Summary
Fasting-induced suppression of the hypothalamic-pituitary-thyroid (HPT) axis is an adaptive response to decrease energy expenditure during food deprivation. Previous studies demonstrate that leptin communicates nutritional status to the HPT axis through thyrotropin-releasing hormone (TRH) in the paraventricular nucleus (PVN) of the hypothalamus. Leptin targets TRH neurons either directly or indirectly via the arcuate nucleus through pro-opiomelanocortin (POMC) and agouti-related peptide/neuropeptide Y (AgRP/NPY) neurons. To evaluate the role of these pathways in vivo, we developed double knockout mice that lack both the melanocortin 4 receptor (MC4R) and NPY. We show that NPY is required for fasting-induced suppression of Trh expression in the PVN. However, both MC4R and NPY are required for activation of hepatic pathways that metabolize T4 during the fasting response. Thus, these signaling pathways play a key role in the communication of fasting signals to reduce thyroid hormone levels both centrally and through a peripheral hepatic circuit.
Introduction
Circulating thyroid hormone (TH) levels are critical regulators of energy expenditure in rodents and man (Barker, 1951; Du Bois, 1936). Thus, their acute regulation in periods of nutritional stress or illness is an important adaptive mechanism to survival. In humans, long-term caloric restriction leads to a fall in TH levels, which is secondary to a fall in leptin levels (Rosenbaum et al., 2005; Rosenbaum et al., 2002). Similar physiology exists in rodents during an acute fast where a drop in leptin levels leads to a fall in both the predominant form of thyroid hormone, thyroxine (T4), and the active form, triiodothyronine (T3), over 24–48 hr (Ahima et al., 1996; Connors et al., 1985; Legradi et al., 1997). Additionally, suppression of the hypothalamic-pituitary-thyroid (HPT) axis during nutritional stress extends to both thyrotropin-releasing hormone (TRH) production in the paraventricular nucleus (PVN) of the hypothalamus and thyroid-stimulating hormone (TSH) production from the pituitary (Blake et al., 1991; Blake et al., 1992; Spencer et al., 1983). To further emphasize the role of leptin in the HPT axis, leptin replacement during food restriction prevented decreases of TH levels in both humans and rodents (Ahima et al., 1996; Legradi et al., 1997; Rosenbaum et al., 2002). Thus, the nutritional regulation of the HPT axis by leptin becomes an important paradigm to mechanistically understand the importance of this axis in regulating energy expenditure and ultimately body weight.
Two possible mechanisms have been proposed to explain leptin’s actions on the HPT axis. These two mechanisms both involve hypothalamic neurocircuitry that regulates TRH production in the PVN: 1. Leptin acts directly through its receptors on hypophysiotropic TRH neurons that project to the median eminence to regulate TSH production in the pituitary (Harris et al., 2001; Nillni et al., 2000; Perello et al., 2006) or 2. Leptin regulates TRH neurons indirectly via its actions on pro-opiomelanocortin (POMC) and agouti-related peptide/neuropeptide Y (AgRP/NPY) neurons in the arcuate nucleus (Bjorbaek and Hollenberg, 2002; Fekete et al., 2001; Fekete et al., 2000a; Fekete et al., 2002b; Legradi et al., 1998). Although these pathways are not mutually exclusive, genetic data suggest that leptin signaling is absolutely required for normal function of the HPT axis as mice with leptin receptor mutations have central hypothyroidism whereas mice that lack the MC4R or NPY have normal T4 levels at baseline (Bates et al., 2004; Erickson et al., 1997; Fekete et al., 2004). Contrary to these observations, the POMC-derived peptide α-melanocyte stimulating hormone (α-MSH) can stimulate Trh expression in the PVN and both AgRP and NPY can suppress Trh expression in the PVN suggesting that neurons within the arcuate nucleus could play a major role in the regulation of the HPT axis through their action on Trh (Fekete et al., 2001; Fekete et al., 2000a; Fekete et al., 2002b). Indeed, ablation of the arcuate nucleus by monosodium L-glutamate in rats prevented a fasting-induced decrease in Trh mRNA, serum TSH and T4 levels strengthening the role of the arcuate nucleus in metabolic regulation of Trh (Legradi et al., 1998). However, because MSG may have more widespread effects including increased leptin insensitivity, this experiment did not rule out other pathways for leptin action (Nillni, 2010). Despite these insights, the exact pathway that regulates leptin’s actions on the HPT axis is not clear.
