Abstract
Background
The role of human papillomavirus (HPV) in the causation of esophageal squamous cell carcinoma is unclear. We examined the associations between esophageal squamous cell carcinoma and 28 centrally measured HPV serological markers in serum from six existing case–control studies conducted in regions with differing background risks of esophageal cancer.
Methods
We used centralized multiplex serology to test serum samples from 1561 case subjects and 2502 control subjects from six case–control studies for antibodies to the major HPV capsid protein (L1) and/or the early proteins E6 and/or E7 of eight high-risk, two low-risk, and four cutaneous HPV types. Study-specific odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated using conditional logistic regression with adjustment for smoking, alcohol consumption, and other potential confounders. Pooled odds ratios and 95% confidence intervals were calculated using either a linear mixed-effects approach or a joint fixed-effects approach. All statistical tests were two-sided.
Results
We found statistically significant associations between esophageal squamous cell carcinoma and antibodies to E6 for HPV16 (OR = 1.89, 95% CI = 1.09 to 3.29, P = .023) and HPV6 (OR = 2.53, 95% CI = 1.51 to 4.25, P < .001) but not for other tested HPV types. There were no statistically significant associations between esophageal squamous cell carcinoma and antibodies to E7 for any of the tested HPV types. Simultaneous seropositivity for HPV16 E6 and E7 was rare (four case subjects, two control subjects; OR = 5.57, 95% CI = 0.90 to 34.35; P = .064). We also found statistically significant associations between esophageal squamous cell carcinoma and capsid antibodies for the high-risk mucosal type HPV33 L1 (OR = 1.30, 95% CI = 1.00 to 1.69; P = .047) and the low-risk mucosal types HPV6 (OR = 1.22, 95% CI = 1.05 to 1.42; P = .010) and HPV11 (OR = 1.30, 95% CI = 1.09 to 1.56, P = .0036).
Conclusions
We found limited serological evidence of an association between esophageal squamous cell carcinoma and HPV in the populations studied. Although HPV does not appear to be an important risk factor for esophageal squamous cell carcinoma, we cannot exclude the possibility that certain HPV types may be involved in a small subset of cancers.
CONTEXT AND CAVEATS
Prior knowledge
Infection with oncogenic human papillomavirus (HPV) types has been linked to various cancers, including cancers of the head and neck. However, the role of HPV in the causation of esophageal squamous cell carcinoma is unclear.
Study design
Centralized multiplex serology was applied to serum samples from 1561 case subjects and 2502 control subjects from six case–control studies to detect circulating antibodies against 28 HPV antigens (18 L1, E6, or E7 antigens from the eight high-risk mucosal HPV types, including HPV16 and HPV33; six L1, E6, or E7 antigens from the two prevalent low-risk mucosal HPV types, HPV6 and HPV11; and four L1 antigens from cutaneous HPV types).
Contribution
There were only a limited number of nominally statistically significant associations between esophageal squamous cell carcinoma and seropositivity for HPV16 E6, HPV6 E6, HPV33 L1, HPV6 L1, and HPV11 L1.
Implications
The limited serological evidence for an association between esophageal squamous cell carcinoma and HPV in the populations studied suggests that HPV is not an important risk factor for esophageal squamous cell carcinoma.
Limitations
Inflation of the type I error rate for observing one or more false statistically significant test results among all tests performed was likely because the analyses were not adjusted for multiple comparisons. The results of the contributing case–control studies are susceptible to reverse causation. Some study-specific and/or general confounders may not have been adequately adjusted for in this analysis. Differences in the rates of undiagnosed cervical cancers between case subjects and control subjects could confound estimates of the associations between seropositivity to the E6 or E7 proteins of the high-risk mucosal HPV types and esophageal squamous cell carcinoma for women.
From the Editors
Cancer of the esophagus was the eighth most frequently occurring type of cancer in 2008, with an estimated 481 400 new diagnoses (1). In the same year, there were an estimated 406 000 deaths from the disease, making it the sixth most common cancer cause of death (1). Despite increasing rates of esophageal adenocarcinomas in many Western countries, squamous cell carcinoma of the esophagus remains the dominant histological type of esophageal cancer worldwide and thus is the focus of this study.
Tobacco smoking and alcohol consumption are considered causal for esophageal squamous cell carcinoma, particularly in developed countries, where exposure to both of these factors has been shown to increase the risk greatly, sometimes multiplicatively (2–4). In certain geographic regions with a high incidence of esophageal squamous cell carcinoma, most notably in developing countries, the risk of esophageal squamous cell carcinoma has been found to be associated with local factors, such as poor socioeconomic conditions (5,6); diets low in fruits, vegetables, and specific micronutrients such as niacin, vitamins A and C, magnesium, and riboflavin (7); low serum selenium levels in China (8); consumption of hot tea in South America (9) and Iran (10); the use of certain traditional medicinal plants (eg, Solanum nigrum) in Transkei, South Africa (11); and consumption of specific opiates (12,13). However, the lack of known prevalent strong risk factors for esophageal squamous cell carcinoma in many high-incidence regions suggests that additional important risk factors are yet to be identified.
One possible risk factor for esophageal squamous cell carcinoma is infection with oncogenic human papillomavirus (HPV) types. HPV type 16 (HPV16) is known to cause the majority of squamous cell carcinomas of the cervix (14–17) and is strongly associated with subgroups of squamous cell carcinomas at other anogenital sites (18,19) and with cancers of the head and neck, particularly the oropharynx (20–22). The possibility that HPV might play an etiologic role in the development of esophageal squamous cell carcinoma was first proposed in 1982 by Syrjänen et al. (23,24) based on histological findings that suggested possible associations between HPV and both malignant and benign squamous cell lesions of the esophagus. Since then, although more than 100 studies have investigated the relationship between esophageal squamous cell carcinoma and HPV, the evidence of an association is inconclusive (25). Arguments in support of an association include 1) the detection of HPV DNA in esophageal squamous cell carcinoma (26); 2) the histological similarities of the oral and esophageal squamous epithelia (27); 3) the proximity of the esophagus and oropharynx and their similar neoplastic responses to smoking and alcohol (26,27); 4) evidence of an association between HPV and bovine esophageal cancer (28); and 5) in vitro transformation of esophageal epithelial cells by HPV (29). Arguments against an association between esophageal squamous cell carcinoma and HPV include the inconsistent conclusions among serological studies of HPV and esophageal squamous cell carcinoma and the wide variations in the prevalence of HPV DNA detected in esophageal squamous cell carcinoma tissue among studies (26).
Serological testing for HPV proteins has been a useful tool for identifying and confirming associations between HPV and various types of squamous cell cancer (16–22). The presence of circulating antibodies to the HPV late capsid protein L1 is considered a marker of cumulative (lifetime) exposure to HPV (30) and high seropositivity for HPV16 L1 has been shown to be associated with increased risks of cancer of the cervix (16,19), the oral cavity (20), and the oropharynx (20,22). A number of serological studies have examined the association between esophageal squamous cell carcinoma and HPV16 L1 or HPV18 L1 antibodies (19,31–36). Although all of those studies measured L1 antibodies using similar methods, the definitions of seropositivity varied among the studies, making comparisons of results between studies difficult. Seropositivity for the E6 and E7 proteins of HPV16 and HPV18 have been shown to be moderately sensitive and highly specific markers for HPV-driven carcinomas of the cervix (17), oropharynx (20,21), and penis (18), and thus appear to be useful markers for identifying patients with HPV-positive tumors. However, the statistical power to detect relatively small underlying associations between specific cancer types and seropositivity for E6 or E7 antibodies is limited in case–control studies because invasive HPV-driven carcinomas are rare in the populations from which control subjects are chosen (17,18,20–22). Thus, it is important to consider the absence of a statistical association between esophageal squamous cell carcinoma and seropositivity to either E6 or E7 for a given HPV type in conjunction with the results for all other serological markers of that HPV type.
The evolution of reliable high-throughput multiplex serological techniques has allowed the simultaneous testing of up to 100 different markers using only 2 μL of serum per sample (37–39). The aim of this collaboration was to examine the associations between the risk of esophageal squamous cell carcinoma and a number of HPV serological markers measured using this technology in serum from existing case–control studies conducted in regions with differing background risks of esophageal cancer, after adjustment for smoking, alcohol consumption, and other potential confounders. To our knowledge, this is the largest study to examine the relationship between esophageal squamous cell carcinoma and HPV antibodies, the first study to compare HPV E6/E7 seroreactivities of esophageal squamous cell carcinoma case subjects with that of control subjects, and the first study to examine the relationships between esophageal squamous cell carcinoma and antibodies to HPV types other than HPV16, HPV18, HPV33, and HPV73 (a very rare type known to be associated with cervical cancer but not examined in this study).
