Abstract
Objective
To describe patterns of HIV infection among stable sexual partnerships across sub-Saharan Africa (SSA).
Methods
The authors defined measures of HIV discordancy and conducted a comprehensive quantitative assessment of discordancy among stable partnerships in 20 countries in SSA through an analysis of the Demographic and Health Survey data.
Results
HIV prevalence explained at least 50% of the variation in HIV discordancy, with two distinct patterns of discordancy emerging based on HIV prevalence being roughly smaller or larger than 10%. In low-prevalence countries, approximately 75% of partnerships affected by HIV are discordant, while only about half of these are discordant in high-prevalence countries. Out of each 10 HIV infected persons, two to five are engaged in discordant partnerships in low-prevalence countries compared with one to three in high-prevalence countries. Among every 100 partnerships in the population, one to nine are affected by HIV and zero to six are discordant in low-prevalence countries compared with 16–45 and 9–17, respectively, in high-prevalence countries. Finally, zero to four of every 100 sexually active adults are engaged in a discordant partnership in low-prevalence countries compared with six to eight in high-prevalence countries.
Conclusions
In high-prevalence countries, a large fraction of stable partnerships were affected by HIV and half were discordant, whereas in low-prevalence countries, fewer stable partnerships were affected by HIV but a higher proportion of them were discordant. The findings provide a global view of HIV infection among stable partnerships in SSA but imply complex considerations for rolling out prevention interventions targeting discordant partnerships.
Keywords: HIV, demographic and health survey, discordancy, sexual partnerships, sub-Saharan Africa, epidemiology, behavioural intervention, AIDS, sexual behaviour, epidemiology, epidemiology, HSV-2, epidemiology (general), homosexuality
Introduction
The HIV epidemic in large swaths of sub-Saharan Africa (SSA) is marked by substantial HIV prevalence in the general population.1 Recent community surveys and data from voluntary counselling and testing programmes in SSA revealed a considerable proportion of individuals living in stable discordant sexual partnerships (SDPs; ie, one partner testing HIV seropositive, while the other testing HIV seronegative).2 3
The levels of HIV discordancy among stable partnerships affected by HIV and the reasons behind the variability in HIV discordancy across different settings remain inadequately established. Moreover, there has been an intense debate about the role of HIV seroconversions among SDPs in the HIV epidemic and the priority of HIV prevention interventions among discordant couples relative to other prevention approaches such as among commercial sex networks.4–7 Recent progress in HIV prevention research has raised this debate to a new height by demonstrating substantial efficacies for several prevention interventions including highly active antiretroviral therapy,8 pre-exposure prophylaxis9 10 and microbicides.11 These promising findings in the context of the debate about the contribution of SDPs to HIV incidence confront us with the urgency to determine the patterns of HIV infection among stable partnerships and the distribution of discordancy across SSA.
The aim of this article is to describe the patterns of discordancy in SSA using an ecological approach to inform the debate about the role of discordancy in the HIV epidemic and to provide a basis upon which HIV prevention programmes among SDPs can be considered.
Methods
Overview and definitions
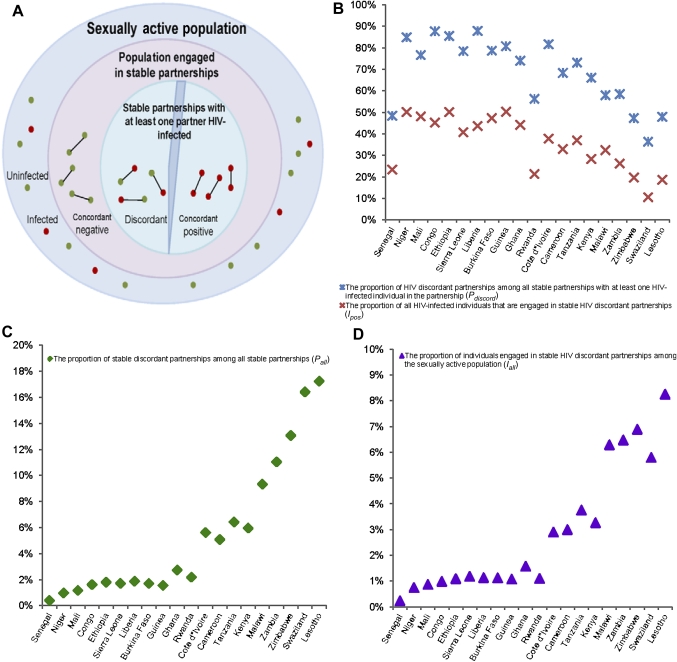
We described the distribution of prevalent HIV infections among stable sexual partnerships in SSA by analysing the Demographic and Health Survey (DHS) data for 20 countries. A stable sexual partnership is defined here as a spousal or cohabiting partnership as per the DHS methodology which defines a couple as a man and a woman living in a consensual union within a household at the time of the cross-sectional DHS survey.12 Every couple in a polygamous partnership is considered as a separate union. We followed an age-based definition for the sexually active population which include women aged 15–49 years and men aged 15–49, or 15–54, or 15–59 years based on the DHS inclusion criteria for each country.13 We characterise different aspects of discordancy by defining several population-level epidemiological measures (figure 1A):
Figure 1.
