placebo-controlled, double-blind, 52-wk extension study; volunteers at selected sites continued with original randomly assigned treatment [placebo, 7.5 mg phentermine/46 mg controlled-release topiramate (7.5/46), or 15 mg phentermine/92 mg controlled-release topiramate (15/92)] to complete a total of 108 wk. All subjects participated in a lifestyle-modification program.
Results: Of 866 eligible subjects, 676 (78%) elected to continue in the extension. Overall, 84.0% of subjects completed the study, with similar completion rates between treatment groups. At week 108, PHEN/TPM CR was associated with significant, sustained weight loss (intent-to-treat with last observation carried forward; P < 0.0001 compared with placebo); least-squares mean percentage changes from baseline in body weight were –1.8%, –9.3%, and –10.5% for placebo, 7.5/46, and 15/92, respectively. Significantly more PHEN/TPM CR–treated subjects at each dose achieved ≥5%, ≥10%, ≥15%, and ≥20% weight loss compared with placebo (P < 0.001). PHEN/TPM CR improved cardiovascular and metabolic variables and decreased rates of incident diabetes in comparison with placebo. PHEN/TPM CR was well tolerated over 108 wk, with reduced rates of adverse events occurring between weeks 56 and 108 compared with rates between weeks 0 and 56.
Conclusion: PHEN/TPM CR in conjunction with lifestyle modification may provide a well-tolerated and effective option for the sustained treatment of obesity complicated by cardiometabolic disease. This trial was registered at clinicaltrials.gov as NCT00796367.
INTRODUCTION
Obesity is a global epidemic (1, 2). This chronic disease increases morbidity and mortality, in large part due to associated comorbidities, including T2D4, CVD, metabolic syndrome, liver disease, and cancer (1–7). The first-line strategy for the treatment of obesity and prevention of cardiometabolic disease is achieving weight loss through lifestyle interventions, which consist of reductions in caloric intake (by 500–1000 calories/d), increases in physical activity, and changes in health behaviors (8). However, adherence to lifestyle changes can be challenging for a wide variety of reasons, such as a lack of readiness for change on the part of the patient, physical restrictions that limit activity, and a shortage of therapeutic venues that include a multidisciplinary health care team essential to treatment effectiveness. When lifestyle changes alone do not provide the desired weight loss, the addition of pharmacotherapy or bariatric surgery provides a viable option for patients meeting eligibility criteria. However, effective pharmacologic options are limited, and indication for bariatric surgery is limited to patients with a high BMI due to the inherent risks of invasive procedures (9, 10). Thus, it is imperative that effective, long-term pharmacologic strategies are identified that may be used in conjunction with lifestyle changes to combat the obesity epidemic.
Currently, orlistat (Xenical; Genentech), a gastric and pancreatic lipase inhibitor, is the only approved agent for the long-term pharmacologic treatment of obesity in the United States (10). Phentermine (Apidex-P; Teva), a central norepinephrine-releasing drug, is approved for short-term (a few weeks) treatment of obesity as monotherapy (37.5 mg/d) (10, 11). Topiramate (Topamax; Janssen Pharmaceuticals), an anticonvulsant (200–400 mg/d) that is also approved for the prophylaxis of migraine headaches (100 mg/d), has shown weight-loss properties but is not currently approved as a therapy for obesity (10, 12–16). A low-dose combination of phentermine plus controlled-release topiramate (PHEN/TPM CR) as an adjunct to lifestyle modification was previously shown to reduce body weight through 56 wk of treatment in the CONQUER study (17). The SEQUEL study, an extension of the CONQUER study, was designed to assess the longer-term efficacy and safety of lifestyle intervention and 2 doses of PHEN/TPM CR for an additional 52 wk (ie, a total treatment duration of 108 wk).
SUBJECTS AND METHODS
Subjects
All subjects were eligible to enroll in the extension study if they completed the CONQUER study on treatment and complied with protocol requirements. Inclusion and exclusion criteria for CONQUER required subjects to have a BMI (in kg/m2) of ≥27 and ≤45 as well as ≥2 weight-related comorbidities, as previously described in detail (17). To continue into the SEQUEL extension study, female subjects of childbearing potential were required to continue contraception in the form of a double-barrier method, stable hormonal contraception plus single barrier, or tubal ligation. Exclusion criteria included having a BMI ≤22 at the completion of the CONQUER study, continuously not taking the study drug for >4 wk at the completion of the CONQUER study, developing a condition during the CONQUER study that would interfere with compliance or attainment of study measures, or participating in another formal weight-loss program.
Study design
SEQUEL was a double-blind, placebo-controlled, 52-wk extension of the CONQUER study. On completion of the 56-wk CONQUER study (17), sites that met the criteria described below were eligible to offer enrollment in SEQUEL. Participants in SEQUEL continued with the original treatment to which they were randomly assigned during CONQUER: lifestyle intervention plus placebo, lifestyle plus 7.5 mg phentermine/46 mg controlled-release topiramate, or lifestyle plus 15 mg phentermine/92 mg controlled-release topiramate, hereafter referred to as placebo, 7.5/46, and 15/92, respectively. Of the 93 CONQUER sites, 36 were selected for the extension study on the basis of their high initial enrollment numbers and rates of retention, and these sites remained blinded to assigned treatment through the end of SEQUEL. This study was conducted between December 2008 and June 2010 and was approved by institutional review boards at each site. All subjects provided written informed consent for participation in the SEQUEL extension. This trial was registered with clinicaltrials.gov as NCT00796367.
Randomization and interventions
Subjects were randomly assigned by using a computer-generated algorithm and were stratified according to their sex and diabetic status. Study drugs and placebo were administered as capsules that were identical in size and appearance. Eligible subjects maintained their originally assigned once-daily treatment from the CONQUER study (2:1:2 randomization for placebo, 7.5/46, or 15/92) (17). Investigators and subjects remained blinded to treatment assignment. The screening visit for the extension study occurred at the final CONQUER visit, and study visits occurred at 4-wk intervals thereafter. All subjects continued to receive standardized diet and lifestyle-modification counseling based on the LEARN (Lifestyle, Exercise, Attitudes, Relationships, and Nutrition) program (18). Dose reductions or interruptions were allowed for subjects who experienced AEs or who had tolerability issues. Subjects discontinuing the study drug were encouraged to remain in the study, complete study assessments, and continue with the lifestyle counseling according to the LEARN program.
Study endpoints
The CONQUER study had 2 predefined, coprimary endpoints, which were retained as the primary outcome measures in the SEQUEL extension study: mean percentage weight loss and percentage of subjects achieving ≥5% weight loss from baseline (week 0 of CONQUER) to week 108. Secondary endpoints included the following: weight loss in kilograms; percentage of subjects achieving ≥10%, ≥15%, or ≥20% weight loss; and change in waist circumference from baseline to week 108. Other efficacy endpoints included changes from baseline to week 108 in blood pressure, serum lipid variables, glycemic measures, concomitant medications for weight-related comorbidities, and rate of progression to diabetes among subjects without diabetes at baseline. All subjects were assessed for diabetic status at baseline per the 2007 American Diabetes Association guidelines, as follows: fasting blood glucose ≥126 mg/dL or 2-h blood glucose ≥200 mg/dL after an oral-glucose-tolerance test (19). Hb A1c concentrations were also obtained to assess degree of hyperglycemia but were not used as a diagnostic criterion for diabetes at baseline (20). Subjects were considered to have progressed to T2D if their blood glucose was ≥126 mg/dL under fasting conditions during ≥2 consecutive measurements and/or ≥200 mg/dL at 2 h after an oral-glucose-tolerance test. Safety assessments included AEs, physical examination, clinical and laboratory measurements, vital signs, and electrocardiography. At each visit, depressive symptoms were assessed by using the 9-item Patient Health Questionnaire self-reported assessment for depressive symptoms, and the presence of suicidal ideation or behavior was assessed by using the C-SSRS, an 11-item, clinician-administered assessment.