Methods
Selection of Studies
Esophageal squamous cell carcinoma case–control studies that had at least 100 case subjects and 100 control subjects were identified through review of published studies and supplemented by discussions with investigators of as yet unpublished studies. Those that had stored participant serum available for serological HPV antibody testing and that had collected data on the major esophageal squamous cell carcinoma risk factors, including smoking and alcohol consumption, were invited to participate in the InterSCOPE collaboration. Six study groups, from South Africa (19), Australia (40), Central and Eastern Europe (41), Brazil (42), Iran (13), and China (43), agreed to participate and forward their serum samples from histopathologically confirmed esophageal squamous cell carcinoma case subjects as well as serum samples from one or two age- and sex-matched control subjects to the German Cancer Research Center (Deutsches Krebsforschungszentrum [DKFZ]) within a prespecified time frame for serological analysis. A total of 1561 case subjects and 2502 control subjects had complete information on all predetermined study-specific confounders (see “Definitions of Exposures and Confounders” below) and were thus selected for inclusion in this analysis. De-identified questionnaire data and serum results were forwarded to the Cancer Epidemiology Research Unit at Cancer Council NSW for statistical analysis. The participating studies covered a wide range of geographical areas and had estimated age-standardized incidence rates of esophageal carcinoma (standardized to the world standard population) that ranged from approximately 5 per 100 000 in Australia to approximately 50 per 100 000 in Shanxi Province, China (Table 1). All six studies and this pooled analysis were approved by the appropriate national or institutional ethics committees or review boards. Written or witnessed oral informed consent was obtained from participants before interview in South Africa. Written consent was obtained from participants before interview in the five remaining studies.
Table 1.
Characteristics of included case–control studies
| Study name (reference) | Study location | Age- standardized incidence rate per 100 000* (reference) | Recruitment period | No. of case subjects/No. of control subjects | Matching | Source of control subjects | Questionnaire administration | Blood collection, processing, and storage |
| South Africa (19) | South Africa: Johannesburg | 15 (44) | 1995–2006 | QV1†: 290/455 | Category matched; age, serum storage time, sex | Hospital, patients with cancers unrelated to smoking, alcohol, or HPV infection | Face-to-face interviews conducted by trained nurses | Collection shortly after diagnosis and before treatment (all subjects). Processed within 3–8 h at room temperature, then stored at −20°C to −35°C. |
| QV2†: 407/655 | ||||||||
| Total: 697/1110 | ||||||||
| Australia (40) | Australia: country wide | 5 (45) | 2001–2005 | 211/424 | Category matched; age, sex | Population control subjects randomly selected from the electoral roll | Self-completed mailed questionnaires | Collection within 3 mo of diagnosis (case subjects) or interview (control subjects). Processed within 2 d at room temperature and then stored at −20°C to −80°C. |
| Central and Eastern Europe (41) | Romania: Bucharest; Poland: Lodz; Russia: Moscow; Czech Republic: Olomouc, Prague | 6 (9) | 1998–2003 | 157/314 | Directly matched; age, serum storage time, sex, study center | Hospital, patients with conditions unrelated to smoking, alcohol, or HPV infection | Face-to-face interviews | Collection shortly after diagnosis and before treatment (all subjects). Processed within a few hours at room temperature and then stored at −80°C. |
| Brazil (42) | Brazil: Rio, Pelotas, Goiania | 8 (1) | 2000–2003 | 157/314 | Directly matched; age, serum storage time, sex, study center | Hospital, patients with conditions unrelated to smoking, alcohol, or HPV infection | Face-to-face interviews | Collection shortly after diagnosis and before treatment (all subjects). Processed within a few hours at room temperature and then stored at −80°C. |
| Iran (13) | Iran: Eastern Golestan Province | 40 (46) | 2003–2007 | 220/221 | Directly matched; age, sex, neighborhood | Neighborhood control subjects randomly selected from family health census | Face-to-face interviews conducted by trained nurses and physicians | Collection shortly after diagnosis and before treatment (case subjects) or at time of interview (control subjects). Processed within 1 h (case subjects) or 12 h (controls) at 4°C and then stored at −80°C. |
| China (43) | China: Shanxi Province | 50 (47) | 1998–2004 | 119/119 | Directly matched; age, sex, neighborhood | Neighborhood control subjects nominated by matched case subject | Face-to-face interviews conducted by trained nurses | Collection shortly after diagnosis and before treatment (case subjects) or attime of interview (control subjects). Processed within 1–4 d of refrigeration (case subjects) or storage in wet ice (control subjects) and then stored at −80°C. |
| Total | 1561/2502 |
Age-standardized incidence rates of esophageal carcinoma (standardized to the world standard population).
QV1 and QV2 represent questionnaire versions one and two, respectively, of the South African study. Version 2 (after 1998) elicited more detailed information on smoking and alcohol consumption than version 1. In the pooled analyses examining exposure to smoking and alcohol, multilevel smoking and alcohol covariates were used for the subset of South African participants who responded to the latter questionnaire version. In the pooled analyses examining serological marker associations, exposure to alcohol and smoking was defined as ever vs never for all South African participants.
Serological Methods
Serum or plasma samples from each study, which were previously stored at temperatures ranging from −20°C to −80°C (Table 1), were sent on dry ice to DKFZ (Heidelberg, Germany) and stored at −20°C until the day of multiplex serological testing, which was performed as described in detail elsewhere (37–39). Briefly, antigens were bacterially expressed as recombinant double fusion proteins with N-terminal glutathione S-transferase (GST) and a C-terminal peptide (tag) consisting of the last 11 amino acids from the large T antigen of simian virus 40 (48). Bacterial pellets were lysed with the use of a high-pressure homogenizer, the lysates were cleared of insoluble components by centrifugation, and the resulting supernatants were stored with 50% glycerol at −20°C. Fusion proteins were characterized by Coomassie-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis, immunoblot analyses using GST- and tag-specific antibodies and GST-capture enzyme-linked immunosorbent assay (48). GST–tag double fusion proteins from cleared lysates were affinity-purified in situ through binding to glutathione casein–coated fluorescence-labeled polystyrene beads. Each fusion protein was bound to a spectrally distinct bead set (SeroMAP Microspheres; Luminex Corp, Austin, TX), and the fusion protein–loaded bead sets were mixed. Sera were pre-incubated at 1:50 dilution in phosphate-buffered saline containing 1 mg/mL casein, 2 mg/mL lysate from bacteria expressing GST-tag alone (to block antibodies directed against residual bacterial proteins and the GST–tag), 0.5% polyvinylalcohol, 0.8% polyvinylpyrrolidone, and 2.5% Superchemiblock (Millipore, Billerica, MA) to suppress nonspecific binding of antibodies to the beads themselves (39). Serum dilutions were incubated with the same volume of mixed bead sets, resulting in a final serum dilution of 1:100. Bound antibodies were detected with biotinylated goat anti-human immunoglobulin G (H + L) secondary antibody and streptavidin-conjugated R-phycoerythrin. A Luminex 100 analyzer (Luminex Corp) was used to identify the internal color of the individual beads and to quantify their fluorescence (expressed as median fluorescence intensity [MFI]) of at least 100 beads per set per serum. A fusion protein consisting of GST and tag without intervening viral antigen served for individual “serum background” determination.