Measures of discordancy among stable sexual partnerships across 20 countries in sub-Saharan Africa. (A) Schematic diagram describing the presence of HIV discordancy among stable sexual partnerships in the sexually active population. (B) The proportion of HIV discordant partnerships among all stable partnerships with at least one HIV-infected individual in the partnership (), and the proportion of all HIV-infected individuals engaged in stable HIV discordant partnerships (). (C) The proportion of stable discordant partnerships among all stable partnerships . (D) The proportion of individuals engaged in stable HIV discordant partnerships . Countries are shown in order of increasing HIV prevalence.
1. The proportion of SDPs among all stable partnerships defined as
This measure conveys the level of discordancy among all stable sexual partnerships in the population.
2. The proportion of SDPs among all stable partnerships with at least one HIV-infected individual in the partnership defined as
This measure conveys the proportion of stable partnerships affected by HIV where the uninfected partner has not acquired the infection yet but is at risk of acquiring it from the infected partner in the future. The complement of (ie, ) provides the proportion of stable partnerships affected by HIV that are seropositive concordant.
3. The proportion of individuals engaged in SDPs among the entire sexually active population defined as
This measure conveys the abundance of individuals who are engaged in SDPs in the population. It can be expressed approximately by
The approximation is valid provided there are minimal differences in the rate of concurrent stable partnerships among the sub-population engaged in SDPs versus the rest of the population engaged in stable partnerships.
The abundance of HIV-infected individuals who can transmit the infection to their uninfected partners can be expressed approximately as . The approximation is valid provided, among the population engaged in SDPs, there are minimal differences in the number of persons who are HIV infected versus those who are not HIV infected. These differences can arise due to concurrency of sexual partnerships.
4. The proportion of HIV-infected individuals engaged in SDPs among the entire HIV-infected population defined as
This measure conveys the level of engagement of HIV-infected individuals in SDPs. It can be expressed approximately by
The approximation is valid provided there are minimal differences in the rate of concurrent stable partnerships among the subpopulation engaged in SDPs versus the subpopulation engaged in stable HIV concordant positive partnerships.
An additional useful measure is the prevalence of ‘positive partnerships’, that is the proportion of partnerships affected by HIV out of all stable partnerships . This measure is not independent of the above measures and can be obtained by
Each of these measures describes discordancy from a different angle as the nature of discordancy, and its implications in terms of prevention efforts cannot be well understood without appreciating the full picture drawn by all these measures simultaneously. The supplementary online appendix includes a heuristic and intuitive illustration of the application of these measures and their approximations in a hypothetical population. The appendix also demonstrates how the approximations provide satisfactorily precise estimates for these measures. It bears notice that each of these approximations is introduced either because the DHS data do not include sufficient information to calculate the measure per its exact definition or because the approximation minimises the effects of survey non-response rates and incompleteness of HIV testing data.
Statistical analyses
Countries were considered for analysis based on the availability of DHS HIV serological biomarker survey. For each country, we analysed only the most recent DHS survey where HIV data were collected. As a result, a total of 20 countries in SSA were included: Burkina Faso (2003), Cameroon (2004), Democratic Republic of Congo (2007), Cote d'Ivoire (2005), Ethiopia (2005), Ghana (2003), Guinea (2005), Kenya (2008–2009), Lesotho (2009), Liberia (2007), Malawi (2004), Mali (2006), Niger (2006), Rwanda (2005), Senegal (2005), Sierra Leone (2008), Swaziland (2006–2007), Tanzania (2007–2008), Zambia (2007) and Zimbabwe (2005–2006).
