Abstract
Mutations in THAP1 have been associated with dystonia 6 (DYT6). THAP1 encodes a transcription factor that represses the expression of DYT1. To further evaluate the mutational spectrum of THAP1 and its associated phenotype, we sequenced THAP1 in 567 patients with focal (n=461), segmental (n=68), or generalized dystonia (n=38). We identified 10 novel variants, including six missense substitutions within the DNA-binding Thanatos-associated protein domain (Arg13His, Lys16Glu, His23Pro, Lys24Glu, Pro26Leu, Ile80Val), a 1bp-deletion downstream of the nuclear localization signal (Asp191Thrfs*9), and three alterations in the untranslated regions. The effect of the missense variants was assessed using prediction tools and luciferase reporter gene assays. This indicated the Ile80Val substitution as a benign variant. The subcellular localization of Asp191Thrfs*9 suggests a disturbed nuclear import for this mutation. Thus, we consider six of the 10 novel variants as pathogenic mutations accounting for a mutation frequency of 1.1%. Mutation carriers presented mainly with early onset dystonia (<12 years in five of six patients). Symptoms started in an arm or neck and spread to become generalized in three patients or segmental in two patients. Speech was affected in four mutation carriers. In conclusion, THAP1 mutations are rare in unselected dystonia patients and functional analysis is necessary to distinguish between benign variants and pathogenic mutations.
Keywords: dystonia, THAP1, mutation, DNA binding, phenotype–genotype
Introduction
Dystonia is a movement disorder, characterized clinically by involuntary twisting, repetitive movements, and abnormal postures.1 Mutations in the THAP1 (THAP domain-containing, apoptosis-associated protein 1) gene were identified to underlie dystonia 6 (DYT6), a form of primary torsion dystonia.2 THAP1 encodes a 213-amino acid transcription factor featuring a specific DNA-binding THAP (Thanatos-associated protein) zinc-finger domain at the N terminus and a nuclear localization signal (NLS) towards the C terminus.2 We and others recently demonstrated in vitro that the DYT1 promoter is a target for the transcription factor activity of THAP1.3, 4 Mutations in DYT1 cause another form of primary torsion dystonia.5
About 50 different THAP1 mutations have been reported to date including missense, nonsense, and frameshift mutations.2, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 In addition to the disease-causing mutations, two variants, c.-237_236delinsTT and c.71+9C>A, in the non-coding region of THAP1 may be associated with dystonia.9, 13
DYT6 typically manifests as early-onset generalized or segmental dystonia, frequently with prominent laryngeal involvement and a rostrocaudal evolution of symptoms.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The phenotype though is highly variable even within a single family ranging from unaffected carriers to generalized dystonia.8, 9, 13 Mutation frequency varies between 0.5% in unselected primary dystonia patients13 and 25% in non-DYT1 multiplex families in whom at least one individual had non-focal involvement and onset of symptoms by < 22 years.7
The clinical relevance of some reported mutations in THAP1 is equivocal. Recently, in-vitro tests have become available to explore the functional consequences of different mutations.3, 4, 18 In the present study, we investigated 567 patients with primary dystonia for mutations in the THAP1 gene and performed an in-vitro assay to assess the putative functional consequences of the mutations. These findings were related to the clinical phenotype of mutation carriers.
Patients and methods
Patients
The study was approved by the local ethics committee and all participants gave written informed consent. Since our initial study in 2009,9 we collected another 498 unrelated patients and included 69 patients who were tested in 2009 for known mutations only (published as Group B9 including patients with sporadic focal dystonia without facial or laryngeal involvement and onset >26 years). Patients were consecutively recruited at different movement disorders centers including the Section of Clinical and Molecular Neurogenetics Lübeck, Germany, the General Hospital Kassel, Germany, the University Hospitals of Hamburg-Eppendorf, Berlin (Charité), Rostock, and Kiel, the Institute of Music Physiology and Musicians' Medicine, University of Music, Drama and Media, Hanover, the University Hospital of Belgrade, Serbia, and the Toronto Western Hospital, Canada. All patients were examined by at least one movement disorder specialist. The diagnosis of primary dystonia was established based on the absence of a history of neuroleptic exposure, head trauma, and anoxia and of any clinical symptoms or signs suggestive of secondary dystonia. Further, secondary causes were excluded by brain MRI. Patients were of German (n=436), Serbian (n=120), or Canadian (n=12) origin. All patients tested negative for the GAG deletion in the DYT1 gene. Patients presented mainly with focal dystonia (n=461; 81%).
