Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a persistent and ubiquitous environmental contaminant. The health impact of TCDD exposure is of great concern to the general public. Recent reports have implied that eicosapentaenoic acid (EPA) might be a potential chemopreventive agent and influence hepatotoxicity. The aim of the current study was to explore the effectiveness of EPA in alleviating the toxicity of TCDD on primary cultured rat hepatocytes. EPA (5, 10 and 20 μM) was added to cultures alone or simultaneously with TCDD (5 and 10 μM). Rat hepatocytes were treated with TCDD and EPA for 48 h, and then cytotoxicity was detected by [3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyltetrazolium bromide] (MTT) assay and lactate dehydrogenase (LDH) release, while total antioxidant capacity (TAC) and total oxidative stress (TOS) levels were determined to evaluate the oxidative injury. The DNA damage was also analyzed by liver micronucleus assay (LMN) and 8-oxo-2-deoxyguanosine (8-OH-dG). The results of MTT and LDH assays showed that TCDD but not EPA decreased cell viability. TCDD also increased TOS level and significantly decreased TAC level in rat hepatocytes in a clear dose dependent manner. On the basis of increasing doses, the dioxin caused significant increases of micronucleated hepatocytes (MNHEPs) and 8-OH-dG as compared to control culture. Whereas, in cultures treated with EPA alone, TOS level did not change and the level of TAC significantly increased. The presence of EPA with TCDD minimized the toxic effects of the dioxin on primary hepatocytes cultures. Noteworthy, EPA has a protective effect against TCDD-mediated DNA damages.
Keywords: Eicosapentaenoic acid, TCDD, Liver, Oxidative stress, Genotoxicity
Introduction
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is an environmental contaminant that elicits a broad spectrum of toxic effects in a species-specific manner (Dere et al. 2011). It displays a wide spectrum of toxic effects, including dermal toxicity, reproductive toxicity, immunotoxicity, hepatotoxicity, carcinogenicity, teratogenicity, neurobehavioral, endocrine, and metabolic alterations (Talorete et al. 2001; Natsume et al. 2005; Hung et al. 2006; Jin et al. 2010). The relative potencies for TCDD have been reported in previous studies (Van den Berg et al. 1998). The studies have suggested that these effects are mediated by the aryl hydrocarbon receptor (AhR) and oxidative stress is an important constituent in the mechanism of TCDD toxicity. The exposures of mice and rats to different doses of TCDD have resulted in increases in the production of reactive oxygen species (ROS), lipid peroxidation (LPO), and DNA damage (Hassoun et al. 2000; Reyes-Hernánde et al. 2010; Aly and Khafagy 2011). Hence, TCDD has been shown to be responsible for multi-site cancers in experimental animals (Bock 1994; Grassman et al. 1998). TCDD is also a potent promoter of cancer in liver (Huff et al. 1994). However, in vivo and in vitro studies of human and animal cells have provided inconsistent findings of TCDD genotoxicity. On the other hand, there are equivocal findings of chromosomal aberrations in humans exposed in vivo to TCDD (IARC 1997) and the increases in production 8-oxo-2-deoxyguanosine (8-OH-dG) in the liver of mice (Hung et al. 2006) Recently, it has been reported that dioxin-like chemicals alter expression of numerous genes in liver, but it remains unknown which lie in pathways leading to major toxicities such as hepatotoxicity, wasting and lethality (Forgacs et al. 2010; Moffat et al. 2010).
Eicosapentaenoic acid (EPA) is an omega-3 fatty acid. Oils from cold-water fish are reported to be rich in (n-3) polyunsaturated fatty acids, in particular EPA and docosahexaenoic acid (DHA) (Eicher and McVey 1995). Evidence presented over the past 20 years has shown that long-chain polyunsaturated fatty acids are beneficial for health (Kruger et al. 2010). Diets rich in n-3 polyunsaturated fatty acids have been associated with a reduced risk of several types of cancer (Slagsvold et al. 2010). EPA was found to be useful for prevention and treatment of heart failure (Kitamura et al. 2011). EPA exhibited significant neuroprotective potential in neurological injuries (Dyall and Michael-Titus 2008). This fatty acid can act as hepatoprotective agent (Roy et al. 2007; El-Mowafy et al. 2011). In the last years, there has been a renewed interest in the biological activities of EPA. It has therapeutic properties, such as anti-inflammatory, immunomodulatory, antioxidant, and anti-tumour activities, among others (Mandal et al. 2010; Gapeyev et al. 2011; Li et al. 2011). Moreover, in a recent study, EPA was found the most effective fatty acid in free radical-scavenging potential (Richard et al. 2008). For this reason, in various tissues it attracts the attention of scientists in the search for new therapeutic usage. Antioxidants play an important role in inhibiting and scavenging free radicals, thus providing protection to humans against infectious and degenerative diseases. (Nader et al. 2010). According to the above data, TCDD is involved in the production of hepatic oxidative stress and supplementation with EPA may protect the animals from the harmful effects of TCDD. However, no attention was paid to the effects of EPA in hepatoprotection against TCDD; in addition, nothing is known on the effects of EPA upon LMNs in hepatocyte cultures. In our present study, we examined the effects of EPA on the viability of cells in TCDD-induced liver injury (with LDH and MTT assays). We also evaluated the role of EPA on antioxidant capacity (with TAC and TOS analysis) and DNA damage (with LMN rates and 8-OH-dG levels) after TCDD-treatment of hepatocyte cultures.
Materials and methods
Test compounds and chemicals
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD; CAS No. 1746-01-6) and eicosapentaenoic acid (EPA; CAS No. 10417-94-4) were purchased from Sigma-Aldrich® (USA). All other chemicals used in the experiments were also purchased from Sigma-Aldrich® and Fluka® (Germany).
Animals
Male rats of Sprague–Dawley strain (obtained from the Medical Experimental Research Center, Atatürk University, Turkey), of 200–300 g body weight, were used throughout the present studies. They were allowed water and standard laboratory chow ad libitum and maintained under standard light, temperature, and relative humidity conditions. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (1996). The study protocol was also approved by the local ethical committee.