We calculated country-specific demographic and epidemiological indicators such as the size of the sexually active population, the proportion of the sexually active population engaged in stable partnerships (using the average rate of self-reported engagement in stable partnerships for men and women), the proportion of HIV-1-infected individuals engaged in stable partnerships (using the average rate of self-reported engagement in stable partnerships for HIV-1-infected men and women), the distribution of partnerships based on HIV-1 serostatus (concordant negative, discordant or concordant positive), the number of HIV-1-infected individuals and HIV-1 population prevalence. For this purpose, we merged the DHS couple data set for each country with the corresponding HIV-1 serostatus data set. We limited our analysis to HIV-1 seropositivity. In addition, couples, where only one of the partners had tested for HIV-1, were excluded from our analyses. Where couple data sets could not be identified, we matched individual data sets for men and women with the corresponding HIV serostatus data set, before merging both data sets using the husband line number as an identifier to form a couple data set with HIV serostatus information. This procedure was performed based on established guidelines for managing DHS data.12 We also applied the sampling weights retrieved from the DHS data sets in our calculations of demographic and epidemiological indicators.
Results
Overall, the mean response rate was high across countries of SSA and varied for the DHS surveys between 86.1% and 97.7% with a mean of 92.9% and for HIV testing between 67.0% and 96.5% with a mean of 83.8%. Women were more likely to participate in the DHS surveys compared with men (mean of 95.2% vs 90.6%), and this difference was statistically significant for all countries. Women were also more likely to undertake HIV testing compared with men (mean of 86.8% vs 80.4%), and this difference was also statistically significant for all countries. The rate of incomplete or missing HIV testing information among couples ranged from 2.2% to 33.8% with an average of 14.2% across the countries. Information was missing for both partners among 5.3% of the couples. In addition, men were more likely than women to have incomplete or missing HIV testing information (5.9% vs 3.0%), and the difference was statistically significant for 13 of the 20 countries.
Statistical analyses of DHS data also revealed variability in the demographic and epidemiological measures of discordancy across SSA. The proportion of adults reporting a current stable sexual partnership varied widely (from 35.3% to 76.3%). In addition to the well-described variation in HIV prevalence across countries (0.5%–23.0%), variations in HIV discordancy measures ranged from 36.3% to 87.8% for (figure 1B), 10.5% to 50.3% for (figure 1B), 0.4% to 17.2% for (figure 1C), 0.2% to 8.3% for (figure 1D) and 0.9% to 45.2% for . Table 1 presents HIV prevalence and discordancy measures for all 20 countries examined in this study.
Table 1.
Key epidemiological indicators across countries in sub-Saharan Africa
| Country | HIV population prevalence | Proportion of HIV discordant partnerships among all stable partnerships | Proportion of HIV discordant partnerships among all stable partnerships with at least one HIV-infected individual | Proportion of partnerships affected by HIV out of all stable partnerships | Proportion of individuals engaged in a stable discordant partnership among the sexually active population | Proportion of all HIV-infected individuals engaged in a stable discordant partnership |
| Low-prevalence countries (%) | ||||||
| Senegal | 0.54 | 0.40 | 48.36 | 0.83 | 0.23 | 23.36 |
| Niger | 0.68 | 0.97 | 84.90 | 1.14 | 0.74 | 50.13 |
| Mali | 1.20 | 1.16 | 76.65 | 1.51 | 0.87 | 48.11 |