Genotyping
To test for mutations, we sequenced all three exons and exon–intron boundaries of the THAP1 gene using a capillary sequencing machine. Sequences were aligned to the reference sequence (NC_000008.10) using the Mutation Surveyor software (SoftGenetics, State College, PA, USA). In addition, the frequency of two known polymorphisms and all novel missense variants were determined in 365 German controls by sequencing. To assess the effect of the missense mutations in silico, the prediction softwares PolyPhen219 (HumVar- tool; http://genetics.bwh.harvard.edu/pph2/) and SIFT20 were used.
Measurement of transcription factor activity
We previously showed that THAP1 represses the expression of DYT1 in a concentration-dependent manner and that DYT6-associated mutations result in decreased repression of DYT1 in Luciferase reporter gene assays.4 Here, we used these reporter gene assays to characterize the novel missense mutations (Lys16Glu, Lys24Glu, Pro26Leu, Ile80Val) within the DNA-binding THAP domain in addition to the two previously tested substitutions (Arg13His and His23Pro).4 In brief, human endothelial cells (HeLa) were transfected with wild-type or mutated THAP1 constructs in the pcDNA3.1/myc-his plasmid (Invitrogen, Darmstadt, Germany) and the DYT1 core promoter fragment4 in the pGL4.10 vector (Promega, Mannheim, Germany) using FuGENE-HD (Roche, Mannheim, Germany). Activity of firefly and renilla luciferase was measured after 24–48 h incubation with the Dual Luciferase Reporter Assay System (Promega) in a Mithras-Luminometer (Bertholdt, Bad Wildbach, Germany). All measurements were verified in a minimum of three independent experiments and as triplicates in each experiment.
Subcellular localization of truncated THAP1
We investigated the subcellular localization of the novel frameshift mutation c.570delA (Asp191Thrfs*9) that is downstream of the predicted NLS. The respective coding regions of wild-type and mutated THAP1 were inserted into the pEGFP-N3 plasmid (Clontech, Mountain View, CA, USA) to generate green fluorescent protein (GFP)-labeled THAP1 fusion proteins. These GFP-labeled constructs were transiently expressed for 48 h in OVCAR-3 cells and localization was determined using confocal laser scanning microscopy as described.18 OVCAR-3 cells are well suited for immunocytochemistry experiments due to their size and handling.
Results
Patients and mutation screening
Clinical details of the patients are presented in Table 1. Among the 567 patients with primary dystonia, we identified 10 novel variants. These included six missense substitutions within the DNA-binding THAP domain (Arg13His, Lys16Glu, His23Pro, Lys24Glu, Pro26Leu, Ile80Val), a 1-bp deletion resulting in a frameshift downstream of the predicted NLS (Asp191Thrfs*9), a base pair substitution in the 5′ untranslated region (UTR; c.-32C>T), and two single base pair substitutions in a single patient in the 3′ UTR (c.*1A>G + c.*10A>T). Clinical and genetic information on mutation carriers is given in Table 2. A detailed case report including description of available family members has been presented elsewhere for the patient with the Arg13His mutation.14 None of the novel substitutions was found among 730 German control chromosomes.
Table 1. Characteristics of the study population.
| Family history (n) | |||||||
|---|---|---|---|---|---|---|---|
| Type of dystonia | Patients (n) | Male (%) | Mean±SD age at onset | Mean±SD age | Positive | Negative | Unknown |
| Generalized | 38 | 61.9 | 19.1±17.8 | 40.9±15.1 | 5 | 26 | 7 |
| Segmental | 68 | 48.4 | 43.5±19.0 | 59.1±12.8 | 6 | 40 | 22 |
| Focal | 461 | ||||||
| Cervical | 155 | 39.9 | 42.8±13.1 | 54.9±13.7 | 22 | 62 | 71 |
| Blepharospasm | 63 | 30.2 | 56.9±10.7 | 68.6±10.3 | 2 | 29 | 32 |
| Writer's cramp | 53 | 54.7 | 41.4±10.9 | 55.4±10.8 | 1 | 22 | 30 |
| Musician's dystonia | 168 | 73.2 | 34.8±10.5 | 43.8±12.0 | 13 | 135 | 20 |
| Spasmodic dysphonia | 18 | 33.3 | 50.9±11.3 | 65.4±11.2 | 0 | 3 | 15 |
| Other | 4 | 50.0 | 58.2±10.9 | 61.5±12.2 | 1 | 3 | 0 |
Table 2. Clinical and genetics information of mutation carriers.