Hepatocyte isolation and cultivation
Rats were sacrificed by CO2 overdose, and the livers were removed immediately. Isolated hepatocytes from rats were prepared by the collagenase perfusion technique (Wang et al. 2002). The liver was perfused through the hepatic portal vein with calcium-free Hanks balanced salt solution to remove blood for about 10 min at a flow rate of 2.5 mL/min. As soon as the liver became grayish brown in color, a second buffer solution containing collagenase (Hank’s balanced salt supplemented with 4 mM calcium chloride and 0.5 mg collagenase/mL) was perfused at the same rate until the liver appeared to have broken up. After treatment the liver minced into 3- to 4-mm pieces with a sterile scalpel. Following mechanical dissociation, the cells were filtered through gauze (100 μm) and centrifuged at 1350 rpm for 5 min. Then, the hepatocytes were collected in medium containing bovine serum albumin and bovine insulin. The cell suspension was filtered through gauze (100 μm) again and allowed to sediment for 20 min to eliminate cell debris, blood, and sinusoidal cells. The cells were then washed three times by centrifugation at 50 g, tested by Trypan blue dye exclusion for viability (always in the range of 82-93%). The hepatocytes were then suspended in a mixture of 75% Eagle’s minimum essential medium and 25% medium 199, supplemented with 10% fetal calf serum containing streptomycin (100 μg/ml), penicillin (100 IU/ml), bovine insulin (10 μg/ml), bovine serum albumin (0.2%) and NaHCO3 (5 mM). For the experimental procedure, hepatocytes were plated in tissue culture plates. The medium was changed 3-4 h later. The effect of TCDD and EPA was studied after 48 h of exposure in cultures maintained with a medium deprived of fetal calf serum but supplemented with hydrocortisone (3 × 10−6 M) hemisuccinate (Rakba et al. 1999). Hepatocytes were cultured for an additional 8 h before treatment.
Treatments
After 8 h of plating, when primary hepatocytes got adhered and attained their epithelial morphology, culture medium was aspirated and replaced with an equal volume of the medium supplemented with different TCDD (5 and 10 μM) and EPA (5, 10 and 20 μM) concentrations followed by incubation in CO2 incubator for 48 h (n = 7). This investigation stems from the previous works (Bechoua et al. 1999; Katic et al. 2010).
MTT assay
Cytotoxicity was assessed by measuring the formation of a formazan from 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) spectrophotometrically test, modified after Mosmann (1983). Hepatocytes were incubated with 0.7 mg/ml MTT for 30 min at 37 °C at the end of the experiment. After washing with PBS the blue formazan was extracted from cells with isopropanol/formic acid (95:5). Cytotoxicity was photometrically determined at 560 nm (Lewerenz et al. 2003).
LDH assay
Lactate dehydrogenase (LDH) activity was measured in the culture medium as an index of cytotoxicity, employing an LDH kit (Bayer Diagnostics®, France) adapted to the auto analyzer (ADVIA 1650, USA). Enzyme activity was expressed as the extra-cellular LDH activity percentage of the total activity on the plates.
TAC and TOS assays
The automated total antioxidant capacity (TAC) and total oxidant status (TOS) assays were carried out in the culture medium by commercially available kits (Rel Assay Diagnostics®, Turkey) in plasma samples obtained from blood cultures for 2 h (Erel 2004).
LMN assay
Liver MN assay was done by using the method of Suzuki et al. (2009). Immediately prior to evaluation, 10–20 μl of hepatocyte suspension was mixed with an equal volume of acridine orange (AO)—4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) staining solution (0.5 mg/ml AO; 10 μg/ml DAPI) for fluorescent staining. Approximately 10–20 μl of the mixture was dropped onto a glass slide and covered with a cover glass. Samples of well-isolated hepatocytes were evaluated with the aid of a fluorescence microscope counting the number of MNHEPs in 2000 hepatocytes for each animal. MNHEPs were defined as hepatocytes with round or distinct MNs that stained like the nucleus, with a diameter of 1/4 or less than that of the nucleus, and confirmed by focusing up and down, and taking into account hepatocyte thickness (performed by one observer: F. Geyikoglu).
Nucleic acid oxidation
DNA oxidation was determined by measuring the amount of 8-OH-dG adducts. DNA was digested by incubation with DNAase I, endonuclease, and alkaline phosphatase (Schneider et al. 1993). The amount of 8-OH-dG was measured by high-performance liquid chromatography (HPLC) with electrochemical detection as described previously (Floyd et al. 1986; Caraceni et al. 1997).
Statistics
The experimental data were analyzed using one-way analysis of variance (ANOVA) and Duncan’s tests to determine whether any treatment significantly differed from the controls or each others. Results are presented as mean ± SD values and the level of 0.05 was regarded as statistically significant.
Results
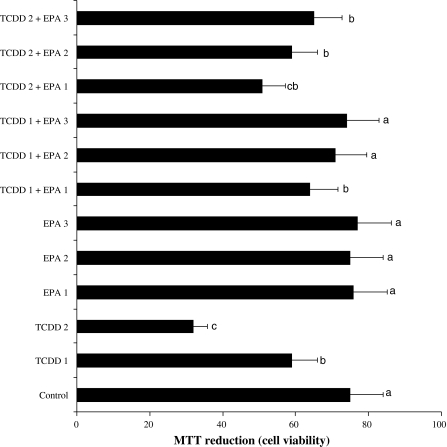
Figure 1 shows the results of cytotoxicity measured by MTT assay. When assayed in vitro on hepatocyte cells using the MTT assay, the values for the 5 and 10 μM TCDD-treated cells ranged from 1.3 and 2.4 fold lower than that for the control cells, respectively. However, the three doses of EPA (5, 10 and 20 μM) improved cellular in vitro activities against the toxicity of TCDD. And no cytotoxicity was observed for control cells.
Fig. 1.