Statistical analysis
Analyses of change in weight, waist circumference, and other continuous efficacy measures were conducted in a modified ITT sample with the LOCF method to impute missing values. The modified ITT sample was defined as all subjects who received at least one study drug dose with at least one postbaseline measurement of body weight. ANCOVA was used to evaluate percentage weight loss, with treatment, sex, and diabetic status as fixed effects and baseline weight as a covariate. LS means, corresponding SEs, 2-sided 95% CIs, and 2-sided P values for percentage weight loss within each treatment group were derived from the ANCOVA. Analyses of percentage of categorical weight loss were conducted by using logistic regression, with treatment, sex, and diabetic status as fixed effects and baseline weight as a covariate. For each treatment comparison, the estimated OR, SE, 95% Wald CI, and P value were determined. Multiple imputation was used as a sensitivity analysis to supplement the ITT-LOCF approach, and an analysis of subjects who completed the study while actively taking the study drug was also performed to understand the effect of treatment after 108 wk of exposure.
The annualized incidence rate of T2D was calculated as the number of newly diagnosed subjects divided by the number of subject-years of follow-up for each treatment group. The number of subject-years of follow-up was calculated as the sum of the number of days across all subjects from the randomization date in CONQUER to the onset date of T2D or to the date of completion or discontinuation from SEQUEL (for subjects who did not develop T2D) divided by 365.25.
Safety analyses were based on incidence of AEs, changes in laboratory evaluations, vital signs, electrocardiograms, physical examination findings, and results from the 9-item Patient Health Questionnaire and the C-SSRS during both the CONQUER and SEQUEL studies. TEAEs were defined as AEs occurring between week 0 of CONQUER and ≤28 d after the last dose of double-blind study drug in SEQUEL. Analysis of TEAEs was also performed from the start date of study drug in the SEQUEL study and ≤28 d after the last dose of study drug in the SEQUEL study. All other safety variables were measured between baseline (week 0 of CONQUER) and week 108 or early termination.
RESULTS
Disposition of study subjects and baseline characteristics
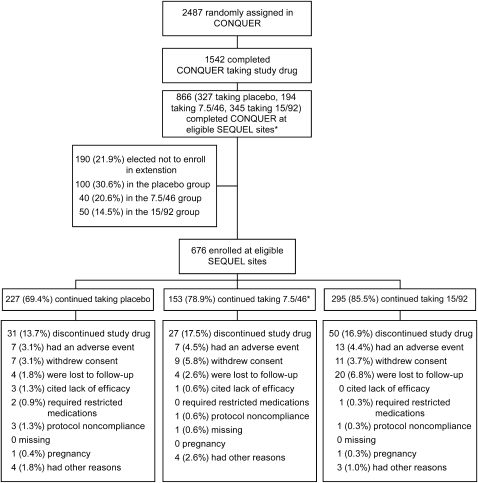
Of the 866 subjects who completed the CONQUER trial at eligible SEQUEL sites, 676 (78.1%) elected to enroll in SEQUEL and continue receiving their blinded treatment as an adjunct to lifestyle modification for an additional 52 wk (Figure 1). A greater proportion of subjects in the 15/92 treatment arm (85.5%) consented to continue with treatment than did subjects in the 7.5/46 treatment arm (79.4%), whereas the placebo group had the lowest proportion of subjects electing to continue in the protocol (69.4%). Overall, 84.0% (568/676) of subjects completed the extension study, including 86.3% (196/227) of those assigned to placebo, 82.5% (127/154) of those assigned to 7.5/46, and 83.1% (245/295) of those in the 15/92 group. Seven (3.1%) subjects in the placebo arm, 7 (4.5%) subjects in the 7.5/46 arm, and 13 (4.4%) in the 15/92 arm, discontinued the study drug due to an AE (Figure 1). Three subjects in the placebo arm and one subject in the 7.5/46 arm stopped treatment due to lack of efficacy, whereas no subjects in the 15/92 arm discontinued due to lack of efficacy. A higher number of subjects were lost to follow-up in the 15/92 group (20 subjects) than in either the placebo or 7.5/46 groups (4 subjects each).
FIGURE 1.
Trial profile. Standardized lifestyle intervention was used across all treatment groups. Of the 27 subjects who discontinued the study drug because of adverse events during the SEQUEL study, the event began during the CONQUER study for 3 subjects (one in each treatment group). For the remaining 24 subjects, the adverse event leading to discontinuation of the study drug began during the SEQUEL study. *One subject in the 7.5/46 group enrolled in the study but discontinued before receiving the study drug. 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; 15/92, 15 mg phentermine/92 mg controlled-release topiramate.
Baseline demographic, anthropometric, and clinical characteristics, including comorbidities, were similar among subjects in all 3 treatment arms of the SEQUEL study, and in each of the treatment arms the characteristics of the subjects in SEQUEL were similar to and representative of the larger CONQUER cohort (Table 1). Whereas the proportion of subjects with hypertension and dyslipidemia was similar between groups and between CONQUER and SEQUEL, a greater percentage of subjects enrolling in SEQUEL had T2D at baseline compared with the original CONQUER cohort (21.5% compared with 15.8%, respectively). In total, 451 (66.8%) subjects in SEQUEL met the American Heart Association and National Heart, Lung, and Blood Institute criteria (21, 22) for metabolic syndrome at baseline, including 152 (67.0%) in the placebo group, 107 (69.9%) in the 7.5/46 group, and 192 (65.1%) in the 15/92 group.
TABLE 1.