Testing was performed by laboratory staff who were blinded to the case–control status of the subjects. Serum samples were analyzed for antibodies to the major capsid protein (L1) and/or the early oncoproteins E6 and/or E7 of the following HPV types: the high-risk mucosal types HPV16 and HPV18 (L1, E6, and E7), and HPV31, HPV33, HPV35, HPV45, HPV52, and HPV58 (L1 and E6 only); low-risk mucosal HPV types HPV6 and HPV11 (L1, E6, and E7); and cutaneous HPV types HPV1, HPV4, HPV49, and HPV77 (L1 only) as described previously (37,38). The 10 high- and low-risk HPV types analyzed in this study were selected based on preexisting evidence of their associations with squamous cell carcinoma of the cervix, oral cavity, or oropharynx or with benign tumors of the larynx and respiratory epithelium. These HPV types were predetermined to be the best candidates for assessing whether HPV plays a role in esophageal squamous cell carcinoma. The four cutaneous HPV types—for which no tumor association was expected—were included primarily as specificity controls. In addition, we performed serological testing for antibodies to three non-HPV antigens as controls: p53 (a positive control in which seropositivity was expected to be associated with esophageal squamous cell carcinoma) and the VP1 capsid proteins of two ubiquitous human polyomaviruses, Merkel cell virus (MCV) and JC virus (JCV) (additional specificity controls) (49). The testing of all sera was performed over three consecutive days in 96-well plates, and each serum sample was tested once. All sera from any one study were tested on the same day. A quality control panel of 188 randomly chosen sera was included on each day of testing to determine interday variation. Pearson correlation coefficients (R) of raw MFI values for the individual antigens in the quality control samples ranged from .76 to .99 (median = .96) for low-prevalence antigens (ie, HPV early proteins E6 and E7) and from .93 to .99 (median = .97) for high-prevalence antigens (ie, HPV L1 and the control antigens). A reference serum with known reactivity to seven antigens (HPV16 L1, HPV49 L1, HPV77 L1, HPV16 E6, HPV16 E7, JCV VP1, and MCV VP1) was included on each plate as a measurement standard. Interplate coefficients of variation for this plate standard for the various antigens across the three assay days ranged from 13.5% to 19.6% (median = 16.4%), indicating low plate-to-plate variation. Autofluorescence of each bead set and background reactions resulting from binding of secondary reagents to the antigen-loaded beads (“bead background”) were determined in one well per plate without human serum. The antigen-specific reactivity of each serum sample was then calculated as the antigen-specific raw MFI value minus the sum of the mean bead background value (averaged over plates) and individual serum background value.
Definitions of Exposures and Confounders
Binary indicators of seropositivity were defined for all serological markers that were assessed. We used previously published cutoff values to define seropositivity for the L1 antigens of HPV1, HPV4, HPV 16, HPV18, HPV31, HPV33, HPV45, HPV49, HPV52, HPV58, and HPV77 (37,50). For the remaining antigens (the L1 antigens of HPV6, HPV11, and HPV35; the E6 antigens of HPV6, HPV11, HPV 16, HPV18, HPV31, HPV33, HPV35, HPV45, HPV52, and HPV58; and the E7 antigens of HPV6, HPV11, HPV 16, and HPV18), all of which were mucosal sexually transmitted HPV types, we used cutoff values to define seropositivity that were derived from the sera of a group of self-reported Korean virgins [algorithm as defined in Clifford et al. (50)]. We also constructed four additional composite binary indicator variables. In the first variable, subjects were classified seropositive if they were seropositive for either HPV16 E6 or E7 or to both (hereafter referred to as HPV16 E6 and/or E7 seropositivity). In the second variable, subjects were classified as seropositive if they were seropositive for both HPV16 E6 and E7 (hereafter referred to as HPV16 E6 and E7 seropositivity). In the third variable, subjects were classified seropositive if they were seropositive for either HPV18 E6 or E7 or to both (hereafter referred to as HPV18 E6 and/or E7 seropositivity). In the fourth variable, subjects were classified as seropositive if they were seropositive for both HPV18 E6 and E7 (hereafter referred to as HPV18 E6 and E7 seropositivity).
Multilevel covariates representing cumulative lifetime consumption of alcohol and tobacco smoking were constructed for the studies that had sufficient data available on these exposures. For these studies, pack-years of smoking were used to represent the cumulative lifetime exposure to tobacco smoke and were calculated by multiplying the number of packs of cigarettes smoked per day by the number of years of smoking. One pack of cigarettes was defined as 20 g of tobacco. Similarly, cumulative exposure to alcohol was represented by drink-years, which were calculated by multiplying the number of alcoholic drinks consumed per day by the number of years of drinking. One drink was defined as 15.7 g of alcohol (ethanol). These definitions of pack-years and drink-years have been used in other studies that examined factors associated with esophageal squamous cell carcinoma (51,52). For use as covariates in regression analyses examining the associations between esophageal squamous cell carcinoma and serological markers, cumulative lifetime consumption of alcohol and tobacco smoking were categorized as never drank alcohol, 1–79, 80–139, 140–199 or 200 or more drink-years and never smoker, ex-smoker who smoked 1–29 pack-years, ex-smoker who smoked 30 or more pack-years, smoker who smoked 1–29 pack-years, or smoker who smoked 30 pack-years or more, respectively. A composite alcohol and smoking covariate was also created to examine the association between esophageal squamous cell carcinoma and combined levels of smoking and alcohol consumption with the following categories: never smoked or drank alcohol, never smoked and 1–79 drink-years of alcohol exposure, never smoked and 80 or more drink-years of alcohol exposure, ever smoked and 1–79 drink-years of alcohol exposure, and ever smoked and 80 or more drink-years of alcohol exposure. Cut points for the above individual and composite alcohol and smoking covariates were chosen so as to provide 1) uniformly defined categories across studies with these measures and 2) sufficient numbers of case and control subjects within each category of each study (to allow reliable estimates of the study-specific effects of smoking and alcohol) while maintaining an adequate number of categories for each covariate (to reduce the potential for residual confounding). The use of various other cut points satisfying the above mentioned criteria did not produce substantive changes in the overall conclusions of this study (data not shown).
Exposure to alcohol was defined as ever vs never for Iran, China, and South Africa. Exposure to smoking was defined as ever vs never for South Africa only (we were also able to construct multilevel smoking and alcohol covariates for a subset of the South African participants who were interviewed using versions of the study questionnaire that were designed after 1998).
Variables representing the highest level of education attained (none, primary, secondary, or tertiary) and remoteness of residence (urban or rural) were uniformly defined and coded across studies. Several local study variables were also constructed, including opium, nass, and hot tea consumption in Iran; maté consumption in Brazil; and snuff use in South Africa.
Statistical Analysis
Study-specific odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated using conditional logistic regression. Fully adjusted study-specific models were adjusted for education level (none, primary, secondary, or tertiary for all studies), remoteness of residence (rural or urban for all studies), opium consumption (never or ever for Iran only), nass consumption (never or ever for Iran only), tea consumption (warm/lukewarm, hot, or very hot for Iran only), maté consumption (never, cold/warm, hot/very hot for Brazil only), and snuff use (never or ever for South Africa only); and by matched design for age (category matched by 5-year age groups for South Africa and Australia; directly matched by ±2 years for Iran, by ±5 years for China, and by ±10 years for Central and Eastern Europe, and Brazil), sex (male, female for all studies), serum storage time (category matched by 2-year serum storage times groups for South Africa; directly matched by ±1 year serum storage times for Central and Eastern Europe, and Brazil only), neighborhood (Iran and China only), and study center (Central and Eastern Europe and Brazil only). Fully adjusted study-specific models examining the associations between esophageal squamous cell carcinoma and serological markers were additionally adjusted for alcohol consumption (never or ever for South Africa, Iran, and China; never drank alcohol, 1–79, 80–139, 140–199, or ≥200 drink-years for Australia, Central and Eastern Europe, and Brazil) and tobacco smoking (never or ever for South Africa; never, ex-smoker who smoked 1–29 pack-years, ex-smoker who smoked ≥30 pack-years, smoker who smoked 1–29 pack-years, or smoker who smoked ≥30 pack-years for Australia, Central and Eastern Europe, Brazil, Iran, and China). The effects of all covariates were modeled categorically.
Pooled odds ratios and 95% confidence intervals were calculated using either a linear mixed-effects approach or a joint fixed-effects approach (53). The linear mixed-effects approach calculates the pooled odds ratio as a weighted average of the study-specific odds ratios with weights equal to the inverse marginal variances. In the presence of statistically significant heterogeneity [assessed using the residual heterogeneity statistic (54)], the marginal variance is calculated as the sum of the study-specific variance and the additional unexplained variance of exposure effects across studies (53). In the absence of statistically significant heterogeneity, the marginal variance is equal to the study-specific variance. Although the linear mixed-effects approach is generally the preferred method for pooling case–control data (53), it cannot be used if one or more studies have undefined odds ratios due to the absence of at least one seropositive or seronegative, case or control subject. In such circumstances, we estimated the pooled odds ratios by analyzing the combined data in a single conditional logistic regression model (also known as a joint fixed-effects approach). Smoking and alcohol exposures were categorized as ever vs never, and all study-specific covariates (snuff, maté, hot tea, nass, and opium consumption) were excluded from analyses using the joint fixed-effects approach because it requires uniformly defined covariates across studies. The nominal P value is shown for each statistical test without adjustment for multiple comparisons. All statistical tests were two-sided, and statistical significance was defined as P less than .05. All analyses were performed using STATA 11 software (StataCorp 2010 Statistical Software: release 11·0, STATA: College Station, TX).
