Abstract
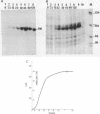
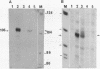
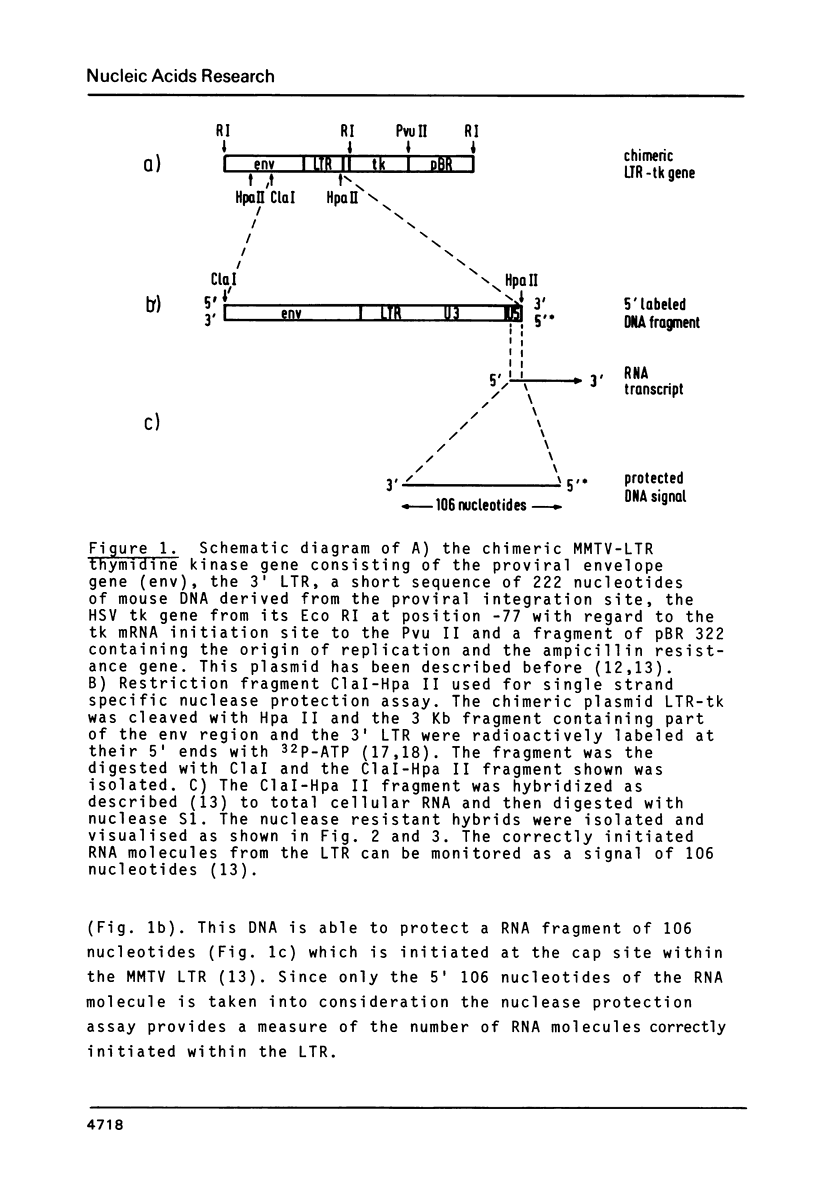
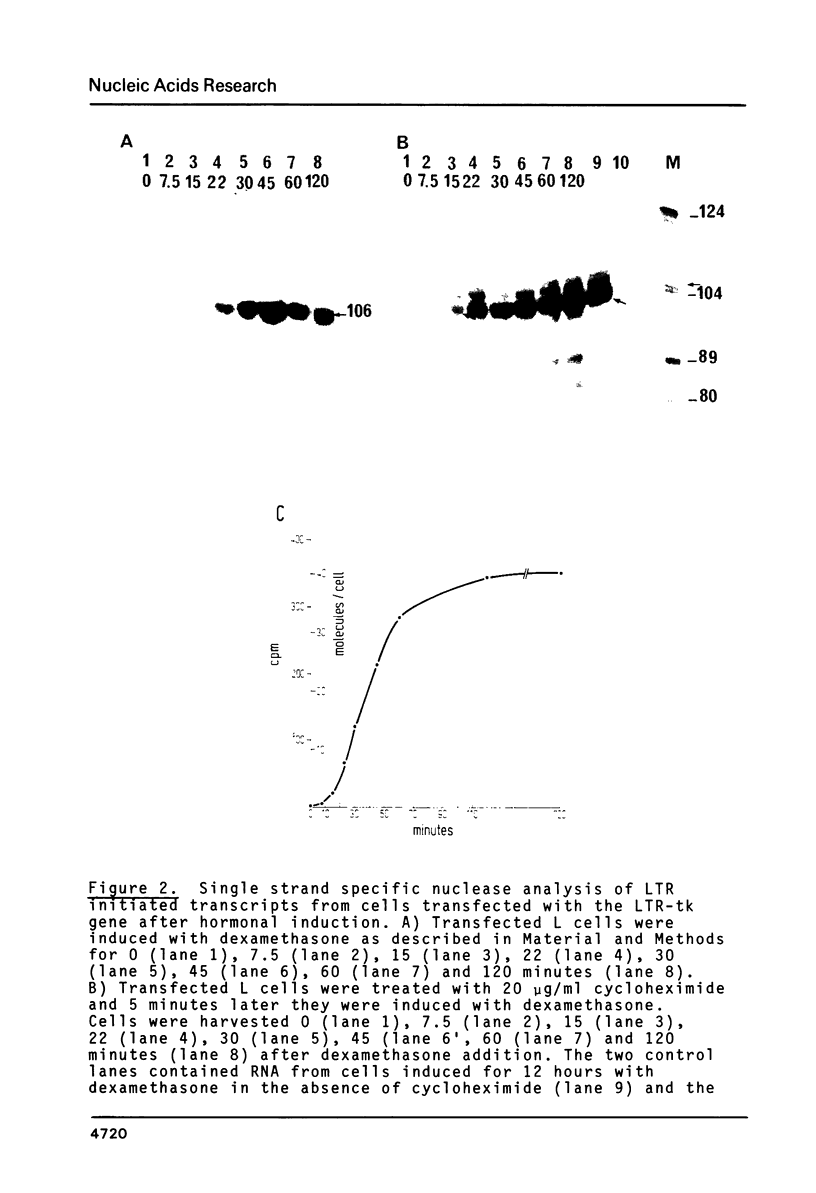
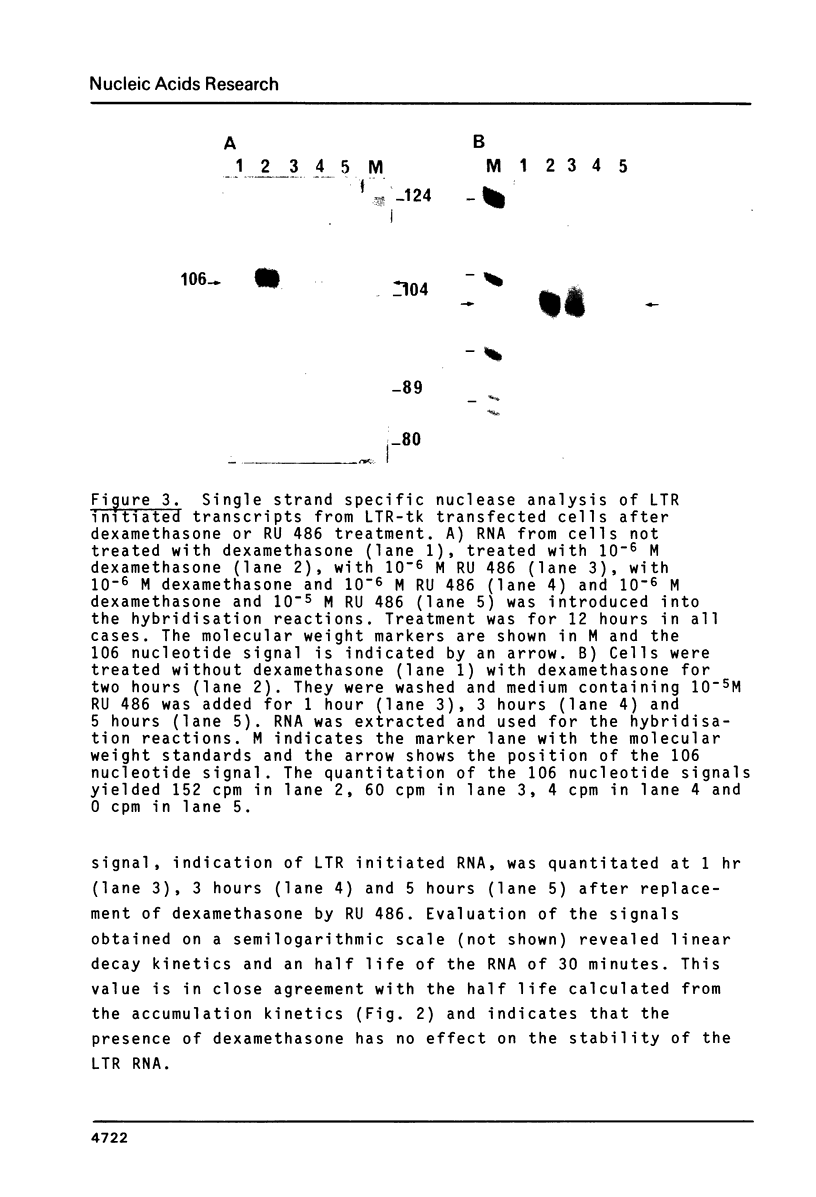
A chimeric gene, recombined in vitro, containing a long terminal repeat (LTR) sequence from the proviral DNA of mouse mammary tumor virus and the thymidine kinase (tk) gene of Herpes Simplex Virus was introduced into L tk- cells. No transcription of LTR RNA was observed in transfected cells when glucocorticoid hormones were absent from the growth medium. Accumulation of LTR initiated RNA was measured upon hormone addition by the single strand specific nuclease RNA mapping procedure. The accumulation was rapid (detectable after 7.5 minutes), independent of simultaneous protein synthesis and mediated by a functional glucocorticoid receptor complex. Glucocorticoid hormones affect LTR transcription at the level of initiation. The rate of initiation (1.8 X 10(-2) molecules/cell/sec) and a half life of about 30 minutes could be calculated for LTR RNA. The half life of LTR RNA is independent of the presence of hormone.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Cloned mouse mammary tumor virus DNA is biologically active in transfected mouse cells and its expression is stimulated by glucocorticoid hormones. Cell. 1981 Feb;23(2):335–345. doi: 10.1016/0092-8674(81)90129-x. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Geisse S., Scheidereit C., Westphal H. M., Hynes N. E., Groner B., Beato M. Glucocorticoid receptors recognize DNA sequences in and around murine mammary tumour virus DNA. EMBO J. 1982;1(12):1613–1619. doi: 10.1002/j.1460-2075.1982.tb01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager L. J., Palmiter R. D. Transcriptional regulation of mouse liver metallothionein-I gene by glucocorticoids. Nature. 1981 May 28;291(5813):340–342. doi: 10.1038/291340a0. [DOI] [PubMed] [Google Scholar]
- Herrmann W., Wyss R., Riondel A., Philibert D., Teutsch G., Sakiz E., Baulieu E. E. Effet d'un stéroide anti-progestérone chez la femme: interruption du cycle menstruel et de la grossesse au début. C R Seances Acad Sci III. 1982 May 17;294(18):933–938. [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Jeep S., Wurtz T., Nguyen-Huu M. C., Giesecke K., Schütz G. Control of cellular content of chicken egg white protein specific RNA during estrogen administration and withdrawal. Biochemistry. 1979 Feb 20;18(4):616–624. doi: 10.1021/bi00571a011. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Rahmsdorf U., Kennedy N., Fabiani L., Michalides R., Nusse R., Groner B. Structure, stability, methylation, expression and glucocorticoid induction of endogenous and transfected proviral genes of mouse mammary tumor virus in mouse fibroblasts. Gene. 1981 Dec;15(4):307–317. doi: 10.1016/0378-1119(81)90174-8. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Palmiter R. D. Glucocorticoid regulation of metallothionein-I mRNA synthesis in cultured mouse cells. J Biol Chem. 1981 Mar 25;256(6):2621–2624. [PubMed] [Google Scholar]