Baseline data by treatment group1
| Standardized lifestyle intervention across all treatment groups |
||||||
| Entire cohort (CONQUER study) |
Long-term treatment (SEQUEL study) |
|||||
| PHEN/TPM CR |
PHEN/TPM CR |
|||||
| Placebo(n = 994) | 7.5/46(n = 498) | 15/92(n = 995) | Placebo(n = 227) | 7.5/46(n = 153) | 15/92(n = 295) | |
| Age (y) | 51.2 ± 10.32 | 51.1 ± 10.4 | 51.0 ± 10.7 | 52.7 ± 9.8 | 52.2 ± 10.6 | 51.2 ± 10.4 |
| Women [n (%)] | 695 (69.9) | 349 (70.1) | 693 (69.6) | 147 (64.8) | 106 (69.3) | 195 (66.1) |
| Race [n (%)] | ||||||
| White | 861 (86.6) | 429 (86.1) | 850 (85.4) | 198 (87.2) | 134 (87.6) | 244 (82.7) |
| African | 114 (11.5) | 56 (11.2) | 122 (12.3) | 28 (12.3) | 17 (11.1) | 44 (14.9) |
| Asian | 6 (0.6) | 5 (1.0) | 11 (1.1) | 0 | 1 (0.7) | 5 (1.7) |
| American Indian or Alaskan native | 4 (0.4) | 6 (1.2) | 8 (0.8) | 2 (0.9) | 1 (0.7) | 0 |
| Native Hawaiian or other Pacific Islander | 2 (0.2) | 2 (0.4) | 3 (0.3) | 0 | 1 (0.7) | 2 (0.7) |
| Other | 12 (1.2) | 5 (1.0) | 8 (0.8) | 1 (0.4) | 0 | 3 (1.0) |
| Ethnicity [n (%)] | ||||||
| Hispanic or Latino | 128 (12.9) | 70 (14.1) | 130 (13.1) | 42 (18.5) | 25 (16.3) | 56 (19.0) |
| Not Hispanic or Latino | 866 (87.1) | 428 (85.9) | 865 (86.9) | 185 (81.5) | 128 (83.7) | 239 (81.0) |
| Weight (kg)3 | 103.3 ± 18.1 | 102.6 ± 18.2 | 103.0 ± 17.6 | 101.1 ± 18.9 | 102.2 ± 18.4 | 101.9 ± 18.9 |
| BMI (kg/m2)3 | 36.7 ± 4.6 | 36.2 ± 4.4 | 36.6 ± 4.5 | 36.0 ± 4.8 | 36.1 ± 4.5 | 36.2 ± 4.7 |
| Waist circumference (cm)3 | 113.4 ± 12.2 | 112.6 ± 12.5 | 113.2 ± 12.2 | 113.0 ± 12.5 | 112.9 ± 12.3 | 112.2 ± 12.3 |
| Blood pressure (mm Hg)3 | ||||||
| Systolic | 128.9 ± 13.5 | 128.3 ± 13.8 | 127.9 ± 13.4 | 128.5 ± 14.3 | 127.8 ± 11.4 | 127.3 ± 13.7 |
| Diastolic | 81.1 ± 9.2 | 80.6 ± 8.8 | 80.1 ± 9.1 | 79.9 ± 9.7 | 80.1 ± 8.8 | 80.1 ± 8.8 |
| Heart rate (bpm)3 | 72.1 ± 9.9 | 72.1 ± 10.1 | 72.6 ± 10.1 | 70.6 ± 10.5 | 72.0 ± 9.6 | 73.0 ± 10.3 |
| Total cholesterol (mg/dL)4 | 205.3 ± 41.7 | 201.6 ± 37.9 | 205.1 ± 40.4 | 203.5 ± 41.9 | 201.8 ± 35.8 | 201.9 ± 38.6 |
| LDL cholesterol (mg/dL)5 | 123.8 ± 36.1 | 120.8 ± 33.8 | 123.7 ± 35.5 | 123.1 ± 36.6 | 121.7 ± 32.7 | 121.5 ± 35.4 |
| HDL cholesterol (mg/dL)6 | 48.8 ± 13.8 | 48.5 ± 12.8 | 49.0 ± 13.7 | 49.5 ± 14.7 | 48.6 ± 13.3 | 48.7 ± 12.8 |
| Triglycerides (mg/dL)7 | 163.6 ± 75.8 | 161.2 ± 75.4 | 162.0 ± 73.4 | 154.4 ± 66.7 | 157.2 ± 71.5 | 158.1 ± 72.0 |
| Fasting glucose (mg/dL)8 | 106.5 ± 23.5 | 105.8 ± 20.7 | 105.9 ± 21.6 | 109.3 ± 24.4 | 110.7 ± 25.3 | 108.2 ± 24.1 |
| Glycated hemoglobin (%)9 | 5.9 ± 0.8 | 5.8 ± 0.7 | 5.9 ± 0.8 | 6.0 ± 0.9 | 6.0 ± 0.9 | 6.0 ± 0.8 |
| Subjects with [n (%)] | ||||||
| Depression history | 179 (18.0) | 81 (16.3) | 165 (16.6) | 42 (18.5) | 35 (22.9) | 55 (18.6) |
| Hypertension10 | 524 (52.7) | 261 (52.4) | 520 (52.3) | 120 (52.9) | 71 (46.4) | 154 (52.2) |
| Hypertriglyceridemia11 | 354 (35.6) | 180 (36.1) | 363 (36.5) | 80 (35.2) | 48 (31.4) | 105 (35.6) |
| T2D12 | 159 (16.0) | 68 (13.7) | 166 (16.7) | 55 (24.2) | 26 (17.0) | 64 (21.7) |
| Metabolic syndrome | 698 (70.2) | 353 (70.9) | 679 (68.2) | 152 (67.0) | 107 (69.9) | 192 (65.1) |
Baseline values for SEQUEL subjects were measured at the start of CONQUER. bpm, beats per minute; PHEN/TPM CR, controlled-release phentermine/topiramate; T2D, type 2 diabetes; 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; 15/92, 15 mg phentermine/92 mg controlled-release topiramate.
Mean ± SD (all such values).
Among CONQUER subjects, baseline weight, BMI, waist circumference, blood pressure, and heart rate values were missing for 2 subjects: one in the placebo group and one in the 15/92 group.
Among CONQUER subjects at baseline, there were missing values for 2 subjects (one for placebo, one for 15/92).
Among CONQUER subjects at baseline, there were missing values for 7 subjects (4 for placebo, 3 for 15/92).
Among CONQUER subjects at baseline, there were missing values for 2 subjects (one for placebo, one for 15/92).
Among CONQUER subjects at baseline, there were missing values for 2 subjects (one for placebo, one for 15/92).
Among CONQUER subjects at baseline, there were missing values for 11 subjects (4 for placebo, 7 for 15/92).
Among CONQUER subjects at baseline, there were missing values for 9 subjects (5 for placebo, 4 for 15/92).
Subjects with hypertension were those with systolic blood pressure ≥140 and ≤160 mm Hg (≥130 and ≤160 mm Hg if diabetic) or diastolic blood pressure ≥90 and ≤100 mm Hg (≥85 and ≤100 mm Hg if diabetic) or who were taking ≥2 antihypertensive medications and had blood pressure <140/90 mm Hg.
Subjects with hypertriglyceridemia were those with fasting triglycerides between 200 and 400 mg/dL or who were taking ≥2 lipid-lowering medications and had fasting triglycerides of <200 mg/dL.
Subjects with diabetes were those with an established diagnosis of T2D, managed with lifestyle measures, metformin therapy, or both.
Weight loss
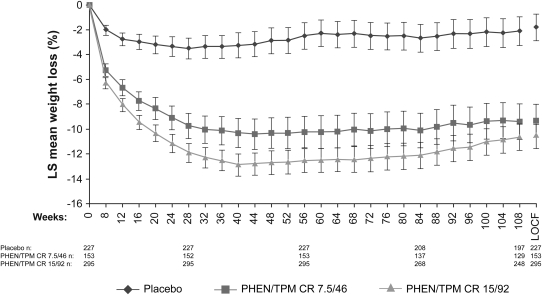
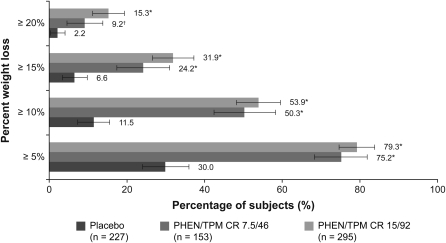
Subjects in both PHEN/TPM CR arms showed significantly greater percentage weight loss than did those in the placebo arm, and the weight loss was sustained during 108 wk (Figure 2; P < 0.0001 compared with placebo at all time points assessed). At week 108, the LS mean percentage changes from baseline in body weight in the ITT-LOCF analysis were significantly greater in the PHEN/TPM CR groups compared with placebo: –1.8%, –9.3%, and –10.5% for placebo, 7.5/46, and 15/92, respectively (P < 0.0001 compared with placebo for all comparisons). For subjects who completed the study while still taking the study drug at week 108, the LS mean percentage changes from baseline in body weight were also significantly greater in the PHEN/TPM CR groups compared with placebo: –2.2%, –9.3%, and –10.7% for placebo, 7.5/46, and 15/92, respectively (P < 0.0001 compared with placebo for all comparisons). Absolute LS mean weight loss using ITT-LOCF data were –2.1, –9.6, and –10.9 kg for the placebo, 7.5/46, and 15/92 groups, respectively (P < 0.0001 compared with placebo for all comparisons). Greater proportions of subjects treated with each dose of PHEN/TPM CR experienced weight losses of ≥5%, ≥10%, ≥15%, and ≥20% when compared with placebo-treated subjects (Figure 3; P < 0.0001 for all comparisons except for weight loss ≥20% for the 7.5/46 group, P = 0.0072; ITT-LOCF). There were also significant reductions in waist circumference at Week 108 in subjects treated with PHEN/TPM CR, with mean reductions of –3.6, –9.8, and –10.6 cm for placebo, 7.5/46, and 15/92 treatment arms, respectively (P < 0.0001 compared with placebo; ITT-LOCF).