MTT reduction in rat hepatocyte cultures maintained 48 h in the presence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), eicosapentaenoic acid (EPA) and their combinations. TCDD 1: 5 μM TCDD; TCDD 2: 10 μM TCDD; EPA 1: 5 μM eicosapentaenoic acid; EPA 2: 10 μM eicosapentaenoic acid; EPA 3: 20 μM eicosapentaenoic acid; bars shown by the same letter are not significantly different from each other at a level of 5%, using Duncan’s test. Also, this is valid for the relative combinations of letters. For example, the bar shown by the letters bc is not different from b, c and ab bars, but different from a or dbars. In other words, the bars having the different letters are different from each other.Values are means ± standard deviation (n = 7)
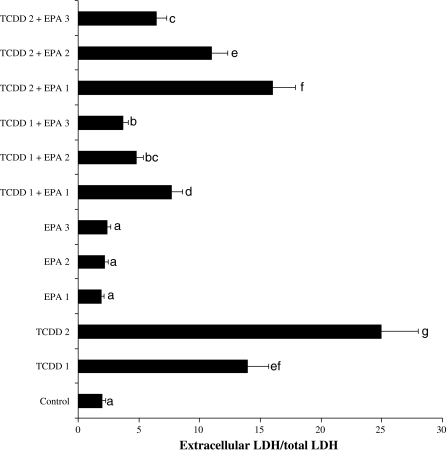
5 and 10 μM TCDD-induced hepatocellular damages were clearly evidenced by five and 12-fold increases in LDH compared with the observations of the controls (Fig. 2). Although LDH was not affected by different doses of EPA alone, the decrease of the level of enzyme reached statistical significance at 5, 10 and 20 μM doses of EPA against TCDD toxicity.
Fig. 2.
Extracellular level of lactate dehydrogenase (LDH) in rat hepatocyte cultures maintained 48 h in the presence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), eicosapentaenoic acid (EPA) and their combinations. Abbreviations are as in Fig. 1
Table 1 shows the effects of EPA on biochemical parameters in the tissue cells of all experimental groups. The hepatic TAC level decreased (p < 0.05) in the TCDD administered group. On the contrary, TOS level was increased by the effect of TCDD. The control hepatocytes maintained optimal value of the antioxidant status. On the other hand, the cells treated with 5, 10 and 20 μM of EPA alone showed increases in the levels of TAC. However, TOS levels were unchanged in both control and EPA treated groups. Moreover, application of EPA at all doses significantly (p < 0.05) increased the reduced TAC ratio by TCDD.
Table 1.
Extracellular total antioxidant capacity (TAC) and total oxidative stress (TOS) levels in cultured rat hepatocytes maintained 48 h in the presence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), eicosapentaenoic acid (EPA) and their combinations
| Treatments | TAC (mmol Trolox Equiv./L) | TOS (μmol H2O2 Equiv./L) |
|---|---|---|
| Control | 5.1 ± 0.5c | 8.3 ± 2.1a |
| TCDD 1 | 4.1 ± 0.6d | 11.4 ± 2.6c |
| TCDD 2 | 3.4 ± 0.4e | 16.4 ± 2.9e |
| EPA 1 | 5.2 ± 0.6c | 8.4 ± 3.1a |
| EPA 2 | 5.8 ± 0.5b | 8.3 ± 2.5a |
| EPA 3 | 6.3 ± 0.7a | 8.2 ± 2.4a |
| TCDD 1 + EPA 1 | 4.3 ± 0.4d | 10.8 ± 3.1c |
| TCDD 1 + EPA 2 | 4.9 ± 0.6c | 9.5 ± 2.7b |
| TCDD 1 + EPA 3 | 5.2 ± 0.5c | 8.5 ± 2.7a |
| TCDD 2 + EPA 1 | 3.8 ± 0.6e | 14.8 ± 3.4d |
| TCDD 2 + EPA 2 | 4.3 ± 0.7d | 12.6 ± 3.1cd |
| TCDD 2 + EPA 3 | 4.5 ± 0.5d | 10.3 ± 2.5c |
Abbreviations are as in Fig. 1. Means shown by the same letter are not significantly different from each other at a level of 5%, using Duncan’s test
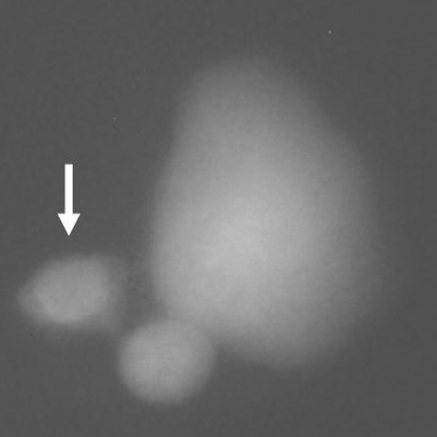
Table 2 shows the results of the LMN assay in rat hepatocyte cultures. The tested doses of TCDD induced statistically significant increases in formations of MNHEPs although EPA (at all doses) did not change the MNHEP numbers as compared to the control group. Moreover, EPA applications minimized the increased MNHEP rates by TCDD (Figs. 3 and 4).
Table 2.
Results of liver micronucleus (LMN) assay in cultured rat hepatocytes maintained 48 h in the presence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), eicosapentaenoic acid (EPA) and their combinations
| Treatments | MNHEP (%)/2000 HEP |
|---|---|
| Control | 0.3 ± 0.1a |
| TCDD 1 | 0.9 ± 0.3c |
| TCDD 2 | 1.7 ± 0.4d |
| EPA 1 | 0.2 ± 0.1a |
| EPA 2 | 0.3 ± 0.1a |
| EPA 3 | 0.2 ± 0.1a |
| TCDD 1 + EPA 1 | 0.8 ± 0.2bc |
| TCDD 1 + EPA 2 | 0.6 ± 0.2b |
| TCDD 1 + EPA 3 | 0.5 ± 0.2b |
| TCDD 2 + EPA 1 | 1.4 ± 0.5d |
| TCDD 2 + EPA 2 | 1.1 ± 0.4c |
| TCDD 2 + EPA 3 | 0.9 ± 0.3c |
HEP Hepatocyte, MNHEPs number of micronucleated hepatocytes. Abbreviations are as in Fig. 1. Means shown by the same letter are not significantly different from each other at a level of 5%, using Duncan’s test
Fig. 3.
A sample hepatocyte from 20 μM of eicosapentaenoic acid (EPA)-treated culture (healthy cell without MN)
Fig. 4.