We performed a series of sensitivity analyses to assess the robustness of the results. First, we estimated minimally adjusted associations between esophageal squamous cell carcinoma and all serological markers (ie, adjusted only for the study matching variables) to examine the overall influence of the full set of potential confounders. Second, associations between esophageal squamous cell carcinoma and seropositivity for each HPV protein that is suspected to cross-react with homologous proteins of phylogenetically related HPV types (ie, species alpha7, comprising HPV types HPV16, HPV31, HPV33, HPV35, HPV52, and HPV58; species alpha9, comprising types HPV18 and HPV45; and species alpha10, comprising types HPV6 and HPV11) were re-estimated after excluding subjects who were seropositive to the protein of interest and one or more potentially cross-reacting proteins. Third, associations between esophageal squamous cell carcinoma and all serological markers were re-estimated after removing data from the largest study (South Africa) and also after removing data from the two studies in which the blood collection procedures differed for the case and control subjects (Iran and China). Fourth, associations between esophageal squamous cell carcinoma and all HPV markers stratified by smoking (ever vs never), alcohol (ever vs never), and sex were estimated using appropriate interaction terms.
Results
A total of 1561 case subjects and 2502 control subjects from the six participating studies were included in the primary analyses (Table 1). The largest proportion of participants was from the South African study (45%), followed by the Australian study (16%). The studies from Iran, Central and Eastern Europe, and Brazil each provided 11%–12% of the participants, and the remaining 6% were provided by the Chinese study. Overall, participants ranged in age from 26 to 91 years and were predominantly male (64%) (Table 2). Compared with control subjects, proportionally fewer case subjects were never smokers (34% vs 50%), were educated at the high school level or beyond (46% vs 57%), or lived in an urban area (62% vs 74%).
Table 2.
Characteristics of case and control subjects in the included studies
| Study | No. of subjects | Median age, y (range) | Female | Never smoker | Never drinker | High school education or beyond | Urban residence |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |||
| South Africa | Control subjects: 1110 | 59 (26–88) | 462 (42) | 616 (55) | 506 (46) | 593 (53) | 975 (88) |
| Case subjects: 697 | 58 (29–90) | 268 (38) | 222 (32) | 234 (34) | 264 (38) | 538 (77) | |
| Australia | Control subjects: 424 | 64 (34–80) | 182 (43) | 209 (49) | 57 (13) | 424 (100) | 271 (64) |
| Case subjects: 211 | 64 (34–79) | 90 (43) | 52 (25) | 29 (14) | 211 (100) | 131 (62) | |
| Central and Eastern Europe | Control subjects: 314 | 59 (39–76) | 36 (11) | 107 (34) | 40 (13) | 300 (96) | 221 (70) |
| Case subjects: 157 | 58 (39–80) | 18 (11) | 19 (12) | 7 (4) | 149 (95) | 80 (51) | |
| Brazil | Control subjects: 314 | 58 (33–89) | 68 (22) | 95 (30) | 127 (40) | 28 (9) | 256 (82) |
| Case subjects: 157 | 60 (33–91) | 34 (22) | 24 (15) | 30 (19) | 7 (4) | 96 (61) | |
| Iran | Control subjects: 221 | 65 (31–85) | 115 (52) | 175 (79) | 214 (97) | 15 (7) | 55 (25) |
| Case subjects: 220 | 64 (30–88) | 114 (52) | 164 (75) | 216 (98) | 5 (2) | 55 (25) | |
| China | Control subjects: 119 | 57 (34–73) | 46 (39) | 53 (45) | 68 (57) | 57 (48) | 71 (60) |
| Case subjects: 119 | 58 (36–70) | 46 (39) | 53 (45) | 71 (60) | 75 (63) | 63 (53) | |
| All studies | Control subjects: 2502 | 60 (26–89) | 909 (36) | 1255 (50) | 1012 (40) | 1417 (57) | 1849 (74) |
| Case subjects: 1561 | 60 (29–91) | 570 (37) | 534 (34) | 587 (38) | 711 (46) | 963 (62) |
The patterns of risk of esophageal squamous cell carcinoma in relation to smoking and alcohol consumption were similar to those reported in other studies among the subset of participants for whom cumulative lifetime consumption of alcohol was available (55,56) (Supplementary Figure 1, available online). Compared with individuals who never smoked or drank alcohol, those who ever smoked and had 80 or more drink-years of alcohol exposure had an elevated risk of esophageal squamous cell carcinoma (OR = 6.13, 95% CI = 4.10 to 9.15). Compared with individuals who never smoked or drank alcohol, those who had ever smoked and reported 1–79 drink-years of alcohol exposure had an odds ratio of 2.09 (95% CI = 1.37 to 3.20). Compared with individuals who never smoked or drank alcohol, never smokers who had 80 or more drink-years of alcohol exposure and never drinker–ever smokers had odds ratios of 1.46 (95% CI = 0.68 to 3.16) and 1.77 (95% CI = 0.76 to 4.12), respectively. Among never smokers, those who had 1–79 drink-years of alcohol exposure had a lower risk of esophageal squamous cell carcinoma compared with never drinkers (OR = 0.60, 95% CI = 0.39 to 0.90).
Associations With Serological Markers
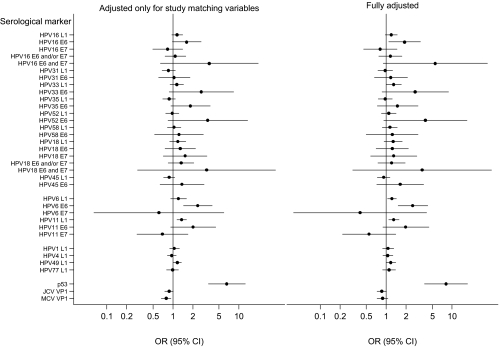
Table 3 shows the pooled fully adjusted odds ratios of esophageal squamous cell carcinoma and 95% confidence intervals associated with each of the 35 serological markers. Figure 1 shows the pooled odds ratios adjusted only for study matching variables (left panel) and after full adjustment (right panel). Supplementary Table 1 (available online) shows the study-specific results.
Table 3.