FIGURE 2.
Mean (95% CI) percentage weight loss from baseline to week 108. LS mean change in the overall study completer sample. Standardized lifestyle intervention was used across all treatment groups. P < 0.0001 compared with placebo at all time points assessed. LOCF, last observation carried forward; LS, least-squares; PHEN/TPM CR 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; PHEN/TPM CR 15/92, 15 mg phentermine/92 mg controlled-release topiramate.
FIGURE 3.
Percentages (and 95% CIs) of subjects achieving ≥5%, ≥10%, ≥15%, or ≥20% weight loss from baseline to week 108 (ITT-LOCF). Standardized lifestyle intervention was used across all treatment groups. *P < 0.0001 compared with placebo; †P = 0.0072 compared with placebo. ITT, intent-to-treat; LOCF, last observation carried forward; PHEN/TPM CR 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; PHEN/TPM CR 15/92, 15 mg phentermine/92 mg controlled-release topiramate.
An analysis of weight loss as a function of baseline BMI category (<30, ≥30 and <35, ≥35 and <40, ≥40 and <45) was performed (see Supplemental Figure 1 under “Supplemental data” in the online issue). PHEN/TPM CR was clearly effective in all BMI categories and produced greater weight loss than did placebo (P ≤ 0.0061). However, a significant treatment effect by baseline BMI category was observed (P = 0.0327). Whereas the 7.5/46 and 15/92 doses were statistically similar in their effectiveness in the lower baseline BMI categories, the 15/92 group showed significantly greater percentage weight loss than did the 7.5/46 group in the most severely obese subjects (baseline BMI ≥40 and <45; P = 0.0016 compared with 7.5/46). Among all subjects with class II obesity or greater at baseline (ie, BMI ≥35) (8), LS mean percentage weight losses at week 108 were significantly greater for PHEN/TPM CR compared with placebo (P < 0.0001; ITT-LOCF): –3.4%, –10.1%, and –12.6% for placebo, 7.5/46, and 15/92 treatment arms, respectively.
In a prespecified subgroup analysis of weight loss in subjects with T2D at baseline, the PHEN/TPM CR–treated subjects also showed greater weight loss when compared with placebo-treated subjects. At week 108, subjects with T2D in the placebo group (n = 55) lost 2.0% of their body weight, whereas subjects with T2D in both the 7.5/46 (n = 26) and 15/92 (n = 64) groups lost 9.0% of their body weight (P = 0.0003 for 7.5/46 and P < 0.0001 for 15/92 compared with placebo).
Changes in weight-related comorbidities
Consistent with study entry criteria, enrolled subjects were generally affected by cardiometabolic disease. Many subjects were receiving medications to control blood pressure, lipid variables, and glucose concentrations, and all subjects were actively managed throughout the trial to control these comorbidities. Blood pressure in subjects with hypertension was generally well controlled with ≥2 antihypertensive medications, the most common of which were angiotensin-converting enzyme inhibitors, thiazide diuretics, and selective β-blockers. More than one-third of subjects with dyslipidemia were receiving statins; specifically, 78 (34.4%), 58 (37.9%), and 93 (31.5%) subjects randomly assigned to the placebo, 7.5/46, and 15/92 subgroups, respectively, were taking statins. The second most common lipid-controlling treatment was fish oil or a specific omega-3 formulation. Subjects with diabetes (n = 145) were required to be managed with a regimen of diet and exercise or metformin monotherapy; 38 (69.1%) subjects with T2D who were randomly assigned to placebo, 15 (57.7%) who were randomly assigned to 7.5/46, and 39 (60.9%) were randomly assigned to 15/92 used metformin during the SEQUEL study. Therefore, to assess effects of PHEN/TPM CR, changes in both cardiometabolic disease variables and medication requirements were assessed.
Blood pressure
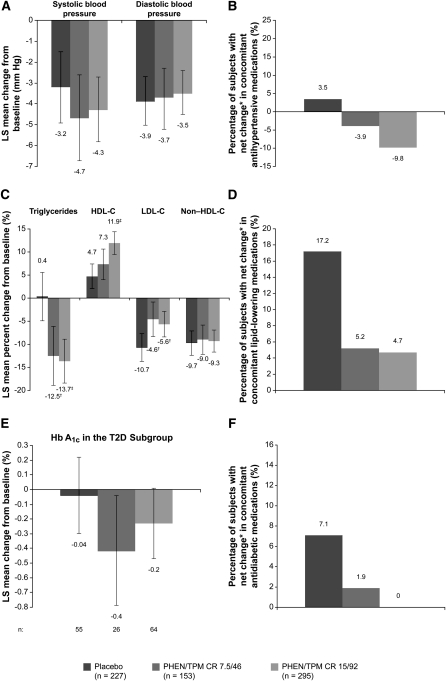
Both systolic and diastolic blood pressure decreased by 3–5 mm Hg at 108 wk compared with baseline in all treatment arms (P < 0.0001 for all comparisons compared with baseline except for placebo compared with baseline for systolic blood pressure, P = 0.0002; NS for PHEN/TPM CR groups compared with placebo; Figure 4A). Although the degree of blood pressure reduction did not differ significantly between treatment arms, subjects randomly assigned to placebo experienced a net increase in the number of antihypertensive medications used, whereas there was a net decrease in the number of medications in subjects receiving 7.5/46 or 15/92 (Figure 4B). Specifically, 7.5% (n = 17) of subjects in the placebo group experienced a decrease in concomitant antihypertensive medication use compared with 13.1% (n = 20) in the 7.5/46 group and 15.6% (n = 46) in the 15/92 group. At the same time, more subjects receiving placebo experienced an increase in antihypertensive medication use than did subjects treated with PHEN/TPM CR: 11.0% (n = 25), 9.2% (n = 14), and 5.8% (n = 17) for the placebo, 7.5/46, and 15/92 groups, respectively.
FIGURE 4.