A sample hepatocyte from 10 μM of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated culture (arrow shows MN formation in micronucleated cell)
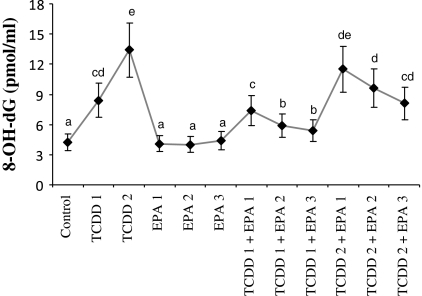
The status of 8-OH-dG in liver cells of control and experimental groups is presented in Fig. 5. Firstly, the hepatocyte levels of 8-OH-dG, a sensitive marker of oxidative DNA damage, were quantified with regard to TCDD treatment. It was observed that TCDD significantly increased 8-OH-dG concentrations in liver cells. Whereas, EPA at increasing doses did not have any affect on 8-OH-dG levels. Moreover, EPA significantly decreased 8-OH-dG concentrations in TCDD-treated hepatocytes in a dose related manner.
Fig. 5.
8-oxo-2-deoxyguanosine (8-OH-dG) adducts in rat hepatocyte cultures maintained 48 h in the presence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), eicosapentaenoic acid (EPA) and their combinations. Abbreviations are as in Fig. 1
Discussion
TCDD increases oxidative stress in hepatic and brain tissues of rats (Hassoun et al. 2000; Shertzer 2010). The greater expression of oxidative stress in liver exposed to TCDD may be due to inactivation of the antioxidant enzymes (Jin et al. 2007). Our study clearly demonstrates that a decreased ability to scavenge ROS may result from TCDD exposure, contributing to oxidative stress and thus to TCDD hepatotoxicity. Recent studies have indicated that the oxidative stress develops when the levels of antioxidants are lowered (Tapiero et al. 2004; Banudevi et al. 2005; Oztopcu-Vatan et al. 2009). Individual antioxidants can be considered very sensitive in revealing a pro-oxidant challenge (Regoli and Winston 1999). And it is emphasized that antioxidants (both enzymatic and non-enzymatic) play a central role in cellular oxidant defense systems that protect cells against damage induced by free radicals, such as superoxide anion and hydrogen peroxide (H2O2) (Cerutti et al. 1994; Valko et al. 2005; Turkez and Geyikoglu 2010). In the present study, important parameters, TAC and TOS are used to monitor the development and extent of liver damage due to oxidative stress. It is observed that TAC is significantly lowered in TCDD-treated rat hepatocytes. As known, TAC comes from non-enzymes like glutathione (GSH), as well as enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) (Murri et al. 2010). GSH is the most abundant and important intracellular antioxidant in animal cells (Kidd 1997). It detoxifies endogenous ROS and/or exogenous oxidative damage (Vina 1990). In mice, exposure to TCDD results in a significant depletion of GSH by oxidative stress in liver (Slezak et al. 2002). GSH is only one part of the antioxidant defense system for liver because there is abundant SOD and CAT in this organ. Therefore, TCDD toxicity can be decreased by SOD and CAT in neutralizing ROS (Wiseman and Chow 2000). SOD is the primary step of the defense mechanism in the antioxidant system against oxidative stress. It catalyzes the dismutation of superoxide radicals (O2−) into molecular oxygen (O2) and H2O2 (Fouchecourt and Riviere 1995; Kakarla et al. 2005). Endogenous H2O2 may be converted to H2O by CAT (Svistunenko 2005). CAT activity is also decreased in hepatocytes during TCDD treatment and its activity is inhibited by O2− (Kono and Fridovich 1982). The suppression of these enzyme activities has been linked to induction of oxidative stress and hepatotoxicity (Latchoumycandane et al. 2003; El-Tawil and Elsaieed 2005). In addition, the observed decreases in GSH-related enzyme activities indicate that TCDD may induce oxidative stress in rat liver by altering GSH metabolic mechanisms at the cellular level (Twaroski et al. 2001). The observed decline in the activity of GSH-Px in TCDD-treated hepatocytes may be ascribed to the reduction in the level of the glutathione and an increase in the level of peroxides (Twaroski et al. 2001). Thus, the balance of this enzyme system may also be essential to remove superoxide anion and peroxides generated in hepatocytes. The relative potency of TCDD is also estimated by comparing the different dose levels for a particular response. The dose dependency of TAC suppression in liver is driven by the range of dose–response data available in our study. The studies provide some evidence that TCDD toxicity generally is related to its increasing dose in tissues (Slezak et al. 2002; Latchoumycandane et al. 2003; Hoffman et al. 1998).
In humans and rodents, a mode of action of TCDD involves events that stem from the initial binding of TCDD to the aryl or aromatic hydrocarbon (Ah) receptor. The Ah receptor is an intracellular protein that acts as a signal transducer and activator for gene transcription (Safe 1990; DeVito et al. 1995). Besides, it is suggested that the relative potency of TCDD in cells may be modulated by binding to other proteins (DeVito et al. 1995). This study supports that TCDD toxicity is highly correlated with the decreasing TAC in liver. In present study, the decreased antioxidant status against increased TOS may be due to TCDD-induced protein carbonylation in rat hepatocytes as a result of oxidative stress or direct inhibition of the enzyme activities by ROS. Protein damage may cause the decline in enzyme activities of the antioxidant system and probably the electron transporting system, then leading to excessive ROS generation in hepatocytes (Meister and Anderson 1983).