To definitively identify the pathway that allows for the fasting-induced fall in TH levels we developed a genetic approach using mice that lacked both MC4R and NPY and thus would be unable to communicate α-MSH, AgRP and NPY signals to the PVN. Importantly, we pair-fed these animals to prevent obesity and to equalize leptin levels. Remarkably, we show that signaling through NPY and MC4R controls the fasting-induced suppression of thyroid hormone levels through two mechanisms: 1. Fasting-induced suppression of the central axis requires NPY and 2. A second pathway based in the liver that enhances the metabolism of TH requires MC4R and NPY. Thus, MC4R and NPY signaling engage both central and peripheral targets to control TH levels during a fast. This engagement of multiple targets underscores the importance of this pathway in the adaptation to nutritional stress.
Results
Obesity prevents suppression of the HPT axis during fasting
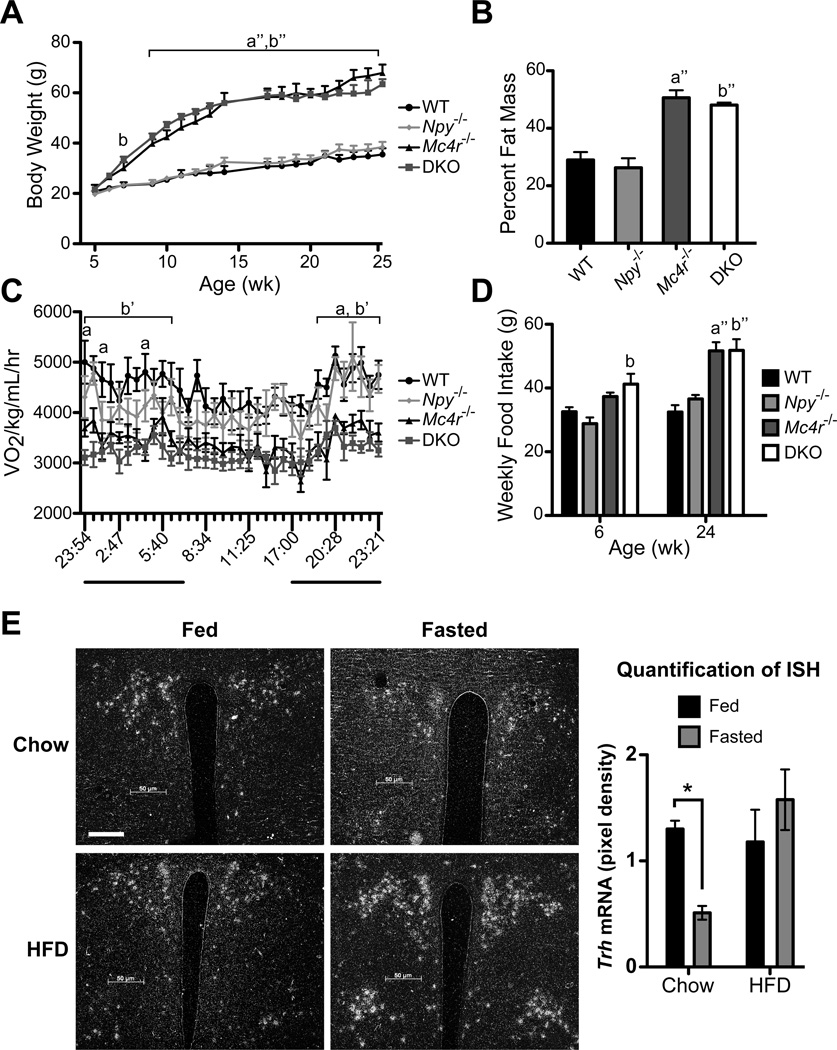
To develop a genetic approach that would determine the importance of POMC and AgRP/NPY signaling in the regulation of the HPT axis in vivo we crossed Npy+/− mice with Mc4r+/− mice to develop WT, Npy−/−, Mc4r−/− and Mc4r−/− Npy−/− (DKO) mice (Balthasar et al., 2005; Erickson et al., 1996). As shown in Figure 1, DKO mice display a phenotype similar to Mc4r−/− mice (Figure 1A) with increased fat mass measured by DEXA (Figure 1B), decreased energy expenditure measured via CLAMS apparatus (Figure 1C), and hyperphagia (Figure 1D) (Balthasar et al., 2005; Huszar et al., 1997). Like their male counterparts, female DKO mice were similar in phenotype to Mc4r−/− mice (data not shown). Interestingly, obese DKO mice had further impaired glucose tolerance compared to Mc4r−/− mice (Figure S1).
