Abstract
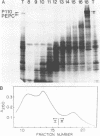
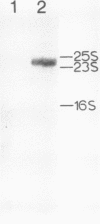
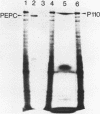
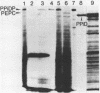
To obtain molecular probes for studies of gene regulation in photosynthetic tissues of maize, we have cloned DNA complementary to poly(A)+RNA extracted from green leaves by insertion into plasmid pBR322 and transformation of E. coli, strain RR1. Colonies were screened by sequential hybridization with 32P-labeled single stranded cDNA synthesized from pooled aliquots of poly(A)+RNA fractionated by sucrose density centrifugation. Among the clones bearing cDNA homologous to high molecular weight poly(A)+RNA, we identified one with an insert of 440 base pairs homologous to mRNA for pyruvate, Pi dikinase, a C-4 carbon cycle protein localized in mesophyll cells of the leaf. Our work indicates that the dikinase subunits are synthesized in the cytoplasm as precursors approximately 13,000 daltons larger than the mature peptide subunits. Leaves of seedlings illuminated during growth have higher levels of pyruvate, Pi dikinase mRNA than leaves of dark-grown plants.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., MacDonald R. J. Cloning of hormone genes from a mixture of cDNA molecules. Methods Enzymol. 1979;68:75–90. doi: 10.1016/0076-6879(79)68007-2. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague D. R., Sims T. L. Evidence for Light-stimulated Synthesis of Phosphoenolpyruvate Carboxylase in Leaves of Maize. Plant Physiol. 1980 Sep;66(3):505–509. doi: 10.1104/pp.66.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa S., Matsunaga K., Sugiyama T. Light induction of phosphoenolpyruvate carboxylase in etiolated maize leaf tissue. Plant Physiol. 1981 Jan;67(1):133–138. doi: 10.1104/pp.67.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Nicodemus C. F. Cloning of alpha 2u globulin cDNA using a high efficiency technique for the cloning of trace messenger RNAs. Gene. 1981 Mar;13(2):145–152. doi: 10.1016/0378-1119(81)90003-2. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Chung S., Zuker C., Lodish H. F. Selection and analysis of cloned developmentally-regulated Dictyostelium discoideum genes by hybridization-competition. Nucleic Acids Res. 1981 Feb 25;9(4):947–963. doi: 10.1093/nar/9.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty K. S., Jr, Vollmer R. T., McCarty K. S. Improved computer program data for the resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1974 Sep;61(1):165–183. doi: 10.1016/0003-2697(74)90343-1. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T. Occurrence of Pyruvate Orthophosphate Dikinase in the Succulent Plant, Kalanchoë daigremontiana Hamet. et. Perr. Plant Physiol. 1975 Nov;56(5):605–607. doi: 10.1104/pp.56.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T. Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry. 1973 Jul 17;12(15):2862–2868. doi: 10.1021/bi00739a014. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfeld P. L., Seeburg P. H., Shine J. The human pro-opiomelanocortin gene: organization, sequence, and interspersion with repetitive DNA. DNA. 1982;1(2):133–143. doi: 10.1089/dna.1.1982.1.133. [DOI] [PubMed] [Google Scholar]