TCDD-induced oxidative stress causes 8-OH-dG and single strand breaks in DNA from liver and brain tissues of rats (Hassoun et al. 2000; Hassoun et al. 2001). In our study, 8-OH-dG significantly increases by the effect of TCDD. In addition, LMN test was performed for the first time and revealed that TCDD exposure increased the rate of MNHEPs in hepatocytes. MN assay is a reliable test indicated chromosomal damage (Yilmaz et al. 2008; Eroglu et al. 2011) and MNHEPs production as 8-OH-dG is one of the key pieces of evidence for the possible involvement of antioxidant activity in oxidative DNA damage (Lee et al. 2010). ROS can alter vital cell components like polyunsaturated fatty acids, proteins and nucleic acids (Halliwell and Gutteridge 1990) The increased production of reactive oxygen species, lipid peroxidation, and DNA and membrane damage are always associated with TCDD exposure (Shertzer et al. 1998; Hung et al. 2006). In the present study, the TCDD also elicited severe instances of liver damage (incresing LDH). LDH in serum as a biological marker for liver damage increased (Park et al. 2010). Cell necrosis leads to a rise in concentration of the LDH enzyme in serum and tissue. LDH released into the medium provides an index of cell death and membrane permeability to LDH and an increase in LDH activity in the medium occurs as a result of cell membrane disintegration and enzyme leakage (Yokogawa et al. 2004). Thus, it is obvious that the degenerative effects of TCDD become more prominent with the increase in the LDH level. In addition, cytotoxicity, the degree to which a chemical can cause cell damage, is assessed in this study by the means of MTT assay. As shown in Fig. 1, the MTT assay results revealed that TCDD was cytotoxic to human liver. Overall, TCDD significantly decreases (P < 0.05) the viability of hepatocytes. Consistent with our finding, MTT assay demonstrated that the viabilities of human adrenocortical, pancreatic and mammary cells were significantly decreased after TCDD treatment (McDougal et al. 1997; Bradshaw et al. 2002; Koliopanos et al. 2002; Andersson et al. 2005. It is reported that the detrimental effects in hepatic tissue of TCDD may lead to disruption in the functional integrity of hepatocytes (Sakamoto et al. 1995; Boverhof et al. 2006; Czepiel et al. 2010; Kopec et al. 2010). Thus, TCDD is known to be responsible for the carcinogenic effects associated with oxidative DNA damage (Hung et al. 2006). Studies have been carried out on the role of antioxidant components in the detoxification of TCDD and limited efficacy of Ah receptor agonists has been established being unable to provide sufficient protection without use of antioxidants (Hung et al. 2006). In this investigation, EPA alone significantly increased TAC capacity and decreased TOS level. Moreover, EPA, which has not previously been used as detoxifier, is demonstrated to display beneficial effects on MNHEPs and 8-OH-dG, by returning their values close to those of the control group after TCDD insult. This suggests the antioxidant activities of EPA.
Sohma et al. (2007) found that EPA supplementation led to an increase of SOD mRNA expression in rat hepatocytes, thereby it protected cells by scavenging superoxide radicals. Again, it was indicated that EPA exerted antioxidative action by reducing the cellular levels of ROS and by maintaining higher GSH levels and antioxidant enzyme activities (Kim and Chung 2007). EPA has been tested for the galactosamine induced liver damage in mice and results indicated that EPA was able to present hepatoprotective effects (Roy et al. 2007). Also, protective effect of EPA was determined against valproate (a drug prescribed for epilepsy, bipolar affective disorder and migraine) hepatotoxicity (El-Mowafy et al. 2011). In the present study, the EPA has also a pronounced effect on the relative potency of TCDD. Noteworthy, it prevents liver damages (with LDH and MTT assays). Taken together these findings constitute evidence that the antioxidative properties of the EPA contribute to the prevention of hepatocyte degenerations and hepatic DNA damages induced by TCDD in rats and this suggests that changes in TAC are related to decrease of oxidative stress. Because effective antioxidants are free radical scavengers that interfere with radical chain reactions, it is possible to protect cellular DNA from oxidative stress by supplementation with antioxidants (Lee et al. 2010).
In summary, our findings clearly reveal that TCDD induces generation of DNA damages and also leads to cell deaths together with hepatocyte damages by a mechanism involving oxidative stress. Our results show that EPA in increasing doses is a promising source for the development of potential protectors against TCDD-induced oxidative stress and especially against MNHEPs and 8-OH-dG; thus, it may reduce the risk of carcinogenesis in liver. Because of the effects of EPA on TAC and TOS levels, this finding may provide new insight into the development of therapeutic and preventive approaches against TCDD toxicity.
Acknowledgments
We thank Dr. Abdulgani Tatar, Dr. Gokhan Yuksel for reading the manuscript and helpful discussion.
References
- Aly HA, Khafagy RM. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced cytotoxicity accompanied by oxidative stress in rat sertoli cells: possible role of mitochondrial fractions of sertoli cells. Toxicol Appl Pharmacol. 2011;252:273–280. doi: 10.1016/j.taap.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Andersson P, Rubio C, Poellinger L, Hanberg A. Gastric hamartomatous tumours in a transgenic mouse model expressing an activated dioxin/Ah receptor. Anticancer Res. 2005;25:903–911. [PubMed] [Google Scholar]
- Banudevi S, Arunkumar A, Sharmila M, Senthilkumar J, Balasubramanian K, Srinivasan N, Aruldhas MM, Arunakaran J. Diallyl disulfide-induced modulation of a few phase I and II drug metabolizing enzymes on Aroclor 1254 toxicity in Rattus norvegicus liver and ventral prostate. J Clin Biochem Nutr. 2005;36:59–65. doi: 10.3164/jcbn.36.59. [DOI] [Google Scholar]