Figure 1. DKO mice have a similar metabolic phenotype as Mc4r−/− mice.
A, Average body weight of male WT, Npy−/−, Mc4r−/− and DKO mice from 5–25 wk of age. B, Percent fat mass as measured by DEXA. C, CLAMS: Mice were placed in a CLAMS apparatus and monitored for oxygen consumption (VO2) over a 24-hr period. The dark bars indicate dark period in the light cycle. D, Weekly food intake at 6 and 24 wk of age. A – D, data are presented as mean ± SEM. n = 6 per genotype. A, C – D were measured with repeated-measures 2-way ANOVA with Bonferroni post hoc test. B was measured with 1-way ANOVA with Tukey’s multiple comparison post hoc test. a = p < 0.05 Mc4r−/− versus WT and Npy−/−; a” = p < 0.001 Mc4r−/− versus WT and Npy−/−; b = p < 0.05 DKO versus WT and Npy−/−; b’ = p < 0.01 DKO versus WT and Npy−/−; b” = p < 0.001 DKO versus WT and Npy−/−. E, ISH was performed brain sections using a 35S-labeled riboprobe against mouse Trh mRNA. Representative images of fed and fasted C57Bl/6 mice on chow or HFD are shown of the PVN at original magnification, x10; scale bar, 50 µm. Quantification of Trh expression (right panel). Data are presented as relative pixel densities. Significance was tested by 2-way ANOVA with Bonferroni post hoc test (n = 4 per diet and fed status). Data are presented as mean ± SEM. * = p < 0.05. See also Figures S1 and S2.
Since both Mc4r−/− and DKO mice were extremely obese, we were concerned with their ability to sense a fast and suppress their HPT axis given their high leptin levels. To test this hypothesis, C57Bl/6 mice were placed either on a chow diet or a high fat diet (HFD) for 14 wk. As expected, HFD-fed mice had increased body weight and leptin levels that were 20-fold higher than WT mice (Figure S2A–B). We then subjected these mice to a 36-hr fast. While leptin levels fell during a fast in HFD-fed mice they were over 20 times higher than fasted leptin levels in chow-fed mice (Figure S2B). Whereas total thyroxine (bound T4 plus free T4 in serum: TT4) and total triiodothyronine (bound T3 plus free T3 in serum: TT3) levels fell significantly in chow-fed mice, they did not change in HFD-fed mice as previously demonstrated in rats (Figure S2C–D) (Goodman et al., 1980). Consistent with these observations, Trh mRNA decreased 60% in the PVN of fasted chow-fed animals but did not fall in the PVN of HFD-fed animals (Figure 1E). Trh mRNA did not change in the lateral hypothalamic area (LHA), anterior hypothalamic area (AHA) or the dorsomedial hypothalamus (DMH) regardless of nutritional status in chow or HFD-fed mice (Figure S2E–J). Taken together these data demonstrate that obesity prevents fasting-induced suppression of the HPT axis at 36 hr fasting. Therefore, to properly interpret the roles of the MC4R and NPY in DKO mice we would need to prevent obesity in these models.