| Congo | 1.27 | 1.61 | 87.69 | 1.84 | 0.99 | 45.25 |
| Ethiopia | 1.43 | 1.80 | 85.50 | 2.11 | 1.09 | 50.12 |
| Sierra Leone | 1.47 | 1.71 | 78.45 | 2.18 | 1.18 | 40.70 |
| Liberia | 1.50 | 1.87 | 87.83 | 2.13 | 1.13 | 43.61 |
| Burkina Faso | 1.54 | 1.69 | 78.68 | 2.15 | 1.13 | 47.34 |
| Guinea | 1.57 | 1.55 | 80.71 | 1.92 | 1.07 | 50.30 |
| Ghana | 2.04 | 2.73 | 74.02 | 3.69 | 1.58 | 44.10 |
| Rwanda | 3.00 | 2.18 | 56.29 | 3.87 | 1.10 | 21.31 |
| Cote d'Ivoire | 4.71 | 5.61 | 81.63 | 6.87 | 2.90 | 37.76 |
| Cameroon | 5.35 | 5.08 | 68.31 | 7.44 | 3.00 | 32.89 |
| Tanzania | 5.73 | 6.42 | 73.12 | 8.78 | 3.76 | 37.00 |
| Kenya | 6.36 | 5.95 | 66.07 | 9.01 | 3.26 | 28.27 |
| Mean | – | 2.72 | 75.21 | 3.70 | 1.60 | 40.02 |
| High prevalence countries (%) | ||||||
| Malawi | 11.74 | 9.33 | 57.93 | 16.11 | 6.29 | 32.39 |
| Zambia | 14.21 | 11.03 | 58.49 | 18.86 | 6.47 | 26.18 |
| Zimbabwe | 18.14 | 13.07 | 47.27 | 27.65 | 6.89 | 19.67 |
| Swaziland | 18.89 | 16.41 | 36.31 | 45.19 | 5.80 | 10.48 |
| Lesotho | 22.97 | 17.23 | 47.90 | 35.97 | 8.25 | 18.61 |
| Mean | – | 13.41 | 49.58 | 28.76 | 6.74 | 21.47 |
Countries are shown in order of increasing HIV prevalence.
Two suggestive patterns of HIV discordancy were discerned based on HIV prevalence being above or below 10%. In countries where HIV prevalence was <10% (low-prevalence countries), the vast majority of stable partnerships affected by HIV were discordant with a mean of 75.2% and a range between 48.4% and 87.8%. On the other hand, around half of these partnerships were discordant in countries where HIV prevalence exceeded 10% (high-prevalence countries), with a mean of 49.6% and a range between 36.3% and 58.5%. Similarly, high levels of were observed in low HIV prevalence countries where a mean of 40.0% of all HIV-infected individuals were engaged in SDPs (range between 21.3% and 50.3%) compared with high-prevalence countries where the mean was 21.5% (range between 10.5% and 32.4%).
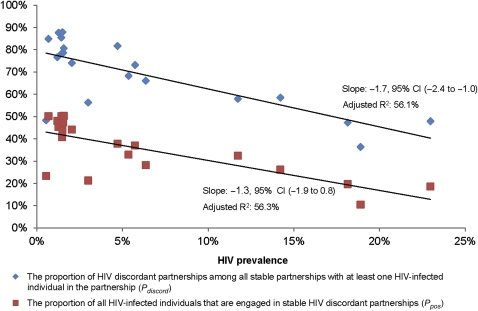
These trends were corroborated by fitting linear regressions revealing statistically significant declines in of −1.7% (95% CI −2.4% to −1.0%) and in of −1.3% (95% CI −1.9% to −0.8%), associated with a 1% increase in HIV prevalence. Figure 2 illustrates the decline in and with respect to HIV prevalence, which solely explains 56.1% and 56.3% of the variations in and , respectively. An exponential fit (not shown) explained 59.7% and 60.7% of the variation in each of these measures, respectively.
Figure 2.
Variability in measures of discordancy with respect to HIV prevalence across 20 countries in sub-Saharan Africa. Variation with respect to HIV prevalence of the proportion of HIV discordant partnerships among all stable partnerships with at least one HIV-infected individual in the partnership () and of the proportion of HIV-infected individuals that are engaged in stable HIV discordant partnerships among the sexually active population ().
Low-prevalence countries had also low levels of , and , whereas high-prevalence countries had high levels of , and . Indeed, out of every 100 stable sexual partnerships, a mean of four were affected by HIV and a mean of three were identified as discordant in low-prevalence countries compared with a mean of 29 and 13, respectively, in high-prevalence countries (table 1). In addition, only a mean of two individuals of every 100 sexually active adults were part of an SDP in low-prevalence countries compared with seven of every 100 in high-prevalence countries (table 1).
We conducted a stratified analysis of the direction of discordancy by sex. Our calculations indicated that women are the infected index partners in 49.4% of SDPs (range between 36.5% and 72.1%). There was, however, no discernable trend in the direction of discordancy by sex with respect to HIV prevalence across SSA (data not shown).
