Abstract
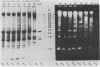
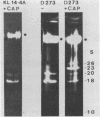
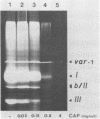
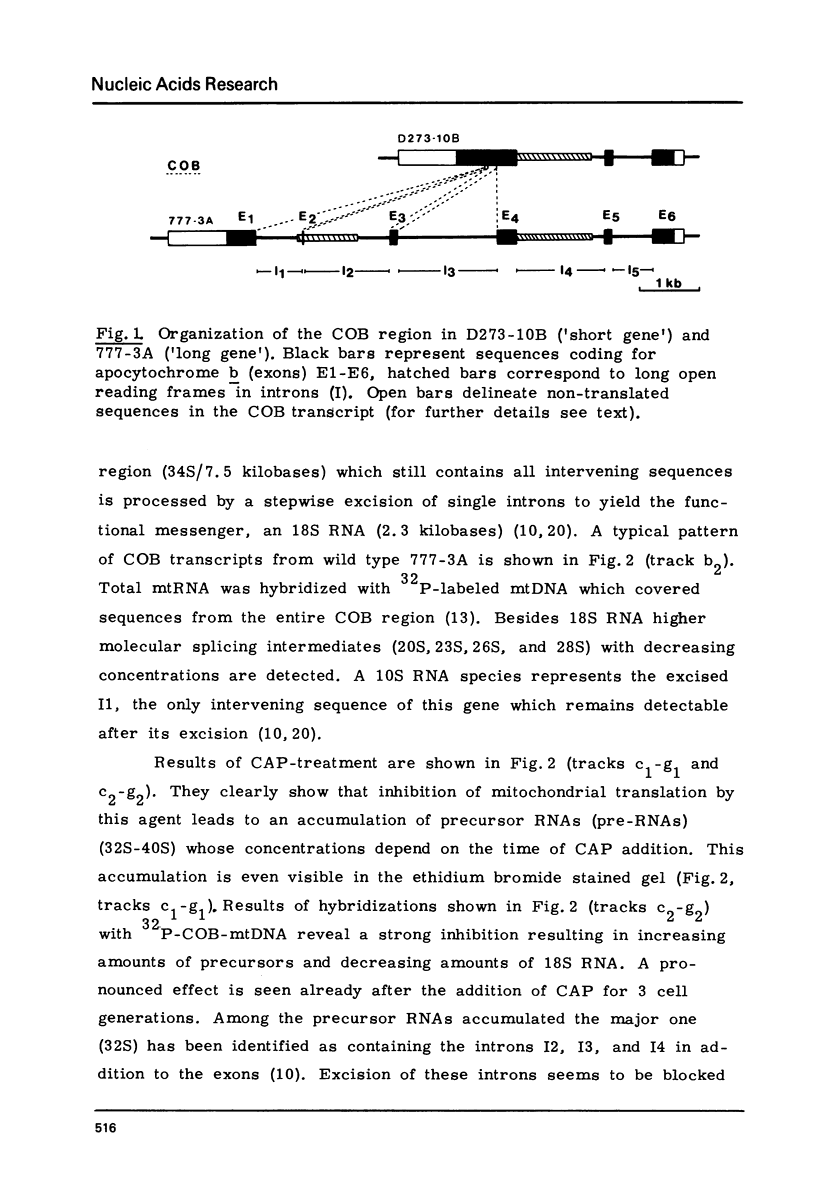
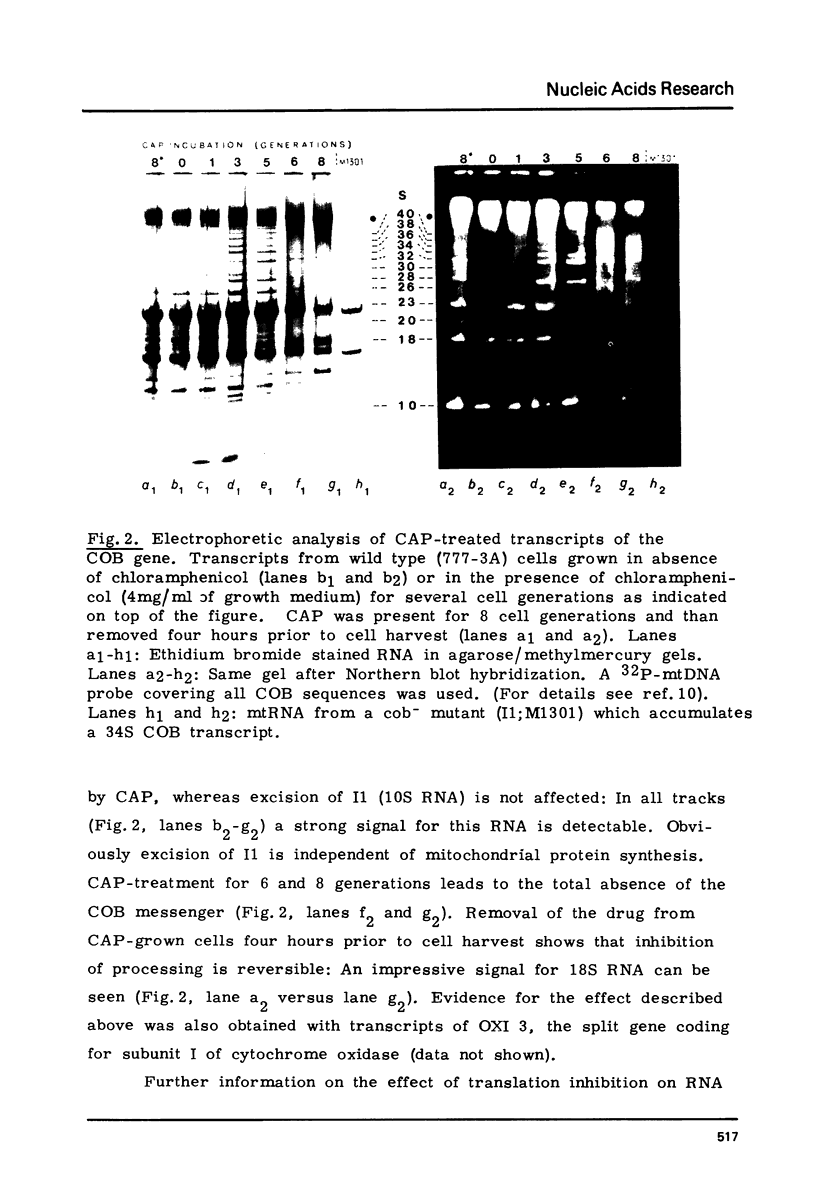
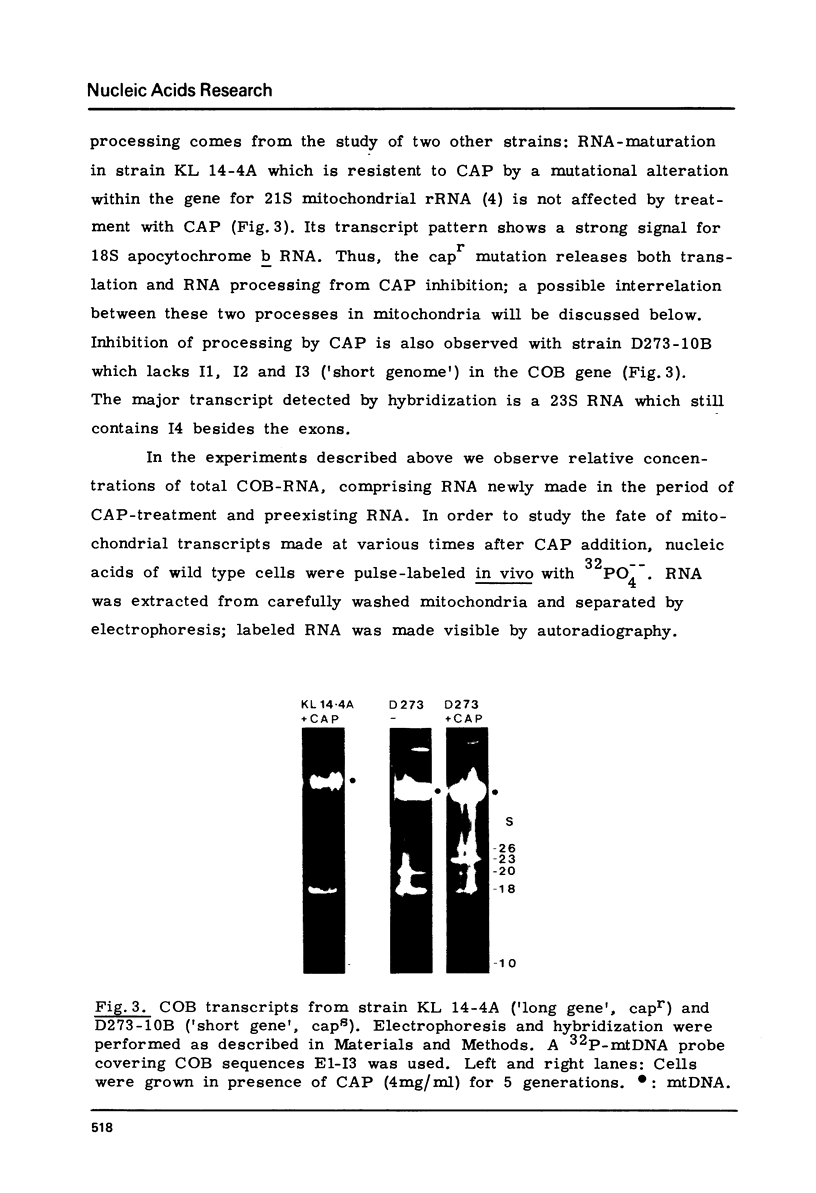
We have investigated the processing of transcripts of the split gene COB in yeast mitochondrial DNA from cells whose mitochondrial translation was blocked by chloramphenicol for several generations of cell growth. First analysis of transcripts by electrophoresis and RNA/DNA-hybridization clearly showed that cell growth in the presence of CAP leads to an inhibition of processing yielding an increasing amount of splicing intermediates of the COB transcript and decreasing amounts of the 18S mRNA coding for apocytochrome b. This observation is in accordance with the now widely favoured idea that mitochondrial proteins are involved in splicing of COB transcripts and that their reduction should hamper processing and - therefore - lead to an accumulation of pre-mRNAs. However, further information obtained by pulse-labeling of pre-mRNA in vivo in the presence of CAP for various times shows that even 30 minutes after addition of CAP a reduction of the processing rate is obtained. Based on these findings we conclude that maturation of mtRNAs is not only dependent on mitochondrial proteins, but also on a more direct interaction of the translation machinery and RNA processing whose nature is so far unknown.