Pair-feeding rescues obese phenotype of Mc4r−/− and DKO mice
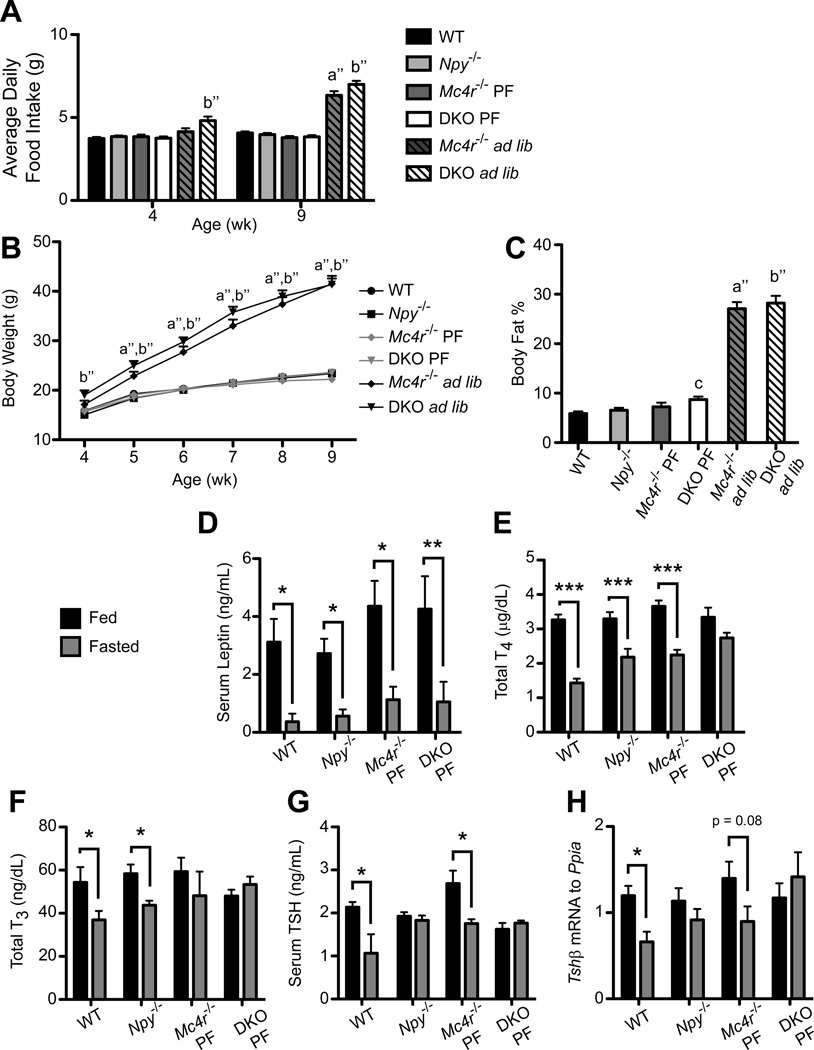
To prevent obesity in Mc4r−/− and DKO animals we developed a pair-feeding paradigm such that the food intake of Mc4r−/− and DKO mice was adjusted to WT levels on a daily basis. Importantly, using this method we were able to deliver identical amounts of food to all genotypes through 9 wk of age (Figure 2A), which allowed body weight (Figure 2B) and fat mass (Figure 2C) to be relatively equalized across genotypes in the pair-fed groups compared to the ad libitum-fed Mc4r−/− and DKO mice. Furthermore, pair-fed Mc4r−/− and DKO mice with a body fat percentage of greater than 15% as measured by MRI were excluded from the study.
Figure 2. Pair-fed mice lacking both NPY and MC4R fail to suppress thyroid hormone and TSH levels.
