Abstract
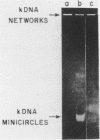
DNA topoisomerase activity detected in cell extracts of the trypanosomatid Crithidia fasciculata interlocks kinetoplast DNA duplex minicircles into huge catenane forms resembling the natural kinetoplast DNA networks found in trypanosomes. Catenation of duplex DNA circles is reversible and equilibrium is affected by ionic strength, and by spermidine. The reaction requires magnesium, is ATP dependent and is inhibited by high concentrations of novobiocin. Extensive homology between duplex DNA rings was not required for catenane formation since DNA circles with unrelated sequences could be interlocked into mixed network forms. Covalently sealed catenaned DNA circles are specifically used as substrates for decatenation. No such preference for covalently sealed duplex DNA rings was observed for catenate formation. Its catalytic properties and DNA substrate preference, suggest a potential role for this eukaryotic topoisomerase activity in the replication of kinetoplast DNA.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldi M. I., Benedetti P., Mattoccia E., Tocchini-Valentini G. P. In vitro catenation and decatenation of DNA and a novel eucaryotic ATP-dependent topoisomerase. Cell. 1980 Jun;20(2):461–467. doi: 10.1016/0092-8674(80)90632-7. [DOI] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Brack C., Delain E., Riou G. Replicating, convalently closed, circular DNA from kinetoplasts of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1642–1646. doi: 10.1073/pnas.69.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. O., Cozzarelli N. R. A sign inversion mechanism for enzymatic supercoiling of DNA. Science. 1979 Nov 30;206(4422):1081–1083. doi: 10.1126/science.227059. [DOI] [PubMed] [Google Scholar]
- Bénard J., Riou G., Saucier J. M. Characterization by sedimentation analysis of kinetoplast DNA from Trypanosoma cruzi at different stages of culture. Nucleic Acids Res. 1979;6(5):1941–1952. doi: 10.1093/nar/6.5.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. Effects of novobiocin on adenovirus DNA synthesis and encapsidation. Nucleic Acids Res. 1980 Apr 11;8(7):1625–1641. doi: 10.1093/nar/8.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Novobiocin inhibition of simian virus 40 DNA replication. Nature. 1980 Jul 31;286(5772):529–531. doi: 10.1038/286529a0. [DOI] [PubMed] [Google Scholar]
- Englund P. T., DiMaio D. C., Price S. S. A nicked form of kinetoplast DNA in Leishmania tarentolae. J Biol Chem. 1977 Sep 10;252(17):6208–6216. [PubMed] [Google Scholar]
- Englund P. T. Free minicircles of kinetoplast DNA in Crithidia fasciculata. J Biol Chem. 1979 Jun 10;254(11):4895–4900. [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Gandini Attardi D., Martini G., Mattoccia E., Tocchini-Valentini G. P. Effect of Xenopus laevis oocyte extract on supercoiled simian virus 40 DNA: formation of complex DNA. Proc Natl Acad Sci U S A. 1976 Feb;73(2):554–558. doi: 10.1073/pnas.73.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Wang J. C. Yeast DNA topoisomerase II. An ATP-dependent type II topoisomerase that catalyzes the catenation, decatenation, unknotting, and relaxation of double-stranded DNA rings. J Biol Chem. 1982 May 25;257(10):5866–5872. [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980 Aug;21(1):115–125. doi: 10.1016/0092-8674(80)90119-1. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Catenation of DNA rings by topoisomerases. Mechanism of control by spermidine. J Biol Chem. 1982 Mar 10;257(5):2687–2693. [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Formation and resolution of DNA catenanes by DNA gyrase. Cell. 1980 May;20(1):245–254. doi: 10.1016/0092-8674(80)90252-4. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. T4 DNA topoisomerase: a new ATP-dependent enzyme essential for initiation of T4 bacteriophage DNA replication. Nature. 1979 Oct 11;281(5731):456–461. doi: 10.1038/281456a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Wolstenholme D. R. Replication of kinetoplast DNA of Crithidia acanthocephali. I. Density shift experiments using deuterium oxide. J Cell Biol. 1976 Aug;70(2 Pt 1):406–418. doi: 10.1083/jcb.70.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J. C., Miller K. G., Englund P. T. Decatenation of kinetoplast DNA by topoisomerases. J Biol Chem. 1980 Jun 10;255(11):4976–4979. [PubMed] [Google Scholar]
- Mattern M. R., Painter R. B. Dependence of mammalian DNA replication on DNA supercoiling. II. Effects of novobiocin on DNA synthesis in Chinese hamster ovary cells. Biochim Biophys Acta. 1979 Jul 26;563(2):306–312. doi: 10.1016/0005-2787(79)90049-2. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Mizuuchi K., Fisher L. M., O'Dea M. H., Gellert M. DNA gyrase action involves the introduction of transient double-strand breaks into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1847–1851. doi: 10.1073/pnas.77.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Isolation and characterization of kinetoplast DNA networks and minicircles from Crithidia fasciculata. J Protozool. 1974 Nov;21(5):774–781. doi: 10.1111/j.1550-7408.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Pulse-labeling of kinetoplast DNA: localization of 2 sites of synthesis within the networks and kinetics of labeling of closed minicircles. J Protozool. 1976 Nov;23(4):583–587. doi: 10.1111/j.1550-7408.1976.tb03846.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Simpson A. M., Wesley R. D. Replication of the kinetoplast DNA of Leishmania tarentolae and Crithidia fasciculata. Biochim Biophys Acta. 1974 May 17;349(2):161–172. doi: 10.1016/0005-2787(74)90077-x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse Y., Wang J. C. E. coli and M. luteus DNA topoisomerase I can catalyze catenation of decatenation of double-stranded DNA rings. Cell. 1980 Nov;22(1 Pt 1):269–276. doi: 10.1016/0092-8674(80)90174-9. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Simpson L. Studies on kinetoplast DNA. I. Isolation of kinetoplast DNA minicircles from Leishmania tarentolae. Biochim Biophys Acta. 1973 Sep 7;319(3):237–253. doi: 10.1016/0005-2787(73)90163-9. [DOI] [PubMed] [Google Scholar]