Effects of PHEN/TPM CR on cardiometabolic variables. LS mean changes (95% CI) in (A) blood pressure, (B) antihypertensive medications, (C) lipid variables, (D) lipid-lowering medications, (E) Hb A1c, and (F) antidiabetic medications from baseline (week 0) to week 108 (ITT-LOCF). Changes in Hb A1c represent the T2D subgroup. Changes in concomitant medications represent the safety study. Standardized lifestyle intervention was used across all treatment groups. *Percentage increase minus percentage decrease; P < 0.05 for between-group differences. †P < 0.01 compared with placebo; ‡P < 0.0001 compared with placebo. Hb A1c, glycated hemoglobin; HDL-C, HDL cholesterol; ITT, intent-to-treat; LDL-C, LDL cholesterol; LOCF, last observation carried forward; LS, least-squares; PHEN/TPM CR 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; PHEN/TPM CR 15/92, 15 mg phentermine/92 mg controlled-release topiramate; T2D, type 2 diabetes.
Lipid variables
Treatment with 7.5/46 and 15/92 led to progressively greater reductions in triglycerides and greater increases in HDL cholesterol than did placebo, despite the fact that the placebo group required a markedly greater net increase in the number of lipid-lowering medications used compared with the PHEN/TPM CR groups. LDL cholesterol decreased in all treatment arms, with the greatest reduction in the placebo group, whereas reduction in non–HDL cholesterol was similar in all groups (Figure 4, C and D). Similar to the changes seen in concomitant antihypertensive medication use, more subjects receiving PHEN/TPM CR had a decrease in lipid-lowering medications than did subjects receiving placebo: 3.1% (n = 7), 5.9% (n = 9), and 5.8% (n = 17) for the placebo, 7.5/46, and 15/92 groups, respectively. Conversely, 20.3% (n = 46) of placebo-treated subjects increased lipid-lowering medication use compared with 11.1% (n = 17) in the 7.5/46 group and 10.5% (n = 31) in the 15/92 group.
Glycemic variables
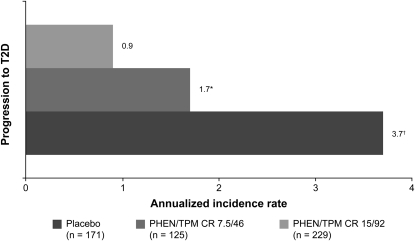
PHEN/TPM CR treatment was associated with beneficial effects on glucose homeostasis at 108 wk (Table 2). When compared with placebo, treatment with PHEN/TPM CR reduced both fasting glucose and fasting insulin concentrations, which is indicative of an improvement in insulin sensitivity (23). In subjects without T2D at baseline, the favorable effects of weight loss on insulin sensitivity and glycemia were associated with decreased progression to T2D during the 2-y course of this study. The annualized incidence rates for progression to T2D among subjects without diabetes at baseline were 3.7%, 1.7%, and 0.9% in the placebo, 7.5/46, and 15/92 treatment groups, respectively. These data indicate a 54% reduction in the progression to T2D in subjects receiving 7.5/46 and a 76% reduction in subjects taking 15/92 compared with placebo (Figure 5).
TABLE 2.
Effects on glucose homeostasis at week 108 (ITT-LOCF)1
| Standardized lifestyle intervention across all treatment groups |
|||
| PHEN/TPM CR |
|||
| Placebo (n = 227) | 7.5/46 (n = 153) | 15/92 (n = 295) | |
| Fasting glucose (mg/dL) | |||
| Baseline | 109.3 ± 24.372 | 110.7 ± 25.28 | 108.2 ± 24.05 |
| LS change at 108 wk | 3.7 (0.8, 6.5)3 | 0.1 (−3.4, 3.7) | −1.2 (−3.8, 1.4) |
| P value vs placebo | N/A | 0.0872 | 0.0048 |
| Fasting insulin (μIU/mL) | |||
| Baseline | 17.5 ± 12.01 | 16.8 ± 12.25 | 17.7 ± 14.61 |
| LS change at 108 wk | −2.6 (−3.9, −1.3) | −5.3 (−6.9, −3.7) | −5.2 (−6.4, −4.0) |
| P value vs placebo | N/A | 0.0051 | 0.0012 |
| Hb A1c (%) | |||
| Baseline | 6.0 ± 0.90 | 6.0 ± 0.90 | 6.0 ± 0.85 |
| LS change at 108 wk | 0.2 (0.09, 0.2) | 0.01 (−0.08, 0.1) | 0.00 (−0.07, 0.07) |
| P value vs placebo | N/A | 0.0042 | 0.0003 |
Values represent changes from baseline (week 0) to week 108 (ITT-LOCF). Hb A1c, glycated hemoglobin; ITT, intent-to-treat; LOCF, last observation carried forward; LS, least-squares; N/A, not available; PHEN/TPM CR, controlled-release phentermine/topiramate; 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; 15/92, 15 mg phentermine/92 mg controlled-release topiramate.
Mean ± SD (all such values).
Mean; 95% CI in parentheses (all such values).
FIGURE 5.
Annualized incidence rate for progression to T2D. Data represent subjects without T2D at baseline. Standardized lifestyle intervention was used across all treatment groups. *P = 0.1514 compared with placebo; †P = 0.0078 compared with placebo. PHEN/TPM CR 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; PHEN/TPM CR 15/92, 15 mg phentermine/92 mg controlled-release topiramate; T2D, type 2 diabetes.
When only subjects with T2D at baseline were considered, the initial mean Hb A1c at week 0 was similar in each of the 3 treatment groups (6.9% in subjects randomly assigned to placebo, 7.3% in the 7.5/46 treatment arm, and 6.9% in the 15/92 treatment arm). At 108 wk, Hb A1c did not substantially change from baseline in the placebo group (0%), whereas treatment with 7.5/46 and 15/92 led to reductions of 0.4% and 0.2%, respectively (Figure 4E). In these actively managed subjects, these reductions in Hb A1c were achieved without any net increase in antidiabetic medications in the 15/92 group [3.1% (n = 9) experienced a decrease and 3.1% (n = 9) experienced an increase] and a small net increment in the 7.5/46 group [0.7% (n = 1) decreased and 2.6% (n = 4) increased use] compared with larger net increases in medications required in the placebo group [1.3% (n = 3) decreased and 8.4% (n = 19) increased use; Figure 4F].
Analysis of all primary and secondary variables by using multiple imputation instead of LOCF to accommodate for dropout yielded results consistent with those presented above (see Supplemental Tables 1 and 2 under “Supplemental data” in the online issue). Similar results were also observed in the set of subjects who completed the study while still taking the study drug (see Supplemental Tables 3 and 4 under “Supplemental data” in the online issue).
Safety and tolerability
AEs
PHEN/TPM CR was well tolerated over 108 wk, as shown by the TEAEs (Table 3). The most commonly reported TEAEs were upper respiratory tract infection, constipation, paraesthesia, sinusitis, and dry mouth. The type of TEAEs occurring between weeks 56 and 108 were similar to those reported in the overall CONQUER sample from weeks 0 to 56 (17). However, as delineated in Table 3, the incidence of individual TEAEs was markedly lower in the second year (weeks 56–108) than in the first year (weeks 0–56). The incidence of SAEs from weeks 0 to 108 was 6.2% for placebo, 5.9% for 7.5/46, and 8.1% for 15/92. The incidence of SAEs during the period of study extension (weeks 56–108) was also similar across treatment groups: 4.0%, 2.6%, and 4.1% for placebo, 7.5/46, and 15/92, respectively—none of which was reported by investigators to be related to study treatment. The percentage of subjects discontinuing due to AEs by week 108 was also similar across treatment groups: 3.1%, 4.5%, and 4.4% of subjects in the placebo, 7.5/46, and 15/92 arms, respectively (Figure 1). There were no deaths in the extension study. During SEQUEL, there were 2 pregnancies, one carried to term in the 15/92 group and one resulting in miscarriage at ~6 wk gestation in the placebo group. The pregnancy that was carried to full term resulted in a healthy macrosomic infant with no observed teratogenic effects.