Average daily food intake (A), Body Weight (B) and Body Fat percentage (C) of combined NPY/MC4R cohorts 2 and 3: WT (n = 40), Npy−/− (n = 38), pair-fed Mc4r−/− (Mc4r−/− PF; n = 20) and DKO (DKO PF; n = 28), and ad libitum Mc4r−/− (Mc4r−/− ad lib; n = 11) and DKO (DKO ad lib; n = 12) mice. A, B were analyzed by repeated-measures 2-way ANOVA with Bonferroni post hoc test. C was analyzed via 1-way ANOVA with Tukey’s multiple comparison post hoc test. For A–C, data are presented as mean ± SEM. a” = p < 0.001 Mc4r−/− ad lib versus WT, Npy−/−, Mc4r−/− PF and DKO PF; b” = p < 0.001 DKO ad lib versus WT, Npy−/−, Mc4r−/− PF and DKO PF; c = p < 0.05 DKO PF versus WT. D, Serum leptin levels were measured in cohort 2 fed and fasted WT (n = 5, n = 7), Npy−/− (n = 10, n = 10), Mc4r−/− PF (n = 4, n = 4), and DKO PF (n = 6, n = 5) mice. E, Combined cohort 2 and 3 Total T4 (TT4) levels of fed and fasted WT (n = 23, n = 17), Npy−/− (n = 21, n = 17), Mc4r−/− PF (n = 10, n = 10), and DKO PF (n = 16, n = 12) mice. F, Total T3 (TT3) concentration of cohort 2 WT (n = 5, n = 7), Npy−/− (n = 10, n = 10), Mc4r−/− PF (n = 4, n = 4), and DKO PF (n = 6, n = 5) mice. G, Serum TSH concentration of cohort 2 WT (n = 4, n = 4), Npy−/− (n = 10, n = 10), Mc4r−/− PF (n = 3, n = 3), and DKO PF (n = 6, n = 5) mice. H, Tshβ levels relative to the control gene Ppia (cyclophilin) were measured via QPCR in cohort 3 WT (n = 18, n = 11), Npy−/− (n = 10, n = 8), Mc4r−/− PF (n = 7, n = 7), and DKO PF (n = 9, n = 7) mice. For D–H, data are presented as mean ± SEM. Significance was measured with 2-way ANOVA with Bonferroni post hoc test. * = p < 0.05; ** = p < 0.01; *** = p < 0.001. See also Figure S3.
We next fasted groups of WT, Npy−/−, and pair-fed Mc4r−/− and DKO mice to determine the response of the HPT axis. As shown in Figure 2D, leptin levels of fed mice were slightly but not significantly higher in pair-fed Mc4r−/− and DKO mice compared to WT and Npy−/− mice but fell equivalently after the fast. A comparison of the leptin levels of pair-fed Mc4r−/− and DKO mice to levels in fed and fasted ad libitum-fed Mc4r−/− and DKO mice (Figure S3A) confirms the effectiveness of the pair-feeding paradigm in establishing normal leptin levels in Mc4r−/− and DKO animals.
Fasting-induced suppression of the HPT axis is impaired in Npy−/− mice and prevented in DKO mice
To determine the contribution of POMC and AgRP/NPY signaling in fasting-induced suppression of the HPT axis, we measured several factors in the HPT axis in fed and fasted WT, Npy−/−, and pair-fed Mc4r−/− and DKO mice. Following 36 hr of fasting, the concentration of TT4 dropped significantly by 56% in WT mice (Figure 2E). As previously described, TT4 fell significantly by 36% in Npy−/− mice, although the drop was not as great as in WT mice with 36 hr of food deprivation (Erickson et al., 1997). TT4 concentrations also fell in pair-fed Mc4r−/− mice, but did not change in pair-fed DKO mice with fasting. As expected, TT4 concentrations measured in ad libitum-fed Mc4r−/− and DKO mice did not change with fasting consistent with the impact of very high levels of leptin on the HPT axis (Figure S3B). Concentrations of TT3 and serum TSH remained unchanged in fasting pair-fed DKO mice compared to the respective 32% and 50% drop seen in WT mice (Figure 2F–G). Whereas TT3 levels fell by 25% in Npy−/− mice, serum TSH did not. Conversely, pair-fed Mc4r−/− mice suppressed serum TSH by 31% but not TT3 with fasting. No changes in TT3 or serum TSH were observed in ad libitum-fed Mc4r−/− and DKO mice (Figure S3C–D).
Since serum TSH does not always accurately reflect TRH action, we confirmed the serum TSH data by measuring Tshβ mRNA levels from the pituitaries of a separate cohort of fed and fasted WT, Npy−/−, and pair-fed Mc4r−/− and DKO mice via quantitative RT-PCR (QPCR). Fasting Npy−/− and pair-fed DKO mice failed to suppress Tshβ compared to WT mice (1.8 fold) while Tshβ expression trended to suppression with fasting (1.5 fold, p = 0.08) in Mc4r−/− mice (Figure 2H). Thus, at the level of the pituitary, NPY appears critical for suppression of Tshβ and serum TSH during fasting, which suggests that NPY may mediate this effect through communication of fasting signals to TRH neurons in the PVN.