Pooled fully adjusted odds ratios for serological markers and esophageal squamous cell carcinoma*
| Serological marker† | Seropositive case subjects, No. (%) | Seropositive control subjects, No. (%) | Fully adjusted OR (95% CI) | P‡ |
| Mucosal high-risk HPV types | ||||
| HPV16 L1 | 235 (15.1) | 333 (13.3) | 1.20 (0.97 to 1.47) | .092 |
| HPV16 E6 | 34 (2.2) | 38 (1.5) | 1.89 (1.09 to 3.29) | .023 |
| HPV16 E7 | 24 (1.5) | 49 (2.0) | 0.82 (0.46 to 1.46) | .50 |
| HPV16 E6 and/or E7 | 54 (3.5) | 85 (3.4) | 1.16 (0.78 to 1.74) | .46 |
| HPV16 E6 and E7§ | 4 (0.3) | 2 (0.1) | 5.57 (0.90 to 34.35) | .064 |
| HPV31 L1 | 132 (8.5) | 237 (9.5) | 0.97 (0.75 to 1.25) | .82 |
| HPV31 E6§ | 22 (1.4) | 34 (1.4) | 1.18 (0.66 to 2.10) | .58 |
| HPV33 L1 | 136 (8.7) | 191 (7.6) | 1.30 (1.00 to 1.69) | .047 |
| HPV33 E6§ | 8 (0.5) | 5 (0.2) | 2.76 (0.86 to 8.82) | .087 |
| HPV35 L1 | 142 (9.1) | 256 (10.2) | 0.97 (0.76 to 1.23) | .80 |
| HPV35 E6§ | 18 (1.1) | 16 (0.6) | 1.49 (0.73 to 3.05) | .27 |
| HPV52 L1 | 137 (8.8) | 217 (8.7) | 1.09 (0.85 to 1.41) | .49 |
| HPV52 E6§ | 6 (0.4) | 3 (0.1) | 3.92 (0.92 to 16.74) | .065 |
| HPV58 L1 | 133 (8.5) | 196 (7.8) | 1.14 (0.88 to 1.47) | .33 |
| HPV58 E6§ | 10 (0.6) | 12 (0.5) | 1.23 (0.50 to 3.05) | .65 |
| HPV18 L1 | 546 (35.0) | 749 (29.9) | 1.28 (0.93 to 1.75) | .13 |
| HPV18 E6§ | 26 (1.7) | 32 (1.3) | 1.24 (0.70 to 2.18) | .46 |
| HPV18 E7§ | 13 (0.8) | 14 (0.6) | 1.31 (0.58 to 2.93) | .52 |
| HPV18 E6 and/or E7§ | 37 (2.4) | 45 (1.8) | 1.21 (0.75 to 1.94) | .43 |
| HPV18 E6 and E7§ | 2 (0.1) | 1 (0.0) | 3.52 (0.31 to 39.48) | .31 |
| HPV45 L1 | 194 (12.4) | 335 (13.4) | 0.92 (0.74 to 1.14) | .43 |
| HPV45 E6§ | 12 (0.8) | 15 (0.6) | 1.64 (0.72 to 3.70) | .24 |
| Mucosal low-risk HPV types | ||||
| HPV6 L1 | 688 (44.1) | 919 (36.7) | 1.22 (1.05 to 1.42) | .010 |
| HPV6 E6§ | 41 (2.6) | 26 (1.0) | 2.53 (1.51 to 4.25) | <.001 |
| HPV6 E7§ | 1 (0.1) | 3 (0.1) | 0.40 (0.04 to 4.09) | .44 |
| HPV11 L1 | 365 (23.4) | 462 (18.5) | 1.30 (1.09 to 1.56) | .0036 |
| HPV11 E6§ | 15 (1.0) | 11 (0.4) | 1.99 (0.89 to 4.44) | .093 |
| HPV11 E | 7 (0.4) | 17 (0.7) | 0.55 (0.22 to 1.40) | .21 |
| Cutaneous HPV types | ||||
| HPV1 L1 | 268 (17.2) | 406 (16.2) | 1.07 (0.88 to 1.30) | .48 |
| HPV4 L1 | 379 (24.3) | 612 (24.5) | 1.06 (0.89 to 1.26) | .48 |
| HPV49 L1 | 427 (27.4) | 586 (23.4) | 1.18 (1.00 to 1.40) | .055 |
| HPV77 L1 | 176 (11.3) | 281 (11.2) | 1.11 (0.88 to 1.39) | .39 |
| Other markers | ||||
| p53 | 266 (17.0) | 111 (4.4) | 8.10 (3.83 to 17.17) | <.001 |
| JCV VP1 | 1139 (73.0) | 1865 (74.5) | 0.86 (0.73 to 1.01) | .072 |
| MCV VP1 | 1261 (80.8) | 2088 (83.4) | 0.88 (0.73 to 1.06) | .18 |
CI = confidence interval; HPV = human papillomavirus; JCV = JC virus; MCV = Merkel cell virus; OR = odds ratio.
Serological marker data were pooled using a linear mixed effects approach except where otherwise indicated.
All P values two-sided.
Serological marker data were pooled using joint fixed effects approach.
Figure 1.
Pooled odds ratios for serological markers and esophageal squamous cell carcinoma adjusted for study matching variables only (left) and fully adjusted (right). Circles represent odds ratios, horizontal lines represent 95% confidence intervals, and the vertical lines represent the odds ratios of unity for the seronegative referent groups. HPV = human papillomavirus; JCV = JC virus; MCV = Merkel cell virus; OR = odds ratio; CI = confidence interval.
HPV Early Protein Markers (E6 and E7).
We found statistically significant associations between esophageal squamous cell carcinoma and seropositivity for E6 for the high-risk mucosal type HPV16 (OR = 1.89, 95% CI = 1.09 to 3.29; P = .023) and for the low-risk mucosal type HPV6 (OR = 2.53, 95% CI = 1.51 to 4.25; P < .001) but not for any of the other HPV types (ORs ranged from 1.18 to 3.92 and P values ranged from .065 to .65) (Table 3). The odds ratios for the associations between esophageal squamous cell carcinoma and E6 antigens of the high-risk mucosal types HPV33 and HPV52 were 2.76 (95% CI = 0.86 to 8.82; P = .087) and 3.92 (95% CI = 0.92 to 16.74; P = .13), respectively. There were no statistically significant associations between esophageal squamous cell carcinoma and E7 seropositivity for HPV types HPV6, HPV11, HPV16, or HPV18. Double seropositivity for HPV16 E6 and E7 was rare and was not associated with esophageal squamous cell carcinoma (4/1561 case subjects, 2/2502 control subjects; OR = 5.57, 95% CI = 0.90 to 34.35; P = .064). Double seropositivity for HPV18 E6 and E7 was even more rare and was also not associated with esophageal squamous cell carcinoma (2/1561 case subjects, 1/2502 control subjects; OR = 3.52, 95% CI = 0.31 to 39.48; P = .31).
HPV Late Protein Markers (L1).
We found statistically significant associations between esophageal squamous cell carcinoma and seropositivity for L1 for the high-risk mucosal type HPV33 (OR = 1.30, 95% CI = 1.00 to 1.69; P = .047) and the low-risk mucosal types HPV6 (OR = 1.22, 95% CI = 1.05 to 1.42; P = .010) and HPV11 (OR = 1.30, 95% CI = 1.09 to 1.56; P = .0036). The odds ratios for the associations between esophageal squamous cell carcinoma and L1 antigens of the high-risk mucosal types HPV16 and HPV18 were 1.20 (95% CI = 0.97 to 1.47; P = .092) and 1.28 (95% CI = 0.93 to 1.75; P = .13), respectively. We found no statistically significant associations between esophageal squamous cell carcinoma and L1 seropositivity for HPV types HPV1, HPV4, HPV31, HPV35, HPV45, HPV49, HPV52, HPV58, or HPV77 (ORs ranged from 0.92 to 1.18, P values ranged from .055 to .82). The association between esophageal squamous cell carcinoma and HPV18 L1 showed statistically significant heterogeneity among the six studies (Pheterogeneity = .0085; Supplementary Table 1, available online); none of the other HPV markers examined using the linear mixed-effects approach showed any statistically significant heterogeneity.
Other Serological Markers.
As expected, we found a strong association between esophageal squamous cell carcinoma and p53 seropositivity (OR = 8.10, 95% CI = 3.83 to 17.17; P < .001). There was statistically significant heterogeneity in the association between p53 seropositivity and esophageal squamous cell carcinoma, with an odds ratio of 3.01 (95% CI = 2.14 to 4.44) in the South Africa study, where control subjects were people with other cancers, and an odds ratio of 40.64 (95% CI = 9.26 to 178.38) in the Australia study, where disease-free control subjects were used (Supplementary Table 1, available online). No positive associations were found between esophageal squamous cell carcinoma and the VP1 capsid protein of human polyomaviruses JCV (OR = 0.86, 95% CI = 0.73 to 1.01; P = .072) or MCV (OR = 0.88, 95% CI = 0.73 to 1.06; P = .18).
Sensitivity Analyses
The pooled fully adjusted odds ratios and 95% confidence intervals were similar to those adjusted only for study matching variables (Figure 1). Associations between HPV serological markers and esophageal squamous cell carcinoma were robust and did not change substantially when we excluded subjects who were seropositive for the HPV protein of interest and one or more phylogenetically related, potentially cross-reacting proteins from the analysis (data not shown). Pooled estimates were largely unaffected by the exclusion of data from the largest study (South Africa) or from the two studies (Iran and China) in which blood collection procedures differed for the case and control subjects (data not shown). There were statistically significant interactions between sex and seropositivity for HPV6 L1 (Pinteraction = .025), HPV11 L1 (Pinteraction = .040), and HPV35 L1 (Pinteraction = .031), with odds ratios for males of 1.40 (95% CI = 1.17 to 1.68; P = .0020), 1.49 (95% CI = 1.20 to 1.86; P < .001), and 1.13 (95% CI = 0.83 to 1.53; P = .42), respectively, and for females, 1.00 (95% CI = 0.78 to 1.27; P = .97), 1.04 (95% CI = 0.79 to 1.35; P = .79), and 0.67 (95% CI = 0.47 to 0.96; P = .030), respectively, but not for any other HPV serological marker (P values ranged from .073 to .86). For the three HPV proteins other than HPV6 L1 and HPV11 L1 that were statistically significantly associated with esophageal squamous cell carcinoma in the combined sex analysis, there was no statistically significant interaction between sex and seropositivity for HPV16 E6 (Pinteraction = .47), HPV33 L1 (Pinteraction = .088), and HPV6 E6 (Pinteraction = .61); the sex-specific odds ratios were 2.03 (95% CI = 1.08 to 3.82; P = .029), 1.50 (95% CI = 1.08 to 2.09; P = .015), and 2.78 (95% CI = 1.48 to 5.22; P = .0015), respectively, for males and 1.39 (95% CI = 0.62 to 3.12; P = .43), 0.97 (95% CI = 0.66 to 1.42; P = .88), and 2.08 (95% CI = 0.84 to 5.17; P = .11), respectively, for females. No statistically significant interactions were found between tobacco smoking or alcohol consumption and seropositivity for any of the HPV markers (data not shown).