TABLE 3.
All adverse events with frequency of ≥5% in any PHEN/TPM CR group1
| Standardized lifestyle intervention across all treatment groups |
||||||
| Weeks 0–56 |
Weeks 56–108 |
|||||
| PHEN/TPM CR |
PHEN/TPM CR |
|||||
| Placebo(n = 227) | 7.5/46(n = 153) | 15/92(n = 295) | Placebo(n = 227) | 7.5/46(n = 153) | 15/92(n = 295) | |
| n (%) | n (%) | |||||
| Constipation | 16 (7.1) | 25 (16.3) | 62 (21.0) | 7 (3.1) | 11 (7.2) | 12 (4.1) |
| Paraesthesia | 6 (2.6) | 21 (13.7) | 62 (21.0) | 0 (0.0) | 1 (0.7) | 10 (3.4) |
| Dry mouth | 5 (2.2) | 21 (13.7) | 59 (20.0) | 1 (0.4) | 1 (0.7) | 4 (1.4) |
| Upper respiratory tract infection | 47 (20.7) | 23 (15.0) | 55 (18.6) | 42 (18.5) | 26 (17.0) | 45 (15.3) |
| Nasopharyngitis | 35 (15.4) | 20 (13.1) | 39 (13.2) | 26 (11.5) | 13 (8.5) | 26 (8.8) |
| Dysgeusia | 4 (1.8) | 18 (11.8) | 39 (13.2) | 0 (0.0) | 1 (0.7) | 3 (1.0) |
| Sinusitis | 19 (8.4) | 17 (11.1) | 39 (13.2) | 18 (7.9) | 12 (7.8) | 28 (9.5) |
| Headache | 21 (9.3) | 8 (5.2) | 28 (9.5) | 6 (2.6) | 4 (2.6) | 12 (4.1) |
| Insomnia | 15 (6.6) | 12 (7.8) | 24 (8.1) | 8 (3.5) | 9 (5.9) | 11 (3.7) |
| Diarrhea | 12 (5.3) | 14 (9.2) | 21 (7.1) | 3 (1.3) | 3 (2.0) | 11 (3.7) |
| Back pain | 19 (8.4) | 11 (7.2) | 21 (7.1) | 7 (3.1) | 9 (5.9) | 15 (5.1) |
| Dizziness | 6 (2.6) | 9 (5.9) | 20 (6.8) | 2 (0.9) | 2 (1.3) | 1 (0.3) |
| Nausea | 13 (5.7) | 5 (3.3) | 19 (6.4) | 4 (1.8) | 10 (6.5) | 4 (1.4) |
| Bronchitis | 8 (3.5) | 9 (5.9) | 17 (5.8) | 7 (3.1) | 8 (5.2) | 10 (3.4) |
| Fatigue | 11 (4.9) | 7 (4.6) | 17 (5.8) | 2 (0.9) | 2 (1.3) | 4 (1.4) |
| Procedural pain | 6 (2.6) | 7 (4.6) | 17 (5.8) | 4 (1.8) | 8 (5.2) | 14 (4.7) |
| Arthralgia | 20 (8.8) | 13 (8.5) | 13 (4.4) | 14 (6.2) | 7 (4.6) | 16 (5.4) |
| Influenza | 11 (4.9) | 11 (7.2) | 13 (4.4) | 8 (3.5) | 10 (6.5) | 19 (6.4) |
| Urinary tract infection | 11 (4.9) | 8 (5.2) | 13 (4.4) | 13 (5.7) | 14 (9.2) | 18 (6.1) |
| Gastroenteritis | 12 (5.3) | 3 (2.0) | 12 (4.1) | 6 (2.6) | 2 (1.3) | 9 (3.1) |
Preferred terms were defined by the MedDRA Coding Dictionary, version 10.1 (26). PHEN/TPM CR, controlled-release phentermine/topiramate; 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; 15/92, 15 mg phentermine/92 mg controlled-release topiramate.
Physical examination and laboratory variables
The reductions in blood pressure at week 108 (Figure 4A) were accompanied by a mean increase in heart rate of 0.4 bpm in placebo subjects, 1.3 bpm in 7.5/46 subjects, and 1.7 bpm in 15/92 subjects; there were no AEs reported that were relevant to changes in heart rate, and there were no associated adverse clinical sequelae. No dose-related changes were observed in shift summaries of selected laboratory variables, and no subjects experienced an SAE or discontinued the study drug due to laboratory abnormalities. At week 108, there was a greater increase in serum bicarbonate with placebo than with PHEN/TPM CR. The mean changes in bicarbonate from baseline to week 108 were 2.2, 0.7, and 0.2 mEq/L for placebo, 7.5/46, and 15/92, respectively (baseline mean bicarbonate concentration was 26.5 mEq/L in all 3 treatment groups). Although absolute changes were small, more subjects treated with PHEN/TPM CR experienced a decrease from baseline in serum bicarbonate of >5 mEq/L at 2 consecutive visits during the 2-y study course than did those in the placebo group; this was observed in 4 subjects (1.8%) in the placebo-treated group, 20 (13.1%) in the 7.5/46-treated group, and 48 (16.3%) in the 15/92-treated group over 108 wk. When looking only at weeks 56–108, 7 (4.6%) subjects in the 7.5/46 group and 12 (4.1%) subjects in the 15/92 group were observed to have a >5-mEq/L decrease in serum bicarbonate from baseline compared with 0 (0%) in the placebo arm. The decreases in bicarbonate generally occurred during the first 3 mo of CONQUER, did not require clinical intervention, and were not progressive during the 2-y study, tending to normalize over time.
Psychiatric effects and suicidal behavior
There was no increase in serious suicidal ideation or suicidal behavior based on the C-SSRS questionnaire during the 108 wk of observation in subjects treated with PHEN/TPM CR. Six subjects responded “yes” to the C-SSRS categories of suicidal ideation and suicidality (3 placebo-treated subjects, 1 7.5/46-treated subject, and 2 15/92-treated subjects, but all were below the threshold for “serious” as defined by the instrument). There were 5 subjects with worsening suicidal ideation (2 in the placebo-, 1 in the 7.5/46-, and 2 in the 15/92-treated groups). During the 2-y period, the incidence of reported anxiety-related AEs correlated with increasing dose: 3.1%, 6.5%, and 9.5% for placebo, 7.5/46, and 15/92 arms, respectively. Most anxiety events were mild in severity. Three subjects in the 15/92 group experienced a severe anxiety-related TEAE, with one subject discontinuing treatment. There were no anxiety-related SAEs. The occurrence of depression-related TEAEs was comparable in the placebo (7.9%) and 15/92 (8.1%) groups and occurred at a lower rate in the 7.5/46 group (3.9%).
DISCUSSION
The previously published CONQUER study reported that treatment of overweight and obese adults with PHEN/TPM CR as an adjunct to lifestyle intervention promoted weight loss and reduced manifestations of cardiometabolic disease over 56 wk when compared with lifestyle intervention plus placebo (17). This extension study, SEQUEL, maintained the blinded treatment groups for an additional 52 wk and showed that these beneficial therapeutic effects were sustained during a 2-y period.