- Bechoua S, Dubois M, Dominguez Z, Goncalves A, Nemoz G, Lagarde M, Prigent AF. Protective effect of docosahexaenoic acid against hydrogen peroxide-induced oxidative stress in human lymphocytes. Biochem Pharm. 1999;57:1021–1030. doi: 10.1016/S0006-2952(99)00012-X. [DOI] [PubMed] [Google Scholar]
- Bock KW. Aryl hydrocarbon or dioxin receptor: biologic and toxic responses. Rev Physiol Biochem Pharmacol. 1994;125:1–42. doi: 10.1007/BFb0030908. [DOI] [PubMed] [Google Scholar]
- Boverhof DR, Burgoon LD, Tashiro C, Sharratt B, Chittim B, Harkema JR, Mendrick DL, Zacharewski T. Comparative toxicogenomic analysis of the hepatotoxic effects of TCDD in Sprague Dawley rats and C57BL/6 mice. Toxicol Sci. 2006;94:398–416. doi: 10.1093/toxsci/kfl100. [DOI] [PubMed] [Google Scholar]
- Bradshaw TD, Trapani V, Vasselin DA, Westwell AD. The aryl hydrocarbon receptor in anticancer drug discovery: friend or foe? Curr Pharm Des. 2002;8:2475–2490. doi: 10.2174/1381612023392784. [DOI] [PubMed] [Google Scholar]
- Caraceni P, Maria N, Ryu HS, Colantoni A, Roberts L, Maidt ML, Pye Q, Bernardi M, Thiel DH, Floyd RA. Proteins but not nucleic acids are molecular targets for the free radical attack during reoxygenation of rat hepatocytes. Free Radic Biol Med. 1997;23:339–344. doi: 10.1016/S0891-5849(96)00571-0. [DOI] [PubMed] [Google Scholar]
- Cerutti P, Ghosh R, Oya Y, Amstad P. The role of the cellular antioxidant defense in oxidant carcinogenesis. Environ Health Perspect. 1994;102:123–129. doi: 10.1289/ehp.94102s10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepiel J, Biesiada G, Gajda M, Szczepański W, Szypuła K, Dabrowski Z, Mach T. The effect of TCDD dioxin on the rat liver in biochemical and histological assessment. Folia Biol (Krakow) 2010;58:85–90. doi: 10.3409/fb58_1-2.85-90. [DOI] [PubMed] [Google Scholar]
- Dere E, Lee AW, Burgoon LD, Zacharewski TR. Differences in TCDD-elicited gene expression profiles in human HepG2, mouse Hepa1c1c7 and rat H4IIE hepatoma cells. BMC Genomics. 2011;12:193. doi: 10.1186/1471-2164-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito MJ, Birnbaum LS, Farland WH, Gasiewicz TA. Comparisons of estimated human body burdens of dioxinlike chemicals and TCDD body burdens in experimentally exposed animals. Environ Health Perspect. 1995;103:820–831. doi: 10.1289/ehp.95103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SC, Michael-Titus AT. Neurological benefits of omega-3 fatty acids. Neuromol Med. 2008;10:219–235. doi: 10.1007/s12017-008-8036-z. [DOI] [PubMed] [Google Scholar]
- Eicher SD, McVey DS. Dietary modulation of Kupffer cell and splenocyte function during a Salmonella typhimurium challenge in mice. J Leukoc Biol. 1995;58:32–39. doi: 10.1002/jlb.58.1.32. [DOI] [PubMed] [Google Scholar]
- El-Mowafy AM, Abdel-Dayem MA, Abdel-Aziz A, El-Azab MF, Said SA (2011) Eicosapentaenoic acid ablates valproate-induced liver oxidative stress and cellular derangement without altering its clearance rate: Dynamic synergy and therapeutic utility. Biochim Biophys Acta 1811:460–467 [DOI] [PubMed]
- El-Tawil OS, Elsaieed EM. Induction of oxidative stress in the reproductive system of rats after subchronic exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Bull Environ Contam Toxicol. 2005;75:15–22. doi: 10.1007/s00128-005-0712-1. [DOI] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Eroğlu HE, Koca I, Yıldırım I (2011) In vitro cytotoxic potential of newly synthesized furo[3,2-c]pyran-4-one derivatives in cultured human lymphocytes. Cytotechnology 63:407–413 [DOI] [PMC free article] [PubMed]
- Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1:163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Forgacs AL, Burgoon LD, Lynn SG, LaPres JJ, Zacharewski T. Effects of TCDD on the expression of nuclear encoded mitochondrial genes. Toxicol Appl Pharmacol. 2010;246:58–65. doi: 10.1016/j.taap.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchecourt M, Riviere J. Activities of cytochrome P450-dependent monooxyganases and antioxidant enzymes in different organs of Norway rats (Rattus norvegicus) inhabiting reference and contaminated sites. Chemosphere. 1995;31:4375–4386. doi: 10.1016/0045-6535(95)00305-R. [DOI] [PubMed] [Google Scholar]
- Gapeyev AB, Kulagina TP, Aripovsky AV, Chemeris NK (2011) The role of fatty acids in anti-inflammatory effects of low-intensity extremely high-frequency electromagnetic radiation. Bioelectromagnetics 32:388–395 [DOI] [PubMed]
- Grassman JA, Masten SA, Walker NJ, Lucier GW. Animal models of human response to dioxins. Environ Health Perspect. 1998;106:761–775. doi: 10.1289/ehp.98106761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-B. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Li F, Abushaban A, Stohs SJ. The relative abilities of TCDD and its congeners to induce oxidative stress in the hepatic and brain tissues of rats after subchronic exposure. Toxicology. 2000;145:103–113. doi: 10.1016/S0300-483X(99)00221-8. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Li F, Abushaban A, Stohs SJ. Production of superoxide anion, lipid peroxidation and DNA damage in the hepatic and brain tissues of rats after subchronic exposure to mixtures of TCDD and its congeners. J Appl Toxicol. 2001;21:211–219. doi: 10.1002/jat.744. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Melancon MJ, Klein PN, Rice CP, Eisemann JD, Spann JW. Comparative developmental toxicity of planar polychlorinated biphenyl congeners in chickens, American kestrels, and common terns. Environ Toxicol Chem. 1998;17:747–757. doi: 10.1002/etc.5620170432. [DOI] [Google Scholar]
- Huff J, Lucier G, Tritscher A. Carcinogenicity of TCDD: experimental, mechanistic, and epidemiologic evidence. Annu Rev Pharmacol Toxicol. 1994;34:343–372. doi: 10.1146/annurev.pa.34.040194.002015. [DOI] [PubMed] [Google Scholar]
- Hung YC, Huang GS, Sava VM, Blagodarsky VA, Hong MY. Protective effects of tea melanin against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced toxicity: antioxidant activity and aryl hydrocarbon receptor suppressive effect. Biol Pharm Bull. 2006;29:2284–2291. doi: 10.1248/bpb.29.2284. [DOI] [PubMed] [Google Scholar]