- Mayo K. E., Warren R., Palmiter R. D. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982 May;29(1):99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Pfahl M. Specific binding of the glucocorticoid-receptor complex to the mouse mammary tumor proviral promoter region. Cell. 1982 Dec;31(2 Pt 1):475–482. doi: 10.1016/0092-8674(82)90140-4. [DOI] [PubMed] [Google Scholar]
- Ponta H., Kennedy N., Herrlich P., Hynes N. E., Groner B. A deletion mutant of mouse mammary tumour virus, lacking 516 nucleotides of the 5' long terminal repeat sequence, can be expressed in a hormone-responsive fashion. J Gen Virol. 1983 Mar;64(Pt 3):567–577. doi: 10.1099/0022-1317-64-3-567. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Glucocorticoid regulation of mouse mammary tumor virus gene expression. Biochim Biophys Acta. 1979 Dec 19;560(4):487–508. doi: 10.1016/0304-419x(79)90014-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Schütz G., Nguyen-Huu M. C., Giesecke K., Hynes N. E., Groner B., Wurtz T., Sippel A. E. Hormonal control of egg white protein messenger RNA synthesis in the chicken oviduct. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):617–624. doi: 10.1101/sqb.1978.042.01.064. [DOI] [PubMed] [Google Scholar]
- Swaneck G. E., Nordstrom J. L., Kreuzaler F., Tsai M. J., O'Malley B. W. Effect of estrogen on gene expression in chicken oviduct: evidence for transcriptional control of ovalbumin gene. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1049–1053. doi: 10.1073/pnas.76.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Young H. A., Scolnick E. M., Parks W. P. Glucocorticoid-receptor interaction and induction of murine mammary tumor virus. J Biol Chem. 1975 May 10;250(9):3337–3343. [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]