| Ind. ID | Age | Sex | Family history | Age at onset | Site at onset | Dystonia at examination | Speech affected | Mutation | PolyPhen-2 prediction19 (score) | SIFT prediction (score) | THAP1 activity (luciferase assay) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L3641 | 59 | F | Pos. | 49 | Neck | Focal | No | c.570delA p. Asp191Thrfs*9 | n.a. | n.a. | n.a. |
| L3841 | 32 | F | Neg. | 8 | Neck | Generalized | Yes (mild) | c.70A>G p.Lys24Glu | Benign (0.100) | Affects function (0.03) | 60 |
| L3969 | 30 | M | Neg. | 6 | Arm | Generalized | Yes | c.38G>A p.Arg13His | Probably damaging (0.989) | Affects function (0.02) | 80 |
| L4071 | 46 | F | Neg. | 11 | Neck | Generalized | Yes | c.46A>G p.Lys16Glu | Probably damaging (0.914) | Affects function (0.03) | 60 |
| L4155 | 33 | M | Pos. | 9 | Arm | Segmental | No | c.68A>C p.His23Pro | Possibly damaging (0.612) | Affects function (0.00) | 20 |
| L4318 | 56 | M | Pos. | 10 | Arm | Segmental | Yes | c.77C>T p.Pro26Leu | Probably damaging (0.994) | Affects function (0.00) | 40 |
| L4325 | 35 | M | Neg. | 33 | Arm | Focal (Musicians' dystonia) | No | c.-32C>T (5′UTR) | n.a. | n.a. | n.a. |
| L4455 | 33 | M | Neg. | 19 | Arm | Generalizeda | Yes (aphonia) | c.(*1A>G (+) *10A>T) (3′UTR) | n.a. | n.a. | n.a. |
| L4457 | 54 | M | Neg. | 41 | Neck | Focal | No | c.238A>G p.Ile80Val | Benign (0.010) | Tolerated (0.79) | 100 |
Improvement of 20% by Levodopa intake.
In our sample, we identified the c.-237_236delinsTT polymorphism only in the heterozygous state. Frequencies were comparable in patients and controls with 4.5% (in German patients), 5.0% (in Serbian patients), and 5.2% (in controls). Frequencies of the variant for different subgroups of dystonia are shown in Table 3.
Table 3. Frequency of the c.-237_236delinsTT polymorphism in different samples.
| Djarmati et al.9 | Replication in Germans | Replication in Serbians | All patients (%) | Replication in controls | |
|---|---|---|---|---|---|
| Total | 19/320 (5.9%) | 17/378 (4.5%) | 6/120 (5.0%) | 42/818 (5.1) | 19/365 (5.2%) |
| Generalized | 0/35 | 1/32 | 0/6 | 3/73 (4.1) | |
| Segmental | 3/60 | 2/55 | 0/12 | 5/127 (3.9) | |
| Focal | 16/225 | 14/291 | 6/102 | 36/618 (5.8) | |
| Cervical | 8/68 | 6/111 | 3/43 | 17/222 (7.7) | |
| Musician's dystonia | 8/94 | 6/122 | 0 | 14/216 (6.5) | |
| Writer's cramp | 0/34 | 0/18 | 2/16 | 2/68 (2.9) | |
| Blepharospasm | 0/29 | 2/32 | 0/30 | 2/91 (2.2) | |
| Spasmodic dysphonia | 0 | 0/5 | 1/13 | 1/18 (5.6) | |
| Focal, different region | 0 | 0/4 | 0 | 0/4 (0.0) |
The other polymorphism that was found more often among dystonia patients (8/1210) compared with controls (1/400),13 c.71+9G>A, was present in 1/567 patients and in 1/365 controls.
Characterization of the coding variants
In a first step, we performed an in-silico analysis of the newly identified non-synonymous variants. Using PolyPhen2, all but Lys24Glu and Ile80Val were predicted to have a possible (His23Pro) or probable (Arg13His, Lys16Glu, Pro26Leu) damaging effect. The SIFT software, predicted an effect on protein function for five of the missense variants but not for Ile80Val (Table 2).