Discussion
We examined levels and variations in HIV discordancy measures among stable sexual partnerships across 20 countries in SSA by statistically analysing DHS data. Our analyses revealed that at least 50% of the variability in the two key discordancy measures ( and ) can be explained by differences in HIV prevalence. Both discordancy measures showed a continuous gradation with a steady decline associated with increasing HIV prevalence. These results can be viewed for simplicity as showing two distinct patterns of HIV discordancy for countries with low compared with high HIV prevalence.
In low-prevalence countries, the majority of stable partnerships with at least one HIV-infected individual were found discordant and a minority were concordant positive. Meanwhile, about half of these partnerships were discordant in high-prevalence countries. Similarly, about one of three HIV-infected individuals was engaged in an SDP in low-prevalence countries (ie, ) compared with about one of five in high-prevalence countries. Our results are in line with distinct empirical evidence from different communities in SSA suggesting a range for between 30% and 90%, with a pattern of about half of partnerships affected by HIV being discordant in high-prevalence areas.3 14–16
Our analyses also revealed that out of every 100 stable partnerships, four are affected by HIV in low-prevalence countries and 29 in high-prevalence countries. Out of all stable partnerships, about one in every 37 is discordant in low-prevalence countries compared with about one in every seven in high-prevalence countries. These results agree with existing empirical evidence suggesting that >10% of partnerships in high-prevalence areas may be discordant.2 3 17 Moreover, our findings show that approximately two of every 100 sexually active adults are engaged in an SDP in low-prevalence countries compared with about seven of every 100 in high-prevalence countries.
In agreement with findings of Eyawo et al14 for 14 countries in SSA, our analyses revealed that women are equally likely as men to be the infected partner in a discordant partnership (46% (95% CI 41% to 51%) in Eyawo et al vs 49.4% (95% CI 44.5% to 54.3%) in our study). Assuming that HIV male to female transmission probability is equal to that of female to male transmission probability, as suggested by empirical data,18 these results suggest that women may be equally likely as men to bring the infection to the stable partnership from sources external to the partnership.
While our study has described discordancy patterns, the nature and balance of drivers behind these observed patterns are not clear and may only be subject to speculative interpretation. Different hypotheses may explain the observed trends. The potential variability in HIV transmission probability per coital act across SSA affects the likelihood of an SDP becoming concordant positive and may explain part of the observed patterns. HIV transmission is dependent on multiple biological factors such as male circumcision,19–21 presence of other sexually transmitted diseases,22 23 presence of tropical co-infections that increase HIV viral load,24 viral factors,25 host genetics25 and host immunology.25 Behavioural factors and uptake of prevention interventions such as frequency of coital acts, temporal changes in sexual behaviour and condom use can also impact the likelihood of transmission within a partnership. All these factors may vary across different settings in SSA.
The likelihood of infection by external partners might also contribute to the observed variations in discordancy measures across SSA. Indeed, the likelihood of acquiring HIV from a source external to the SDP increases with HIV population prevalence.
The chance of partnership formation between infected and uninfected partners may also explain part of the observed dynamics. Assuming random mixing in a population with an HIV prevalence of ‘p’, , , and . Accordingly, the dependence on HIV prevalence of these expressions may explain part of the scale and variability of the discordancy measures.
One hypothesis might be that HIV incidence rate, that is, the annual risk of infection for an individual from all sources, might have declined in some countries with high HIV prevalence. In a setting where HIV incidence rate is declining, it is possible that the rate at which partnerships involving uninfected partners become discordant is less than the rate at which existing SDPs become concordant positive. This potential imbalance in the flow between concordant negative partnerships becoming discordant versus SDPs becoming concordant positive can lead to lower and as existing SDPs become concordant positive at a faster rate than new partnerships become discordant. This suggestion is consistent with empirical evidence for declining HIV incidence in several countries at high HIV prevalence.1 26 27 Mathematical models would be able to examine whether this could be consistent with a fuller analysis of the data and through an examination of data collected in future surveys.
Several study limitations might have affected our findings. First, our selection of the DHS survey for the different countries was constrained by the availability of HIV biomarker information at any particular survey. This limited our ability to consider more countries in SSA for analysis with more recent DHS surveys. In addition, we explored the variation in discordancy measures by HIV prevalence across different countries in SSA through an ecological analysis which uses aggregate rather than individual level data and, hence, limits our ability to establish causality. Furthermore, intra-country epidemic heterogeneity is present and may affect the validity of a national-level analysis using DHS data.