Discussion
In this collaboration of six large case–control studies, we used advanced serological methods to detect circulating antibodies against a total of 28 HPV antigens (18 L1, E6, or E7 antigens from the eight high-risk mucosal HPV types that are most prevalent in cervical cancer; six L1, E6, or E7 antigens from two prevalent low-risk mucosal HPV types; and four L1 antigens from cutaneous HPV types) and found five nominally statistically significant associations with esophageal squamous cell carcinoma risk (ie, HPV16 E6, HPV33 L1, HPV6 E6, HPV6 L1, and HPV11 L1). Non-statistically significant but elevated odds ratios were also found for the E6 proteins of HPV33 and HPV52.
To our knowledge, seven previous studies have examined the associations between esophageal squamous cell carcinoma and HPV serological markers (19,31–36). In these studies, the serological markers examined for associations with esophageal squamous cell carcinoma were HPV16 L1 (all seven studies), HPV18 L1 (32–34), HPV33 L1 (33), and HPV73 L1 (32). Four studies found no statistically significant association between esophageal squamous cell carcinoma and HPV16 L1 seropositivity after adjustment for various risk factors, with estimated odds ratios of 3.0 (95% CI = 0.6 to 14) (33), 0.9 (95% CI = 0.3 to 2.4) (34), 0.8 (95% CI = 0.3 to 2.0) (35), and 1.6 (95% CI = 0.8 to 3.3) (32), whereas three studies did, with estimated odds ratios of 1.5 (95% CI = 1.1 to 2.1) (19), 4.5 (95% CI = 1.8 to 11.9) (36), and 13.1 (95% CI = 1.6 to 108) (31). The odds ratio estimates of the latter two studies, although appearing somewhat inconsistent with those of the other studies, are notable for their high degree of statistical imprecision. The pooled odds ratio for HPV16 L1 of 1.20 (95% CI = 0.97 to 1.47) estimated in this analysis is relatively small when compared with previously observed odds ratios measuring the associations between HPV16 L1 seropositivity and squamous cell carcinoma of the cervix (OR ∼8) (16) and oropharynx (OR ∼3.5) (20) but similar in magnitude to odds ratios observed for squamous cell carcinoma of the oral cavity (OR ∼1.5.) (20). Similarly, the statistically significant odds ratio of 1.89 (95% CI = 1.09 to 3.29) that we observed for the association between esophageal squamous cell carcinoma and HPV16 E6 seropositivity is notably smaller than previously observed corresponding odds ratios for squamous cell carcinoma of the cervix (OR ∼146) (17) and oropharynx (OR ∼9.9) (20) but similar in magnitude to that observed for squamous cell carcinoma of the oral cavity (OR ∼2.6) (20). Although squamous cell carcinomas of the cervix, oropharynx, and oral cavity have also been shown to be associated with serum antibodies against HPV16 E7 (20), we found no statistically significant associations between esophageal squamous cell carcinoma and antibodies to E7 for the four HPV types tested (ie, HPV16, HPV18, HPV6, and HPV11). Additional data suggesting a causal association between HPV16 and other squamous cell cancers include the strong serological response to both the E6 and E7 proteins [squamous cell carcinoma of the cervix, OR ∼335 (17), the oropharynx, OR ∼67 (20), and the oral cavity, OR ∼4 (20)]. In this study population, we found about a sixfold increase in the odds of esophageal squamous cell carcinoma when antibodies to both HPV16 E6 and E7 were present; however, this “double seropositive” response was found in only four (0.26%) of 1561 case subjects and in two (0.08%) of 2502 control subjects in the entire study. Thus, the overall results for HPV16 from this study taken together with evidence from previous serological studies suggest that if cases of esophageal squamous cell carcinoma are caused by HPV16, they must be rare.
The three previous studies that examined the relationship between esophageal squamous cell carcinoma and circulating HPV18 L1 antibodies found no association (32–34). Consistent with these results are the non-statistically significant odds ratios we observed in this study for each of the five HPV18 serological markers that were assessed. In the only previous study to examine the relationship between esophageal squamous cell carcinoma and HPV33 L1 antibodies, Bjørge et al. (33) did not find an association. This result, however, was based on only 12 HPV33 L1–seropositive case subjects, suggesting that there was probably insufficient statistical power to detect small to moderate effects. In this study, we observed a statistically significant odds ratio of 1.30 (95% CI = 1.00 to 1.69, P = .047) for HPV33 L1 seropositivity, which is similar in magnitude to the non-statistically significant corresponding estimated odds ratio of 1.8 (95% CI = 0.4 to 7.2, P = .041) observed by Bjørge et al. (33). We did not find a statistically significant association between esophageal squamous cell carcinoma and seropositivity for HPV33 E6, although the odds ratio was elevated (OR = 2.76, 95% CI = 0.86 to 8.82; P = .087). Overall, our findings in combination with previous evidence suggest that if HPV33 is a risk factor for esophageal squamous cell carcinoma, it is unlikely to be major risk factor.
Whereas our findings for HPV16 and HPV33 are consistent, to some degree, with their causative role in other cancers, our findings for HPV6 and HPV11 are more difficult to interpret. These HPV types are known to cause benign tumors of the larynx and respiratory epithelium in children and adolescents (57); however, based on their absence as single infections in cervical cancer (14), they are currently classified as low-risk types. For this reason and because this study is the first to compare the prevalence of low-risk HPV antibodies between esophageal squamous cell carcinoma case subjects and control subjects, the statistically significant associations we observed for HPV6 E6, HPV6 L1, and HPV11 L1 should be viewed as hypothesis-generating findings to be confirmed or refuted in further studies.
In addition to HPV serology, another widely used method for assessing the role of HPV in esophageal squamous cell carcinoma has been to search for HPV DNA in the tissue of the tumors. However, the findings from studies using this method have been remarkably inconsistent. For example, a review published in 2002 showed that the proportions of esophageal squamous cell carcinoma tissues found to contain DNA varied from 0% to 100% among studies (26). These proportions varied considerably even among the larger studies that examined tissues from the same country and that used the same techniques to detect HPV DNA. It has been suggested that imperfect tissue handling and processing protocols resulting in HPV DNA contamination of esophageal squamous cell carcinoma specimens is likely a primary source of the between-study variability in the HPV DNA detection rates (58). It is worth noting, however, that a recent study that claims to have taken extreme care to prevent HPV DNA contamination concluded that HPV is not involved in the development of esophageal squamous cell carcinoma in China (59).
This study has several limitations. First, although we examined the associations between esophageal squamous cell carcinoma and 32 HPV serological markers (including four E6 and E7 composite markers), we did not adjust for multiple comparisons. We believed that a better approach, as recommended by others (60–62), was to evaluate the results in the context of previous supporting evidence, biological plausibility, the number of tests performed, and the strengths of the observed associations. However, we acknowledge that although the P values were nominal for individual tests, the type I error rate for observing one or more false statistically significant test results among all tests performed was likely to be inflated. Second, all of the contributing studies had a case–control design, and thus their results are susceptible to reverse causation. Third, we cannot exclude the possibility that some study-specific and/or general confounders were not adequately adjusted for in this analysis. However, we did attempt to adjust for all previously identified study-specific and general confounders as completely as was permitted by the available data. Moreover, whereas the possibility of unknown confounding factors can never be completely excluded, substantial confounding due to improperly defined known confounders appears unlikely given the consistency between the minimally and fully adjusted pooled estimates (Figure 1). Fourth, because infection with one or more high-risk mucosal types of HPV is a necessary cause of cervical cancer (15), estimates of the associations between seropositivity to the E6 or E7 proteins of the high-risk mucosal HPV types and esophageal squamous cell carcinoma for women are vulnerable to confounding if the rates of undiagnosed cervical cancers differ between case subjects and control subjects. In this study, however, such confounding is likely to be small given that we found no statistically significant differences between males and females with regard to associations involving either E6 or E7 antibodies.