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bechmann H., Haid A., Schweyen R. J., Mathews S., Kaudewitz F. Expression of the "split gene" COB in yeast mtDNA. Translation of intervening sequences in mutant strains. J Biol Chem. 1981 Apr 10;256(7):3525–3531. [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Church G. M., Slonimski P. P., Gilbert W. Pleiotropic mutations within two yeast mitochondrial cytochrome genes block mRNA processing. Cell. 1979 Dec;18(4):1209–1215. doi: 10.1016/0092-8674(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Grivell L. A., Reijnders L., de Vries H. Altered mitochondrial ribosomes in a cytoplasmic mutant of yeast. FEBS Lett. 1971 Aug 15;16(3):159–163. doi: 10.1016/0014-5793(71)80121-7. [DOI] [PubMed] [Google Scholar]
- Haid A., Schweyen R. J., Bechmann H., Kaudewitz F., Solioz M., Schatz G. The mitochondrial COB region in yeast codes for apocytochrome b and is mosaic. Eur J Biochem. 1979 Mar;94(2):451–464. doi: 10.1111/j.1432-1033.1979.tb12913.x. [DOI] [PubMed] [Google Scholar]
- Halbreich A., Pajot P., Foucher M., Grandchamp C., Slonimski P. A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intron-derived circular DNA. Cell. 1980 Feb;19(2):321–329. doi: 10.1016/0092-8674(80)90506-1. [DOI] [PubMed] [Google Scholar]
- Heyting C., Meijlink F. C., Verbeet M. P., Sanders J. P., Bos J. L., Borst P. Fine structure of the 21S ribosomal RNA region on yeast mitochondria DNA. I. Construction of the physical map and localization of the cistron for the 21S mitochondrial ribosomal RNA. Mol Gen Genet. 1979 Jan 11;168(3):231–246. doi: 10.1007/BF00271496. [DOI] [PubMed] [Google Scholar]
- Jacq C., Lazowska J., Slonimski P. P. Sur un nouveau mécanisme de la régulation de l'expression génétique. C R Seances Acad Sci D. 1980 Jan 14;290(2):89–92. [PubMed] [Google Scholar]