Given the multiple logistical difficulties in conducting DHS surveys, some of our discordancy measures may be biased due to inherent biases in the data such as the variability in response rates to HIV testing28 29 where, in countries such as Malawi and Zimbabwe, low response rates of 67% and 70%, respectively, have been recorded. Moreover, the higher likelihood of women to undertake HIV testing might have also reduced the probability of identifying partnerships affected by HIV.
Our findings might also be sensitive to non-random selection bias where urban populations and individuals with prior HIV testing may be more likely to refuse testing.30 31 We conducted further comparative analyses to detect underlying non-random epidemiological or socio-demographic differences between couples with and without complete HIV serostatus information. The comparisons indicated that for most countries, no significant differences exist between both groups with respect to age. Yet, couples with higher education and living in urban areas were less likely to have complete HIV serostatus information. Despite these limitations, the DHS surveys are among the most methodologically rigorous surveys available in SSA.32
Discordant couples are a key population for HIV prevention programmes and several major randomised clinical trials have established substantial efficacies of several prevention interventions that could benefit this population group.8–11 Our findings have implications for how such prevention interventions can be translated into programmes for SDPs, and in the population at large, and form a base upon which the impact of future HIV intervention programmes can be quantified. Our results suggest complex considerations for the implementation of HIV prevention interventions among SDPs. While the majority of partnerships affected by HIV are discordant in low HIV prevalence settings, their enrolment in prevention interventions constitute a major logistical challenge since the absolute number and the proportion of all sexual partnerships that are discordant are small. Conversely, while the number and proportion of all sexual partnerships that are discordant are large in high-prevalence settings, only about half of partnerships affected by HIV are discordant and only about a fifth of all HIV-infected individuals are in SDPs.
Although prevention interventions aiming at the protection of the seronegative individual in an SDP would reduce HIV incidence among discordant partnerships, quantifying the impact of such interventions at the population level requires further empirical epidemiological evidence and thorough mathematical modelling analyses. Moreover, the feasibility of prevention interventions targeting individuals engaged in SDPs must factor the abundance of discordancy, the ease and expense of reaching these individuals and the labour-intensiveness and long-term effectiveness of these interventions.
Key messages.
At least 50% of the variation in HIV discordancy can be explained by differences in HIV prevalence.
In high HIV prevalence countries, which make up the large majority of HIV-infected individuals, most HIV-infected individuals are not in stable discordant couples.
In high HIV prevalence countries, a large fraction of stable partnerships are affected by HIV and half are discordant.
In low HIV prevalence countries, a small fraction of stable partnerships are affected by HIV, but the vast majority of them are discordant.
Complex considerations exist for rolling out prevention interventions targeting discordant partnerships.
Supplementary Material
Footnotes
Funding: Qatar National Research Fund (QNRF) (NPRP 08-068-3-024), the Biostatistics, Epidemiology, and Biomathematics Research Core at the Weill Cornell Medical College in Qatar (WCMC-Q), The Wellcome Trust, and the National Institutes of Health (R01 AI083034).
Competing interests: None.