In this serological study, to our knowledge the largest one to date to examine the association between esophageal squamous cell carcinoma and HPV, we found limited evidence of such an association. We cautiously describe the evidence as “limited” because the strongest evidence of associations (in terms of P values) observed in this study were for HPV types that we had relatively low a priori expectations of having a causal role in esophageal squamous cell carcinoma (ie, HPV6 and HPV11). HPV types with which we had higher a priori expectations of having a causal role showed relatively weak or no evidence of an association with esophageal squamous cell carcinoma (eg, HPV16 and HPV18). We therefore cannot exclude the possibility that our statistically significant findings may have been due to chance. Although HPV does not appear to be an important risk factor for esophageal squamous cell carcinoma, we cannot exclude the possibility that certain HPV types may be involved in a small subset of cancers.
Funding
The InterSCOPE collaboration was partially funded by the intramural research programs of the US National Cancer Institute; the German Cancer Research Center; and Cancer Council New South Wales, Australia; and by grants from the Deutsche Krebshilfe. Institutions who funded the field work of the original studies were Brazilian Multicentric Case–Control Study: European Commission (grant no IC18-CT97-0222); Central and Eastern European Case–Control Study: World Cancer Research Fund, European Commission (Contract No. IC15-CT98-0332); The Golestan Case–Control Study: Digestive Disease Research Center, Tehran University of Medical Sciences (Grant Number 82-603); The Australian Cancer Study: National Health and Medical Research Council (Program Grant No. 199600) (Australia); Johannesburg Cancer Case–Control Study: South African National Health Laboratory Service and Medical Research Council; University of the Witwatersrand; Cancer Research UK; Shanxi Case–Control Study: the US National Cancer Institute (Contract NO2-SC-66211).
Supplementary Material
Footnotes
We are grateful to Monika Oppenländer, Ute Koch, and Naomi Crain for their work on this project. Freddy Sitas produced the initial draft of the article. Sam Egger performed the statistical analyses. Tim Waterboer performed the serological analyses. Freddy Sitas, Sam Egger, Tim Waterboer, Sanford M. Dawsey, Margaret I. Urban, Philip R. Taylor, Christian C. Abnet, Paolo Boffetta, Dianne L. O’Connell, David C. Whiteman, Paul Brennan, Reza Malekzadeh, Michael Pawlita, and the other InterSCOPE collaborators contributed to the writing and commented on the statistical analyses.
The study sponsors did not have any role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the article, or the decision to submit the article for publication.
The InterSCOPE Collaboration: Johannesburg Cancer Case–Control Study: Margaret I. Urban, NHLS/MRC Cancer Epidemiology Research Group, National Health Laboratory Service, and University of the Witwatersrand, Johannesburg, South Africa; The Australian Cancer Study: David C. Whiteman, Penelope M. Webb, Adèle C. Green, Nicholas K. Hayward, Queensland Institute of Medical Research; Central and Eastern European Case–Control Study: David Zaridze (Institute of Carcinogenesis, Moscow, Russia), Ivana Holcatova (Charles University, Prague, Czech Republic), Dana Mates (National Institute of Public Health, Romania), Neonila Szeszenia-Dabrowska (Nofer Institute of Occupational Health, Lodz, Poland), Gilles Ferro (IARC, Lyon, France), Vladimir Janout (Faculty of Medicine, Palacky University, Olomouc, Czech Republic); Brazilian Multicentric Case–Control Study: Maria Paula Curado (International Prevention Research Institute IARC, Lyon, France, and University of Goiania, Brazil), Ana Maria Menezes (Federal University of Pelotas, Brazil), Sergio Koifman (Fiocruz Foundation, National School of Public Health, Rio de Janeiro, Brazil); The Golestan Case–Control Study: Farhad Islami, Dariush Nasrollahzadeh, Reza Malekzadeh (Digestive Disease Research Center, Tehran University of Medical Sciences, Iran); Shanxi Case–Control Study: Nan Hu, Alisa M. Goldstein, Ying Gao (National Cancer Institute, Bethesda, MD), Ti Ding (Shanxi Cancer Hospital, China); US National Cancer Institute, Division of Cancer Epidemiology and Genetics: Philip R. Taylor, Farin Kamangar, Christian C. Abnet, Sanford M. Dawsey; Cancer Council NSW, Cancer Epidemiology Research Unit: Freddy Sitas, Sam Egger, Dianne L. O’Connell; German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ): Michael Pawlita, Tim Waterboer (TW current affiliation: Boehringer Ingelheim GmbH); International Agency for Research on Cancer: Paul Brennan; The Tisch Cancer Institute, Mount Sinai School of Medicine, and International Prevention Research Institute: Paolo Boffetta.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Lyon, France: International Agency for Research on Cancer; 2010. Available from http://globocan.iarc.fr. Accessed March 15, 2011. [Google Scholar]
- 2.International Agency for Research on Cancer Working Group. Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 83. Tobacco Smoke and Involuntary Smoking. Lyon, France:: IARC; 2004. [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165(12):1424–1433. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 4.Baan R, Straif K, Grosse Y, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8(4):292–293. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 5.Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113(3):456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 6.Islami F, Kamangar F, Nasrollahzadeh D, et al. Socio-economic status and esophageal cancer: results from a population-based case–control study in a high-risk area. Int J Epidemiol. 2009;38(4):978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blot WF, McLaughlin JK. Chapter 8, Cancer of the Esophagus in Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 8.Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92(21):1753–1763. doi: 10.1093/jnci/92.21.1753. [DOI] [PubMed] [Google Scholar]
- 9.Sewram V, De Stefani E, Brennan P, Boffetta P. Mate consumption and the risk of squamous cell esophageal cancer in Uruguay. Cancer Epidemiol Biomarkers Prev. 2003;12(6):508–513. [PubMed] [Google Scholar]
- 10.Islami F, Pourshams A, Nasrollahzadeh D, et al. Tea drinking habits and esophageal cancer in a high risk area in northern Iran: population based case–control study. BMJ. 2009;338:b929. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sammon AM. A case–control study of diet and social factors in cancer of the esophagus in Transkei. Cancer. 1992;69(4):860–865. doi: 10.1002/1097-0142(19920215)69:4<860::aid-cncr2820690404>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Ghadirian P, Vobecky J, Vobecky JS. Factors associated with cancer of the esophagus: an overview. Cancer Detect Prev. 1988;11(3–6):225–234. [PubMed] [Google Scholar]
- 13.Nasrollahzadeh D, Kamangar F, Aghcheli K, et al. Opium, tobacco, and alcohol use in relation to esophageal squamous cell carcinoma in a high-risk area of Iran. Br J Cancer. 2008;98(11):1857–1863. doi: 10.1038/sj.bjc.6604369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiological classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 15.Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Naucler P, Chen HC, Persson K, et al. Seroprevalence of human papillomaviruses and Chlamydia trachomatis and cervical cancer risk: nested case–control study. J Gen Virol. 2007;88(pt 3):814–822. doi: 10.1099/vir.0.82503-0. [DOI] [PubMed] [Google Scholar]
- 17.Meschede W, Zumbach K, Braspenning J, et al. Antibodies against early proteins of human papillomaviruses as diagnostic markers for invasive cervical cancer. J Clin Microbiol. 1998;36(2):475–480. doi: 10.1128/jcm.36.2.475-480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heideman DA, Waterboer T, Pawlita M, et al. Human papillomavirus-16 is the predominant type etiologically involved in penile squamous cell carcinoma. J Clin Oncol. 2007;25(29):4550–4556. doi: 10.1200/JCO.2007.12.3182. [DOI] [PubMed] [Google Scholar]