- Kreike J., Bechmann H., Van Hemert F. J., Schweyen R. J., Boer P. H., Kaudewitz F., Groot G. S. The identification of apocytochrome b as a mitochondrial gene product and immunological evidence for altered apocytochrome b in yeast strains having mutations in the COB region of mitochondrial DNA. Eur J Biochem. 1979 Nov;101(2):607–617. doi: 10.1111/j.1432-1033.1979.tb19755.x. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Levens D., Ticho B., Ackerman E., Rabinowitz M. Transcriptional initiation and 5' termini of yeast mitochondrial RNA. J Biol Chem. 1981 May 25;256(10):5226–5232. [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Rubin G. M. Three forms of the 5.8-S ribosomal RNA species in Saccharomyces cerevisiae. Eur J Biochem. 1974 Jan 3;41(1):197–202. doi: 10.1111/j.1432-1033.1974.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Haid A., Grosch G., Schweyen R. J., Kaudewitz F. Pathways of transcript splicing in yeast mitochondria. Mutations in intervening sequences of the split gene COB reveal a requirement for intervening sequence-encoded products. J Biol Chem. 1981 Jul 25;256(14):7610–7619. [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Séquence nucléotidique du gène de l'ARN ribosomique 15S mitochondrial de la levure. C R Seances Acad Sci D. 1980 Dec 8;291(12):933–936. [PubMed] [Google Scholar]
- Van Ommen G. J., Boer P. H., Groot G. S., De Haan M., Roosendaal E., Grivell L. A., Haid A., Schweyen R. J. Mutations affecting RNA splicing and the interaction of gene expression of the yeast mitochondrial loci cob and oxi-3. Cell. 1980 May;20(1):173–183. doi: 10.1016/0092-8674(80)90245-7. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]