Contributors: HC managed the databases, conducted the data analyses and wrote the first draft of the paper. LJA-R conceived and led the design and conduct of the study and contributed to the data analyses. All authors contributed to the interpretation of the results and drafting of the manuscript. All authors were involved in the finalisation of the manuscript and approved the final version.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.UNAIDS UNAIDS Report on the Global AIDS Epidemic. http://www.unaids.org/globalreport/Global_report.htm, 2010 [Google Scholar]
- 2.Guthrie BL, De Bruyn G, Farquhar C. HIV-1-discordant couples in sub-Saharan Africa: explanations and implications for high rates of discordancy. Curr HIV Res 2007;5:416–29 [DOI] [PubMed] [Google Scholar]
- 3.Lingappa JR, Lambdin B, Bukusi EA, et al. Regional differences in prevalence of HIV-1 discordance in Africa and enrollment of HIV-1 discordant couples into an HIV-1 prevention trial. PLoS One 2008;3:e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunkle K, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet 2008;371:2183–91 [DOI] [PubMed] [Google Scholar]
- 5.Matovu JK. Preventing HIV transmission in married and cohabiting HIV-discordant couples in sub-Saharan Africa through combination prevention. Curr HIV Res 2010;8:430–40 [DOI] [PubMed] [Google Scholar]
- 6.Gray R, Ssempiija V, Shelton J, et al. The contribution of HIV-discordant relationships to new HIV infections in Rakai, Uganda. AIDS 2011;25:863–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shelton JD. A tale of two-component generalised HIV epidemics. Lancet 2010;375:964–6 [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thigpen MC, Kebaabetswe PM, Smith DK, et al. Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study. 6th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention; 17–20 July, Rome, Italy: International AIDS Society, 2011 [Google Scholar]
- 10.Partners PrEP Study Team Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women: The Partners PrEP Study. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 July, Rome, Italy: International AIDS Society, 2011 [Google Scholar]
- 11.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329:1168–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutstein S, Rojas G. Guide to DHS Statistics. Calverton, Maryland, 2006. http://www.measuredhs.com/pubs/pub_details.cfm?ID=718&title=Guide%20to%20DHS%20Statistics [Google Scholar]
- 13.Demographic and Health Surveys. Calverton: ICF Macro; http://www.measuredhs.com/ (accessed 19 May 2010). [Google Scholar]
- 14.Eyawo O, de Walque D, Ford N, et al. HIV status in discordant couples in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:770–7 [DOI] [PubMed] [Google Scholar]
- 15.De Walque D. Sero-discordant couples in five African countries: implications for prevention strategies. Popul Dev Rev 2007;33:501–23 [Google Scholar]
- 16.Were WA, Mermin JH, Wamai N, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr 2006;43:91–5 [DOI] [PubMed] [Google Scholar]
- 17.Lurie MN, Williams BG, Zuma K, et al. Who infects whom? HIV-1 concordance and discordance among migrant and non-migrant couples in South Africa. AIDS 2003;17:2245–52 [DOI] [PubMed] [Google Scholar]
- 18.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005;191:1403–9 [DOI] [PubMed] [Google Scholar]
- 19.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med 2005;2:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 2007;369:643–56 [DOI] [PubMed] [Google Scholar]
- 21.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 2007;369:657–66 [DOI] [PubMed] [Google Scholar]
- 22.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008;3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korenromp EL, de Vlass SJ, Nagelkerke NJ, et al. Estimating the magnitude of STD cofactor effects on HIV transmission: how well can it be done? Sex Transm Dis 2001;28:613–21 [DOI] [PubMed] [Google Scholar]
- 24.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science 2006;314:1603–6 [DOI] [PubMed] [Google Scholar]
- 25.Kaul R, Cohen CR, Chege D, et al. Biological factors that may contribute to regional and racial disparities in HIV prevalence. Am J Reprod Immunol 2011;65:317–24 [DOI] [PubMed] [Google Scholar]
- 26.Gregson S, Gonese E, Hallett TB, et al. HIV decline in Zimbabwe due to reductions in risky sex? Evidence from a comprehensive epidemiological review. Int J Epidemiol 2010;39:1311–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehle TM, Hallett TB, Shisana O, et al. A decline in new HIV infections in South Africa: estimating HIV incidence from three national HIV surveys in 2002, 2005 and 2008. PLoS One 2010;5:e11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marston M, Harriss K, Slaymaker E. Non-response bias in estimates of HIV prevalence due to the mobility of absentees in national population-based surveys: a study of nine national surveys. Sex Transm Infect 2008;84(Suppl 1):i71–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra V, Barrere B, Hong R, et al. Evaluation of bias in HIV seroprevalence estimates from national household surveys. Sex Transm Infect 2008;84(Suppl 1):i63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reniers G, Eaton J. Refusal bias in HIV prevalence estimates from nationally representative seroprevalence surveys. AIDS 2009;23:621–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnighausen T, Bor J, Wandira-Kazibwe S, et al. Correcting HIV prevalence estimates for survey nonparticipation using Heckman-type selection models. Epidemiology 2011;22:27–35 [DOI] [PubMed] [Google Scholar]
- 32.Mishra V, Vaessen M, Boerma JT, et al. HIV testing in national population-based surveys: experience from the Demographic and Health Surveys. Bull World Health Organ 2006;84:537–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.