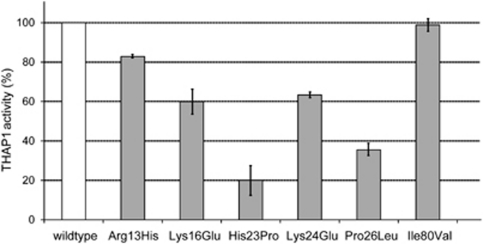
As THAP1 regulates the expression of the DYT1 gene,4 we used reporter gene assays as a readout of mutant THAP1 function. We analyzed the six missense variants within the DNA-binding THAP domain. THAP1 activity was most prominently reduced (80%) for His23Pro, around 50% for Lys16Glu, Lys24Glu, and Pro26Leu, and about 20% for Arg13His. In our assay, the Ile80Val variant did not have any effect on the THAP1 activity (Figure 1; Table 2).
Figure 1.
Effect of missense changes in the DNA-binding domain on THAP1 activity. Activity of THAP1 was measured by repression of the TOR1A promoter in a luciferase reporter gene assay. Wild-type THAP1-mediated repression of the TOR1A core promoter activity was set as 100% (lane 1). The THAP1 activity of Ile80Val was comparable to the wild-type protein. All other missense changes resulted in lower THAP1 activity of 20 to 80%. Bars indicate standard error.
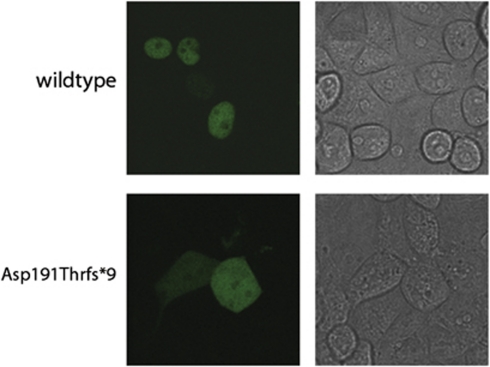
To determine the effect of the Asp191Thrfs*9 frameshift mutation, we assessed its subcellular localization by confocal laser scanning microscopy. While wild-type THAP1–GFP fusion protein was exclusively located in the nuclei, truncated THAP1 Asp191Thrfs*9 showed an impaired nuclear import and was also detected in the cytoplasm (Figure 2).
Figure 2.
Subcellular localization of Asp191Thrfs*9. Subcellular localization of GFP-labeled fusion constructs of THAP1 (green signal; left panel) transiently expressed in OVCAR-3 cells visualized by confocal laser scanning microscopy. Microscopic detection of THAP1-GFP demonstrates an exclusive nuclear distribution of THAP1 wild-type protein (upper panel), whereas Asp191Thrfs*9 mutant is also present in the cytosol (lower panel). Corresponding cells are shown by transmitted-light microscopy (right panel).
For none of the samples with rare variants in the non-coding regions, ie 5′ or 3′ UTR or intron 1, RNA was available to test for a potential effect on RNA stability or splicing. Affected family members were not available to test for segregation of the variants. It was not possible to reveal whether the two substitutions in individual L4455 were located on the same chromosome.
Discussion
We screened a group of 567 dystonia patients and identified 10 novel variants (1.8%), including six missense (1.1%) and a frameshift variant (0.2%). Based on functional analysis, we consider six of them (Arg13His, Lys16Glu, His23Pro, Lys24Glu, Pro26Leu, Asp191Thrfs*9) to represent mutations, ie to be pathogenic (1.1%). The detected non-coding variants are unlikely to be pathogenic as they do not affect the protein sequence. However, it is conceivable that they alter the expression efficiency but proof is lacking due to unavailability of the respective biological material such as RNA or affected family members to test for segregation. The missense variant Ile80Val seems to represent a benign alteration as indicated by the remaining full THAP1 activity in the in-vitro assay and as also calculated by both prediction tools. This finding is supported by three additional notions: first, the amino acids isoleucine and valine have a similar structure and both belong to the unpolar amino acids. Second, Campagne et al21 revealed lysine 70 as the most C-terminal amino acid residue responsible for DNA binding by detailed structural determination of the THAP domain. Thus, isoleucine 80 should not be involved in DNA binding. Third, the patient has a rather late age at onset (41 years) and speech is not affected (see below). On the other hand, lack of an effect in the reporter gene assay might be specific to the tested target promoter or, alternatively, isoleucine 80 may be involved in another function of THAP1, such as protein–protein interactions.
THAP1 contains a bipartite NLS spanning 16 amino acids (aa 146–162) in the C-terminal part of THAP1.18 Although the frame-shift mutation Asp191Thrfs*9 does not affect the NLS itself, we demonstrated an impaired nuclear import of mutant THAP1 in vitro. This altered intracellular distribution of THAP1 may be explained by a protein misfolding affecting at least the C-terminal region of THAP1, which disturbs the formation of the bipartite NLS and results in a reduced interaction with nuclear importing proteins. Alternatively, it is also conceivable that there is remaining THAP1 nuclear import and function in vivo and the variant is benign. The DYT6-atypical phenotype in our patient with late onset (49 years) and focal dystonia without involving speech actually supports this possibility.