- IARC, The International Agency for Research on Cancer (1997) Polychlorinated dibenzopara-dioxins and polychlorinated dibenzofurans. IARC, WHO, Lyon
- Jin X, Lok E, Bondy G, Caldwell D, Mueller R, Kapal K, Armstrong C, Taylor M, Kubow S, Mehta R, Chan HM. Modulating effects of dietary fats on methylmercury toxicity and distribution in rats. Toxicol. 2007;230:22–44. doi: 10.1016/j.tox.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Jin MH, Hong CH, Lee HY, Kang HJ, Han SW. Toxic effects of lactational exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) on development of male reproductive system: involvement of antioxidants, oxidants, and p53 protein. Environ Toxicol. 2010;25:1–8. doi: 10.1002/tox.20466. [DOI] [PubMed] [Google Scholar]
- Kakarla P, Vadluri G, Reddy KS. Response of hepatic antioxidant system to exercise training in aging female rat. J Exp Zool A Comp Exp Biol. 2005;303:203–208. doi: 10.1002/jez.a.149. [DOI] [PubMed] [Google Scholar]
- Katic J, Cemeli E, Baumgartner A, Laubenthal J, Bassano I, Stolevik SB, Granum B, Namork E, Nygaard UC, Lovik M, Leeuwen D, Loock KV, Anderson D, Fucic A, Decordier I. Evaluation of the genotoxicity of 10 selected dietary/environmental compounds with the in vitro micronucleus cytokinesis-block assay in an interlaboratory comparison. Food Chem Toxicol. 2010;48:2612–2623. doi: 10.1016/j.fct.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Kidd PM. Glutathione: systemic protectant against oxidative and free radical damage. Altern Med Rev. 1997;1:155–176. [Google Scholar]
- Kim YJ, Chung HY. Antioxidative and anti-inflammatory actions of docosahexaenoic acid and eicosapentaenoic acid in renal epithelial cells and macrophages. J Med Food. 2007;10:225–231. doi: 10.1089/jmf.2006.092. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Shibata R, Tsuji Y, Shimano M, Inden Y, Murohara T. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am J Physiol Heart Circ Physiol. 2011;300:814–821. doi: 10.1152/ajpheart.00771.2010. [DOI] [PubMed] [Google Scholar]
- Koliopanos A, Kleeff J, Xiao Y, Safe S, Zimmermann A, Büchler MW, Friess H. Increased arylhydrocarbon receptor expression offers a potential therapeutic target for pancreatic cancer. Oncogene. 2002;21:6059–6070. doi: 10.1038/sj.onc.1205633. [DOI] [PubMed] [Google Scholar]
- Kono Y, Fridovich I. Superoxide radicals inhibit catalase. J Biol Chem. 1982;257:5751–5754. [PubMed] [Google Scholar]
- Kopec AK, Burgoon LD, Ibrahim-Aibo D, Mets BD, Tashiro C, Potter D, Sharratt B, Harkema JR, Zacharewski TR. PCB153-elicited hepatic responses in the immature, ovariectomized C57BL/6 mice: Comparative toxicogenomic effects of dioxin and non-dioxin-like ligands. Toxicol Appl Pharma. 2010;243:359–371. doi: 10.1016/j.taap.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger MC, Coetzee M, Haag M, Weiler H. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res. 2010;49:438–449. doi: 10.1016/j.plipres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Chitra KC, Mathur PP. 2, 3, 7, 8-Tetrachlorodibenze-p-dioxin (TCDD) induces oxidative stress in theepididymis and epididymal sperm of adult rats. Arch Toxicol. 2003;77:280–284. doi: 10.1007/s00204-003-0439-x. [DOI] [PubMed] [Google Scholar]
- Lee TK, O’Brien KF, Wang W, Johnke RM, Sheng C, Benhabib SM, Wang T, Allison RR. Radioprotective effect of American ginseng on human lymphocytes at 90 minutes post-irradiation: a study of 40 cases. J Altern Complement Med. 2010;16:561–567. doi: 10.1089/acm.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz V, Hanelt S, Nastevska C, El-Bahay C, Ro¨hrdanz E, Kahl R. Antioxidants protect primary rat hepatocyte cultures against acetaminophen-induced DNA strand breaks but not against acetaminophen-induced cytotoxicity. Toxicology. 2003;191:179–187. doi: 10.1016/S0300-483X(03)00256-7. [DOI] [PubMed] [Google Scholar]
- Li M, Zhu Q, Hu C, Giesy JP, Kong Z, Cui Y. Protective effects of eicosapentaenoic acid on genotoxicity and oxidative stress of cyclophosphamide in mice. Environ Toxicol. 2011;26:217–223. doi: 10.1002/tox.20546. [DOI] [PubMed] [Google Scholar]
- Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 2010;402:602–607. doi: 10.1016/j.bbrc.2010.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal A, Wilson C, Safe S. Inhibition of 7, 12-dimethylbenz[a]anthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonists. Cancer Lett. 1997;120:53–63. doi: 10.1016/S0304-3835(97)00299-1. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Moffat ID, Boutros PC, Chen H, Okey AB, Pohjanvirta R. Aryl hydrocarbon receptor (AHR)-regulated transcriptomic changes in rats sensitive or resistant to major dioxin toxicities. BMC Genomics. 2010;26:11–263. doi: 10.1186/1471-2164-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murri M, Garcia-Delgado R, Alcázar-Ramirez J, Linde F, Fernández-Ramos A, Cardona F, Tinahones FJ. Assessment of cellular and plasma oxidative stress in SAHS patients before and after continuous positive airway pressure treatment. Clin Lab. 2010;56:397–406. [PubMed] [Google Scholar]
- Nader MA, El-Agamy DS, Suddek GM. Protective effects of DHA and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res. 2010;33:637–643. doi: 10.1007/s12272-010-0420-1. [DOI] [PubMed] [Google Scholar]
- Guide for the care and use of laboratory animals. Washington: National Academy Press; 1996. [Google Scholar]
- Natsume Y, Satsu H, Hamada M, Kitamura K, Okamoto N, Shimizu M. In vitro system for assessing dioxin absorption by intestinal epithelial cells and for preventing this absorption by food substances. Cytotechnology. 2005;47:79–88. doi: 10.1007/s10616-005-3753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztopcu-Vatan P, Kabadere S, Uyar R. The effect of pretreatment or combined treatment of quercetin on menadione toxicity in rat primary mixed glial cells in vitro. Cytotechnology. 2009;61:11–16. doi: 10.1007/s10616-009-9235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CM, Cha YS, Youn HJ, Cho CW, Song YS. Amelioration of oxidative stress by dandelion extract through CYP2E1 suppression against acute liver injury induced by carbon tetrachloride in Sprague-Dawley rats. Phytother Res. 2010;24:1347–1353. doi: 10.1002/ptr.3121. [DOI] [PubMed] [Google Scholar]