After 108 wk, the addition of PHEN/TPM CR to a standardized lifestyle modification led to substantial weight loss that coincides with the weight-loss target of 10% recommended by the National Heart, Lung, and Blood Institute for overweight and obese individuals (8). The percentage changes in body weight from baseline were –1.8%, –9.3%, and –10.5% in subjects treated with placebo, 7.5/46, and 15/92, respectively (ITT-LOCF); and 10% weight loss was achieved by >50% of PHEN/TPM CR–treated subjects, whereas <12% of subjects receiving placebo met this goal. Importantly, both doses of PHEN/TPM CR were significantly more effective than placebo regardless of baseline BMI and were similarly effective at baseline BMI values extending from <30 to <40. In those subjects with class III obesity (BMI ≥40), the 15/92 dose produced an even more pronounced degree of weight loss, exceeding that observed with 7.5/46. These data are indicative of therapeutic efficacy for PHEN/TPM CR over a wide range of initial BMI, although higher doses might be more effective in cases of more severe obesity.
By design, subjects in the SEQUEL study were highly affected by cardiometabolic disease, and many were treated with numerous concomitant medications to control blood pressure, lipid variables, and glycemic variables, which were actively managed throughout the trial. PHEN/TPM CR improved these comorbidities and decreased the need for associated medications in comparison with the placebo group. For example, after 2 y of therapy, diastolic and systolic blood pressure showed equal reductions in the placebo and PHEN/TPM CR groups; however, this was accompanied by a net decrease in concomitant antihypertensive medication use in PHEN/TPM CR treatment groups, whereas antihypertensive medications were increased in the placebo group. Reducing the need for medications used to specifically control these comorbidities not only reduces the medication burden associated with cardiometabolic disease but could also improve subject compliance by decreasing their medication regimen complexity and reducing the overall treatment costs (24, 25).
When compared with placebo, PHEN/TPM CR–treated subjects exhibited lower fasting glucose and fasting insulin values compared with subjects receiving placebo, which is indicative of an improvement in insulin sensitivity (23), and experienced greater reductions in waist circumference, a measure of central adiposity related to increased morbidity and mortality (21, 22, 27). Because insulin resistance and central adiposity are integral to the development of cardiometabolic disease, it appears that PHEN/TPM CR–induced weight loss is accompanied by favorable effects on pathophysiologic processes that could reduce risk of metabolic syndrome, T2D, and CVD (8, 21, 22). In support of this, we observed that, among subjects without T2D at baseline, treatment with PHEN/TPM CR reduced progression to T2D by 54% with the 7.5/46 dose and by 76% with the 15/92 dose when compared with the placebo intervention. Previous studies have shown that lifestyle-intervention programs reduce progression to T2D among high-risk individuals with impaired glucose tolerance, and the degree of protection correlated with the amount of weight loss (28–31). Along these same lines, incremental improvements in cardiometabolic disease risk factors have also been shown to correlate with an increasing degree of weight loss, together with associated reductions in morbidity and mortality (5, 32, 33).
Approximately 20% of subjects had T2D at study entry and had been treated with lifestyle modifications alone, single-agent metformin, or both. In the T2D subgroup, PHEN/TPM CR led to significant reductions in Hb A1c after 2 y compared with placebo. Improvements in fasting glucose, fasting insulin, and Hb A1c were experienced without any net change in concomitant antidiabetic medications in subjects with T2D who were randomly assigned to 15/92 and a modest net increase in medications in those assigned to 7.5/46, whereas the placebo group experienced a substantial net increase in required antidiabetic medications to achieve guideline-dictated goals. Thus, weight loss associated with PHEN/TPM CR had a favorable impact on glycemic control in the subjects with T2D, without a need for added oral hypoglycemic agents.
Discontinuation rates during the extension study were similar between placebo and PHEN/TPM CR–treated subjects. The type of AEs reported during weeks 56–108 in the extension study were similar to those in the first 56 wk of the study; however, the incidence rates were lower in the second year of the study. In some subjects, PHEN/TPM CR treatment was associated with reductions in serum bicarbonate, particularly in the first 3 mo of the study, which is likely a manifestation of the carbonic anhydrase activity of topiramate. However, this effect was not progressive in these subjects, and serum bicarbonate tended to return toward normal during the remaining 2 y of the study without the need for clinical intervention. PHEN/TPM CR increased mean heart rate by 1.3–1.7 bpm over baseline; this increase was not accompanied by any related AE reporting and was accompanied by reductions in systolic and diastolic blood pressure. Rigorous assessments of suicidality were conducted by using the C-SSRS, which showed no increase in suicidal ideation associated with PHEN/TPM CR and no difference from placebo in AE reporting for incident depression. PHEN/TPM CR was associated with a dose-dependent increase in the incidence of anxiety. However, these anxiety-related TEAEs were mostly mild in nature and led to only one discontinuation of study drug. Finally, during the 108-wk trial, there were 2 pregnancies, with one pregnancy carried to term in a subject who was randomly assigned to 15/92, resulting in a healthy infant without any observed congenital malformations.
Limitations of this study were that not all CONQUER subjects were eligible to enroll into the SEQUEL extension because only high-enrolling centers were used. Furthermore, participation in the second-year extension study was optional. These aspects may have resulted in bias toward the inclusion of subjects with positive treatment outcomes, because one would expect that subjects who achieved satisfactory weight loss would be more likely to enroll for a second year. However, the baseline clinical characteristics of the subgroup entering the SEQUEL study were similar to those of the CONQUER cohort, with the exception of a greater percentage of subjects with T2D in SEQUEL. Another point pertains to the higher rate of subjects lost to follow-up in the 15/92 arm than in the placebo or 7.5/46 arms. Because the real reason for study discontinuation in these subjects is not known, this could result in an underestimation of important reasons for patients dropping out, including AEs or lack of efficacy. A third limitation of this study is that hyperglycemia, high blood pressure, and dyslipidemia were actively managed on the basis of national treatment guidelines, resulting in the confounding impact of medication changes on the secondary cardiometabolic variables. Actual treatment effects may have been different if related medications and doses were required to be fixed, thereby better isolating an effect of PHEN/TPM CR.
Medical options to promote sustained weight loss are limited. The lipase inhibitor orlistat is currently the only approved medication for chronic treatment of obesity in the United States (10). Bariatric surgery is an option for long-term weight loss but is generally limited to selected subjects with complicated or severe obesity because of the inherent risks of invasive surgical procedures and the requirements for long-term medical follow-up (9, 10, 34, 35). Results of this study suggest that PHEN/TPM CR together with lifestyle measures may be an additional therapeutic option for achieving long-term weight-loss in moderately to severely obese subjects.
In conclusion, PHEN/TPM CR used as an adjunct to lifestyle intervention for the treatment of obesity was well tolerated and produced significant, dose-related weight loss that was maintained during a 108-wk period. PHEN/TPM CR was also associated with sustained improvements in the clinical manifestations of weight-related cardiometabolic disease, including hyperglycemia, dyslipidemia, and elevated blood pressure, despite reduced use of concomitant medications. Importantly, these effects of PHEN/TPM CR led to a reduction in the rate of progression to T2D, with the greatest benefits seen in subjects receiving PHEN/TPM CR 15/92. Thus, PHEN/TPM CR may provide a well-tolerated, effective, and sustainable treatment option for obese subjects with cardiometabolic disease. Furthermore, the unmet clinical need for effective weight-loss medications, together with the favorable risk-benefit profile in the current study, suggests that PHEN/TPM CR in conjunction with a lifestyle-intervention program could be a valuable therapeutic approach to counteract increasing rates of obesity and its related complications.