The results of prediction programs for functional effects of the mutations and the effect size measured in vitro clearly correspond for four of the six missense changes including the Ile80Val variant. For Arg13His, however, an effect on function was predicted by both programs but experimental evidence suggests a rather mild effect. The results for the Lys24Glu mutation were more conflicting as experimental evidence indicates a medium effect but one of the prediction programs (PolyPhen2) predicted no functional change at all.
We detected a THAP1 mutation in 1.1% of our mainly focal dystonia patients. Mutation frequency in the literature ranges from about 0.5–1.8% for mainly unselected primary dystonia patients.9, 10, 11, 12, 13, 15 Thus, THAP1 mutations are rare in dystonia patients. About 50 different THAP1 mutations have been reported to date.2, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 22 One-third of the mutations represents missense mutations located in the THAP domain and is thought to interrupt DNA binding. Another one-third of the mutations are considered to disturb the NLS, such as nonsense mutations, small insertions/deletions, or missense mutations within the NLS. For these proteins, the nuclear import is disturbed resulting in impaired transcriptional activity. Thus, in about 70% of the reported mutations, the DNA binding of mutant THAP1 is considered to be affected. However, functional proof for most of the reported mutations is missing. In the present study, we show that the rare missense variant Ile80Val is probably not pathogenic.
Regarding, the non-coding variants c.-237_236delinsTT, we confirm that there is no significant association with dystonia. Interestingly, however, most studies demonstrate a higher (but not significantly higher) frequency of the variant in dystonia patients compared with controls.9, 10, 23, 24 The association may occur only in subtypes of dystonia. To date, the total number of carriers is too small to obtain significant differences for this rare variant. A differential effect in dystonia subtypes may also be the explanation for the lack of any trend in another study.11 In contrast to our study design, these authors included only about 20% patients with focal dystonia and 75% of the patients had an age of onset <30 years.11 These inclusion criteria are likely to preferentially select patients with monogenic causes of dystonia rather than those carrying polygenic risk factors, thereby masking a possible association. The c.71+9C>A was too rare in our sample to evaluate any trend of a possible association with dystonia.
Among the about 100 described mutation carriers, most presented with an early onset and cervical or arm dystonia at onset.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 In more than 80% of the patients dystonia spreads to other body parts. Speech was affected in about 70% of patients. Clinical phenomenology in mutation carriers reported here was similar. All had onset of symptoms in the neck or arm. All but one mutation carrier had an age at onset between 6 and 11 years and presented with generalized (n=3) or segmental (n=2) dystonia. Only the carrier of the frameshift mutation close to the end of THAP1 had a late onset at the age of 49 years and dystonia remained focal for 10 years after onset. It can be speculated that the effect of this mutation may be rather mild compared with the missense mutations as a considerable proportion of the protein can still enter the nucleus. We also verified the high frequency of laryngeal involvement (4/6) ranging from mild spasmodic dysphonia to aphonia.
Finally, we aimed to correlate the severity of missense changes in the DNA-binding domain with clinical features. Interestingly, the carriers of the benign Ile80Val variant had a rather mild, DYT6-atypical phenotype with a late age at onset, focal dystonia, normal speech, and a negative family history. Further, the carriers of the two mutations with the highest effect on protein function were the only two with a positive family history (Table 2), suggesting that these mutations may exhibit a higher penetrance. To date, these correlations are highly speculative and investigations of larger samples will reveal if in-vitro functional analyses can be correlated to the phenotypic presentation.
Taken together, we report 10 novel variants in THAP1 and demonstrate a functional effect for six of them, underlining that functional analysis is necessary to distinguish between benign variants and pathogenic mutations.
Acknowledgments
This study was supported by governmental and institutional funding, ie by a grant from the Deutsche Forschungsgemeinschaft (to KL, SAS, and FJK; LO 1555/3-1), the Volkswagen Foundation (Lichtenberg Grant to CK), the Hermann and Lilly Schilling Foundation (to CK), and the Else Kröner Fresenius Foundation (to AAK; EKMS).
The authors declare no conflict of interest.