- Rakba N, Melhaoui A, Loyer P, Delcros JG, Morel I, Lescoat G. Bgugaine, a pyrrolidine alkaloid from Arisarum vulgare, is a strong hepatotoxin in rat and human liver cell cultures. Toxicol Lett. 1999;104:239–248. doi: 10.1016/S0378-4274(98)00375-0. [DOI] [PubMed] [Google Scholar]
- Regoli F, Winston GW. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicol Appl Pharmacol. 1999;156:96–105. doi: 10.1006/taap.1999.8637. [DOI] [PubMed] [Google Scholar]
- Reyes-Hernánde OD, Mejía-García A, Sánchez-Ocampo EM, Cabañas-Cortés MA, Ramírez P, Chávez-González L, Gonzalez FJ, Elizondo G. Ube2l3 gene expression is modulated by activation of the aryl hydrocarbon receptor: Implications for p53 ubiquitination. Biochem Pharmacol. 2010;80:932–940. doi: 10.1016/j.bcp.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008;57:451–455. doi: 10.1016/j.phrs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Roy R, Chandrasekhar D, Pujari P. Dietary fish oil as hepatoprotective agent in Mus musculus. Indian J Exp Biol. 2007;45:367–370. [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Crit Rev Toxicol. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- Sakamoto MK, Mima S, Tanimura T. A morphological study of liver lesions in Xenopus larvae exposed to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) with special reference to apoptosis of hepatocytes. J Environ Pathol Toxicol Oncol. 1995;14:69–82. [PubMed] [Google Scholar]
- Schneider JE, Phillips JR, Jr, Pye Q, Maidt ML, Price S, Floyd RA. Methylene blue and rose bengala photoinactivation of RNA bacteriophages: comparative studies of 8-oxoguanine formation in isolated RNA. Arch Biochem Biophys. 1993;301:91–97. doi: 10.1006/abbi.1993.1119. [DOI] [PubMed] [Google Scholar]
- Shertzer HG. Protective effects of the antioxidant 4b, 5, 9b, 10-tetrahydroindeno[1, 2-b]indole against TCDD toxicity in C57BL/6 J mice. Int J Toxicol. 2010;29:40–48. doi: 10.1177/1091581809352885. [DOI] [PubMed] [Google Scholar]
- Shertzer HG, Nebert DW, Puga A, Ary M, Sonntag D, Dixon K, Robinson LJ, Cianciolo E, Dalton TP. Dioxin causes a sustained oxidative stress response in the mouse. Biochem Biophys Res Commun. 1998;253:44–48. doi: 10.1006/bbrc.1998.9753. [DOI] [PubMed] [Google Scholar]
- Slagsvold JE, Pettersen CH, Størvold GL, Follestad T, Krokan HE, Schønberg SA. DHA alters expression of target proteins of cancer therapy in chemotherapy resistant SW620 colon cancer cells. Nutr Cancer. 2010;62:611–621. doi: 10.1080/01635580903532366. [DOI] [PubMed] [Google Scholar]
- Slezak BP, Hamm JT, Reyna J, Hurst CH, Birnbaum LS. TCDD-mediated oxidative stress in male rat pups following perinatal exposure. J Biochem Mol Toxicol. 2002;16:49–52. doi: 10.1002/jbt.10024. [DOI] [PubMed] [Google Scholar]
- Sohma R, Takahashi M, Takada H, Takada H, Kuwayama H. Protective effect of n-3 polyunsaturated fatty acid on primary culture of rat hepatocytes. J Gastroenterol Hepatol. 2007;22:1965–1970. doi: 10.1111/j.1440-1746.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takasawa H, Kobayashi K. Evaluation of a liver micronucleus assay with 12 chemicals using young rats (II): a study by the collaborative study group for the micronucleus test/Japanese Environmental Mutagen Society–Mammalian Mutagenicity Study Group. Mutagen. 2009;24:9–16. doi: 10.1093/mutage/gen047. [DOI] [PubMed] [Google Scholar]
- Svistunenko DA. Reaction of haem containing proteins and enzymes with hydroper- oxides: the radical view. Biochim Biophys Acta. 2005;1707:127–155. doi: 10.1016/j.bbabio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Talorete TP, Isoda H, Maekawa T. Alkylphenolic compounds and their effect on the injury rate, survival and acetylcholinesterase activity of the rat neuronal cell line PC12. Cytotechnology. 2001;36:163–169. doi: 10.1023/A:1014024516821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H, Townsend DM, Tew KD. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother. 2004;58:100–110. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F. Boric acid: a potential chemoprotective agent against aflatoxin b(1) toxicity in human blood. Cytotechnology. 2010;62:157–165. doi: 10.1007/s10616-010-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroski LW, O’Brien ML, Robertson LW. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes and selenium status: implications for oxidative stress. Biochem Pharmacol. 2001;62:273–278. doi: 10.1016/S0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Berg M, Birnbaum L, Bosveld ATC, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, Leeuwen FXR, Liem AKD, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Warn F, Zacharewski T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J. Glutathione: metabolism and physiologic functions. Boca Raton, FL: CRC; 1990. [Google Scholar]
- Wang HX, Ma XC, Deng QL. Cytotoxicity of flutamide and 2-hydroxyflutamide and their effects on CYP1A2 mRNA in primary rat hepatocytes. Acta Pharmacol Sin. 2002;23:562–566. [PubMed] [Google Scholar]
- Wiseman H, Chow SC. Biomolecular free radical toxicity: causes and prevention. Chichester: Wiley; 2000. [Google Scholar]
- Yilmaz S, Aksoy H, Unal F, Celik M, Yüzbaşloğlu D. Genotoxic action of fungicide Conan 5FL (hexaconazole) on mammalian cells in vivo and in vitro. Genetika. 2008;44:323–328. [PubMed] [Google Scholar]
- Yokogawa K, Watanabe M, Takeshita H, Nomura M, Mano Y, Miyamoto K. Serum aminotransferase activity as a predictor of clearance of drugs metabolized by CYP isoforms in rats with acute hepatic failure induced by carbon tetrachloride. Int J Pharm. 2004;269:479–489. doi: 10.1016/j.ijpharm.2003.09.045. [DOI] [PubMed] [Google Scholar]