Supplementary Material
Acknowledgments
We acknowledge and thank the SEQUEL subjects, investigators, and study coordinators; the Medpace team (study Contract Research Organization); The Lockwood Group (for manuscript development assistance); and Vivus Inc internal contributors.
The authors’ responsibilities were as follows—KMG, DBA, DHR, MS, CAP, WWD, and CHB: designed the research; WTG, KMG, and ML: conducted the research; WTG, KMG, DBA, DHR, CAP, MS, WWD, and CHB: analyzed and interpreted data; WTG, DHR, ML, KMG, DBA, CAP, MS, WWD, and CHB: drafted and edited the manuscript; and MS: had primary responsibility for final content and is the guarantor of the manuscript, having had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. The external authors as well as those employed by the funding sponsor participated in protocol design, data analyses, interpretation, and preparation of the manuscript. WTG participated in clinical trials with Merck & Co, Inc; Amylin Pharmaceuticals; Vivus Inc; Abbott; and Daiichi-Sankyo. He has served as an advisor, consultant, and/or speaker for Abbott Nutrition, Daiichi-Sankyo, Johnson & Johnson, LipoScience, Tethys, and Vivus Inc. He is a scientific advisory board member for Daiichi-Sankyo and Tethys, and he holds stock in Bristol-Myers Squibb; Isis/Genzyme; Merck & Co, Inc; Pfizer Inc; Novartis Pharmaceuticals Corp; and Vivus Inc. DHR served as a consultant for Vivus but did not receive any payment for service. ML is a scientific advisory board member for Vivus Inc. KMG received research support from Forest Laboratories, the National Institute of Diabetes and Digestive and Kidney Diseases, and Vivus Inc and owns stock in Orexigen Therapeutics. DBA received grants, honoraria, donations, and consulting fees from numerous food, beverage, and pharmaceutical companies, as well as other commercial and nonprofit entities with interests in obesity, including but not limited to Arena Pharmaceuticals, Jason Pharmaceuticals Inc, Pfizer Inc, and Vivus Inc. He served as a board member for the Pfizer Inc VPO program. WWD, CHB, and CAP are employees of Vivus Inc. MS was employed as the lead statistician for Medpace Inc, the study Contract Research Organization, throughout the study design, execution, and analysis; he had no additional conflicts to disclose.
Footnotes
Abbreviations used: AE, adverse event; bpm, beats per minute; C-SSRS, Columbia Suicide Severity Rating Scale; CVD, cardiovascular disease; Hb A1c, glycated hemoglobin; ITT, intent-to-treat; LOCF, last observation carried forward; LS, least-squares; PHEN/TPM CR, controlled-release phentermine/topiramate; SAE, serious adverse event; TEAE, treatment-emergent adverse event; T2D, type 2 diabetes; 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; 15/92, 15 mg phentermine/92 mg controlled-release topiramate.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 2.Pi-Sunyer X. The medical risks of obesity. Postgrad Med 2009;121:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaet D, Schauer D. Obesity in adults. Clin Evid (Online) 2011. Available from: http://clinicalevidence.bmj.com/ceweb/index.jsp (cited 10 October 2011) [Google Scholar]
- 4.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7 [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007;298:2028–37 [DOI] [PubMed] [Google Scholar]
- 6.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH; American Heart Association Council on Nutrition, Physical Activity, and Metabolism Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 2004;110:2952–67 [DOI] [PubMed] [Google Scholar]
- 7.Logue J, Murray HM, Welsh P, Shepherd J, Packard C, Macfarlane P, Cobbe S, Ford I, Sattar N. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart 2011;97:564–8 [DOI] [PubMed] [Google Scholar]
- 8.National Heart, Lung, and Blood Institute, NIH, US Department of Health and Human Services Identification, evaluation, and treatment of overweight and obesity in adults. Washington, DC: National Heart, Lung, and Blood Institute, 2000. (NIH publication no. 00-4048.) [Google Scholar]
- 9.Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev 2009;CD003641 [DOI] [PubMed] [Google Scholar]
- 10.Hussain SS, Bloom SR. The pharmacological treatment and management of obesity. Postgrad Med 2011;123:34–44 [DOI] [PubMed] [Google Scholar]
- 11.Melnikova I, Wages D. Anti-obesity therapies. Nat Rev Drug Discov 2006;5:369–70 [DOI] [PubMed] [Google Scholar]
- 12.Bray GA, Hollander P, Klein S, Kushner R, Levy B, Fitchet M, Perry BH. A 6-month randomized, placebocontrolled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res 2003;11:722–33 [DOI] [PubMed] [Google Scholar]
- 13.Rosenstock J, Hollander P, Gadde KM, Sun X, Strauss R, Leung A. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care 2007;30:1480–6 [DOI] [PubMed] [Google Scholar]
- 14.Tonstad S, Tykarski A, Weissgarten J, Ivleva A, Levy B, Kumar A, Fitchet M. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol 2005;96:243–51 [DOI] [PubMed] [Google Scholar]
- 15.Toplak H, Hamann A, Moore R, Masson E, Gorska M, Vercruysse F, Sun X, Fitchet M. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2007;31:138–46 [DOI] [PubMed] [Google Scholar]
- 16.Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord 2004;28:1399–410 [DOI] [PubMed] [Google Scholar]
- 17.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341–52 [DOI] [PubMed] [Google Scholar]
- 18.Brownell K. The LEARN program for weight management. Dallas, TX: The Life Style Company, 2000 [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes—2007. Diabetes Care 2007;30(suppl 1):S4–41 [PubMed] [Google Scholar]
- 20.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(suppl 1):S11–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5 [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. ; American Heart Association; National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52 [DOI] [PubMed] [Google Scholar]
- 23.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–607 [DOI] [PubMed] [Google Scholar]
- 24.Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120:713–9 [DOI] [PubMed] [Google Scholar]
- 25.Petrilla AA, Benner JS, Battleman DS, Tierce JC, Hazard EH. Evidence-based interventions to improve patient compliance with antihypertensive and lipid-lowering medications. Int J Clin Pract 2005;59:1441–51 [DOI] [PubMed] [Google Scholar]
- 26.Medical Dictionary for Regulatory Activities. Available from: http://www.meddramsso.com/ (cited 10 December 2010) [Google Scholar]
- 27.Leitzmann MF, Moore SC, Koster A, Harris TB, Park Y, Hollenbeck A, Schatzkin A. Waist circumference as compared with body-mass index in predicting mortality from specific causes. PLoS ONE 2011;6:e18582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The DaQing IGT and Diabetes Study. Diabetes Care 1997;20:537–44 [DOI] [PubMed] [Google Scholar]
- 30.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50 [DOI] [PubMed] [Google Scholar]
- 31.Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J; Finnish Diabetes Prevention Study Group The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–6 [DOI] [PubMed] [Google Scholar]
- 32.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L; Look AHEAD Research Group Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzotzas T, Evangelou P, Kiortsis DN. Obesity, weight loss and conditional cardiovascular risk factors. Obes Rev 2011;12:e282–9 [DOI] [PubMed] [Google Scholar]
- 34.DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med 2007;356:2176–83 [DOI] [PubMed] [Google Scholar]
- 35.International Diabetes Federation Consensus Panel Bariatric surgical and procedural interventions in the treatment of obese patients with type 2 diabetes: a position statement from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Available from: http://www.sicob.org/00_materiali/IDF_Position_Statement.pdf (cited 10 October 2011)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.