References
- Fahn S, Bressman SB, Marsden CD. Classification of dystonia. Adv Neurol. 1998;78:1–10. [PubMed] [Google Scholar]
- Fuchs T, Gavarini S, Saunders-Pullman R, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- Gavarini S, Cayrol C, Fuchs T, et al. Direct interaction between causative genes of DYT1 and DYT6 primary dystonia. Ann Neurol. 2010;68:549–553. doi: 10.1002/ana.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser FJ, Osmanoric A, Rakovic A, et al. The dystonia gene DYT1 is repressed by the transcription factor THAP1 (DYT6) Ann Neurol. 2010;68:554–559. doi: 10.1002/ana.22157. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Bonetti M, Barzaghi C, Brancati F, et al. Mutation screening of the DYT6/THAP1 gene in Italy. Mov Disord. 2009;24:2424–2427. doi: 10.1002/mds.22861. [DOI] [PubMed] [Google Scholar]
- Bressman SB, Raymond D, Fuchs T, Heiman GA, Ozelius LJ, Saunders-Pullman R. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 2009;8:441–446. doi: 10.1016/S1474-4422(09)70081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clot F, Grabli D, Burbaud P, et al. Screening of the THAP1 gene in patients with early-onset dystonia: myoclonic jerks are part of the dystonia 6 phenotype. Neurogenetics. 2011;12:87–89. doi: 10.1007/s10048-010-0264-3. [DOI] [PubMed] [Google Scholar]
- Djarmati A, Schneider SA, Lohmann K, et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol. 2009;8:447–452. doi: 10.1016/S1474-4422(09)70083-3. [DOI] [PubMed] [Google Scholar]
- Groen JL, Ritz K, Contarino MF, et al. DYT6 dystonia: mutation screening, phenotype, and response to deep brain stimulation. Mov Disord. 2010;25:2420–2427. doi: 10.1002/mds.23285. [DOI] [PubMed] [Google Scholar]
- Houlden H, Schneider SA, Paudel R, et al. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 2010;74:846–850. doi: 10.1212/WNL.0b013e3181d5276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söhn AS, Glockle N, Doetzer AD, et al. Prevalence of THAP1 sequence variants in German patients with primary dystonia. Mov Disord. 2010;25:1982–1986. doi: 10.1002/mds.23207. [DOI] [PubMed] [Google Scholar]
- Xiao J, Zhao Y, Bastian RW, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittel S, Moll CK, Bruggemann N, et al. Clinical neuroimaging and electrophysiological assessment of three DYT6 dystonia families. Mov Disord. 2010;25:2405–2412. doi: 10.1002/mds.23279. [DOI] [PubMed] [Google Scholar]
- Cheng FB, Wan XH, Feng JC, Wang L, Yang YM, Cui LY. Clinical and genetic evaluation of DYT1 and DYT6 primary dystonia in China. Eur J Neurol. 2011;18:497–503. doi: 10.1111/j.1468-1331.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- De Carvalho Aguiar P, Fuchs T, Borges V, et al. Screening of Brazilian families with primary dystonia reveals a novel THAP1 mutation and a de novo TOR1A GAG deletion. Mov Disord. 2010;25:2854–2857. doi: 10.1002/mds.23133. [DOI] [PubMed] [Google Scholar]
- Blanchard A, Roubertie A, Simonetta-Moreau M, et al. Singular DYT6 phenotypes in association with new THAP1 frameshift mutations Mov Disord 2011. e-pub ahead of print 25 April 2011. [DOI] [PubMed]
- Osmanovic A, Dendorfer A, Erogullari A, et al. Truncating mutations in THAP1 define the nuclear localization signal Mov Disord 2011. e-pub ahead of print 14 April 2011. [DOI] [PubMed]
- Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagne S, Saurel O, Gervais V, Milon A. Structural determinants of specific DNA-recognition by the THAP zinc finger. Nucleic Acids Res. 2010;38:3466–3476. doi: 10.1093/nar/gkq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SA, Ramirez A, Shafiee K, et al. Homozygous THAP1 mutations as cause of early-onset generalized dystonia. Mov Disord. 2011;26:858–861. doi: 10.1002/mds.23561. [DOI] [PubMed] [Google Scholar]
- Groen JL, Yildirim E, Ritz K, et al. Mov Disord. e-pub ahead of print 29 April 2011; 2011. THAP1 mutations are infrequent in spasmodic dysphonia. [DOI] [PubMed] [Google Scholar]
- Xiao J, Zhao Y, Bastian RW, et al. The c.-237_236GA>TT THAP1 sequence variant does not increase risk for primary dystonia. Mov Disord. 2011;26:549–553. doi: 10.1002/mds.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]