- 19.Sitas F, Urban M, Stein L, et al. The relationship between anti-HPV-16 IgG seropositivity and cancer of the cervix, anogenital organs, oral cavity and pharynx, esophagus and prostate in a black South African population. Infect Agent Cancer. 2007;2(6) doi: 10.1186/1750-9378-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 21.Zumbach K, Hoffmann M, Kahn T, et al. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in patients with head-and-neck squamous-cell carcinoma. Int J Cancer. 2000;85(6):815–818. doi: 10.1002/(sici)1097-0215(20000315)85:6<815::aid-ijc14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza G, Kreimer AR, Viscidi R, et al. Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 23.Syrjänen K, Pyrhönen S, Aukee S, et al. Squamous cell papilloma of the esophagus: a tumour probably caused by human papilloma virus (HPV) Diagn Histopathol. 1982;5(4):291–296. [PubMed] [Google Scholar]
- 24.Syrjänen KJ. Histological changes identical to those of condylomatous lesions found in esophageal squamous cell carcinomas. Arch Geschwulstforsch. 1982;52(4):283–292. [PubMed] [Google Scholar]
- 25.IARC Monographs Working Group. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: International Agency for Research on Cancer. Volume 100 Part B: Biological Agents. Lyon, France: IARC; 2011. [Google Scholar]
- 26.Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55(10):721–728. doi: 10.1136/jcp.55.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillison ML, Shah KV. Chapter 9: the role of mucosal human papillomavirus in nongenital cancers. J Natl Cancer Inst Monogr. 2003;(31):57–65. doi: 10.1093/oxfordjournals.jncimonographs.a003484. [DOI] [PubMed] [Google Scholar]
- 28.Campo MS. Papillomas and cancer in cattle. Cancer Surv. 1987;6(1):39–54. [PubMed] [Google Scholar]
- 29.Shen Z, Cen S, Shen J, et al. Study of immortalization and malignant transformation of human embryonic esophageal epithelial cells induced by HPV18 E6E7. J Cancer Res Clin Oncol. 2000;126(10):589–594. doi: 10.1007/PL00008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dillner J. The serological response to papillomaviruses. Semin Cancer Biol. 1999;9(6):423–430. doi: 10.1006/scbi.1999.0146. [DOI] [PubMed] [Google Scholar]
- 31.Dillner J, Knekt P, Schiller JT, Hakulinen T. Prospective seroepidemiological evidence that human papillomavirus type 16 infection is a risk factor for oesophageal squamous cell carcinoma. BMJ. 1995;311(7016):1346. doi: 10.1136/bmj.311.7016.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamangar F, Qiao YL, Schiller JT, et al. Human papillomavirus serology and the risk of esophageal and gastric cancers: results from a cohort in a high-risk region in China. Int J Cancer. 2006;119(3):579–584. doi: 10.1002/ijc.21871. [DOI] [PubMed] [Google Scholar]
- 33.Bjørge T, Hakulinen T, Engeland A, et al. A prospective, seroepidemiological study of the role of human papillomavirus in esophageal cancer in Norway. Cancer Res. 1997;57(18):3989–3992. [PubMed] [Google Scholar]
- 34.Lagergren J, Wang Z, Bergstrom R, Dillner J, Nyren O. Human papillomavirus infection and esophageal cancer: a nationwide seroepidemiologic case–control study in Sweden. J Natl Cancer Inst. 1999;91(2):156–162. doi: 10.1093/jnci/91.2.156. [DOI] [PubMed] [Google Scholar]
- 35.Van Doornum GJ, Korse CM, Buning-Kager JC, et al. Reactivity to human papillomavirus type 16 L1 virus-like particles in sera from patients with genital cancer and patients with carcinomas at five different extragenital sites. Br J Cancer. 2003;88(7):1095–1100. doi: 10.1038/sj.bjc.6600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han C, Qiao G, Hubbert NL, et al. Serologic association between human papillomavirus type 16 infection and esophageal cancer in Shaanxi Province, China. J Natl Cancer Inst. 1996;88(20):1467–1471. doi: 10.1093/jnci/88.20.1467. [DOI] [PubMed] [Google Scholar]
- 37.Michael KM, Waterboer T, Sehr P, et al. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog. 2008;4(6):e1000091. doi: 10.1371/journal.ppat.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 39.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309(1–2):200–204. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the esophagus. Gut. 2008;57(2):173–180. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 41.Sapkota A, Hsu CC, Zaridze D, et al. Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer Causes Control. 2008;19(10):1161–1170. doi: 10.1007/s10552-008-9183-0. [DOI] [PubMed] [Google Scholar]
- 42.Szymanska K, Matos E, Hung RJ, et al. Drinking of maté and the risk of cancers of the upper aerodigestive tract in Latin America: a case–control study. Cancer Causes Control. 2010;21(11):1799–1806. doi: 10.1007/s10552-010-9606-6. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y, Hu N, Han X, et al. Family history of cancer and risk for esophageal and gastric cancer in Shanxi, China. BMC Cancer. 2009;9(269) doi: 10.1186/1471-2407-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mqoqi N, Kellett P, Sitas F, Jula M. Incidence of Histologically Diagnosed Cancer in South Africa, 1998-1999. National Cancer Registry of South Africa. Johannesburg, South Africa: National Health Laboratory Service; 2004. [Google Scholar]
- 45.Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality Books. 2006. http://www.aihw.gov.au/cancer/data/acim_books/index.cfm. Accessed November 15, 2010. [Google Scholar]
- 46.Semnani S, Sadjadi A, Fahimi S, et al. Declining incidence of esophageal cancer in the Turkmen Plain, eastern part of the Caspian Littoral of Iran: a retrospective cancer surveillance. Cancer Detect Prev. 2006;30(1):14–19. doi: 10.1016/j.cdp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Liu B, Li G, Rong S, Cao D, editors. Atlas of Cancer Mortality in the People's Republic of China. 1st ed. Shanghai, China: China Map Press; 1979. [Google Scholar]
- 48.Sehr P, Muller M, Hopfl R, et al. HPV antibody detection by ELISA with capsid protein L1 fused to glutathione S-transferase. J Virol Methods. 2002;106(1):61–70. doi: 10.1016/s0166-0934(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 49.Kjaerheim K, Roe OD, Waterboer T, et al. Absence of SV40 antibodies or DNA fragments in prediagnostic mesothelioma serum samples. Int J Cancer. 2007;120(11):2459–2465. doi: 10.1002/ijc.22592. [DOI] [PubMed] [Google Scholar]
- 50.Clifford GM, Shin HR, Oh JK, et al. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1874–1879. doi: 10.1158/1055-9965.EPI-07-0349. [DOI] [PubMed] [Google Scholar]
- 51.Lee CH, Lee JM, Wu DC, et al. Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int J Cancer. 2008;122(6):1347–1356. doi: 10.1002/ijc.23264. [DOI] [PubMed] [Google Scholar]
- 52.Antonsson A, Nancarrow DJ, Brown IS, et al. High-risk human papillomavirus in esophageal squamous cell carcinoma. Australian Cancer Study. Cancer Epidemiol Biomarkers Prev. 2010;19(8):2080–2087. doi: 10.1158/1055-9965.EPI-10-0033. [DOI] [PubMed] [Google Scholar]
- 53.Stukel TA, Demidenko E, Dykes J, Karagas MR. Two-stage methods for the analysis of pooled data. Stat Med. 2001;20(14):2115–2130. doi: 10.1002/sim.852. [DOI] [PubMed] [Google Scholar]
- 54.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franceschi S, Bidoli E, Negri E, Barbone F, La Vecchia C. Alcohol and cancers of the upper aerodigestive tract in men and women. Cancer Epidemiol Biomarkers Prev. 1994;3(4):299–304. [PubMed] [Google Scholar]
- 56.Lagergren J, Bergstrom R, Lindgren A, Nyren O. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the esophagus and gastric cardia. Int J Cancer. 2000;85(3):340–346. [PubMed] [Google Scholar]
- 57.Mammas IN, Sourvinos G, Spandidos DA. Human papilloma virus (HPV) infection in children and adolescents. Eur J Pediatr. 2009;168(3):267–273. doi: 10.1007/s00431-008-0882-z. [DOI] [PubMed] [Google Scholar]
- 58.Hubbard RA. Human papillomavirus testing methods. Arch Pathol Lab Med. 2003;127(8):940–945. doi: 10.5858/2003-127-940-HPTM. [DOI] [PubMed] [Google Scholar]
- 59.Koshiol J, Wei WQ, Kreimer AR, et al. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer. 2010;127(1):93–100. doi: 10.1002/ijc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 61.Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142(9):904–908. doi: 10.1093/oxfordjournals.aje.a117737. [DOI] [PubMed] [Google Scholar]
- 62.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.