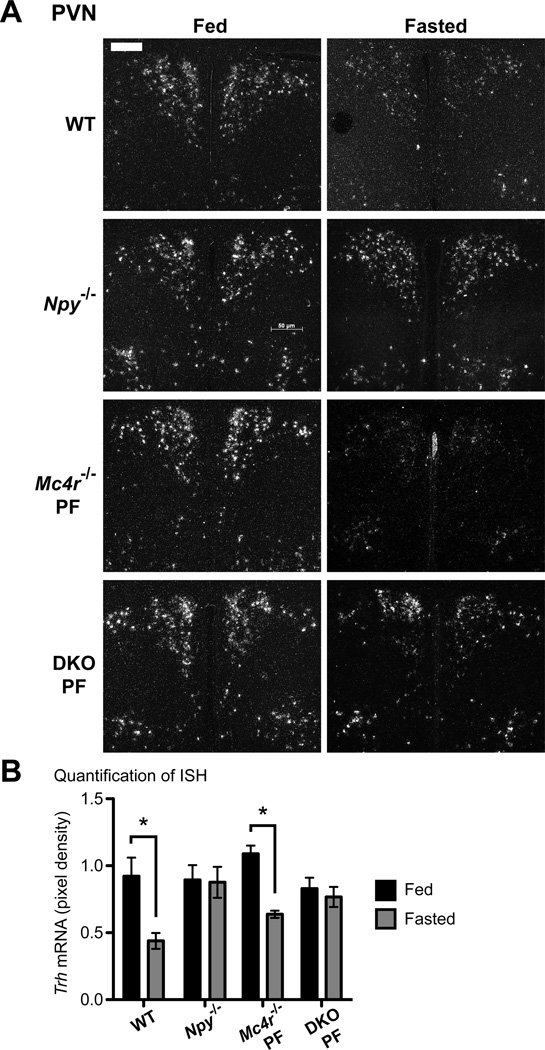
To test this hypothesis, we performed in situ hybridization for Trh expression in the PVN of the hypothalamus, the location of hypophysiotropic neurons that project from the PVN to the median eminence to release TRH peptide. As has been previously demonstrated, Trh expression was significantly decreased by 53% in fasted WT mice when compared to fed controls (Figure 3A–B) (Blake et al., 1991). Similarly, fasted pair-fed Mc4r−/− mice also repressed Trh expression by 42% in the PVN when compared to fed-controls. Importantly, Trh was only regulated during fasting in WT and Mc4r−/− mice in the PVN and not in other areas of the hypothalamus where Trh is expressed, which includes the AHA, the LHA and the DMH (Figure S4A–C). Remarkably, fasting did not regulate Trh levels in the PVN of Npy−/− mice. Similar results were seen in pair-fed DKO mice validating the key requirement for NPY in mediating the fasting-induced suppression of Trh (Figure 3). Furthermore, Trh expression did not change in fasting ad libitum-fed Mc4r−/− and DKO mice, again highlighting the impact of obesity on the HPT axis (Figure S4D). Taken together, NPY signaling is the major contributor to communicating fasting signals to the central HPT axis, as Trh, Tshβ and serum TSH remained unchanged in fasted Npy−/− and pair-fed DKO mice. Despite this, TT4 and TT3 levels still drop in fasted Npy−/− mice, but not in pair-fed DKO mice. This suggests that the MC4R plays a role in suppression of thyroid hormone levels during fasting through a TRH-independent pathway. This is further supported by the failure of pair-fed Mc4r−/− mice to fully suppress TT4 levels and TT3 levels during a fast.
Figure 3. NPY is required for fasting-induced suppression of Trh expression.
ISH was performed on brain sections using a 35S-labeled riboprobe against mouse Trh mRNA. A. Representative images of fed and fasted WT (n = 5, n = 6), Npy−/− (n = 8, n = 9) and pair-fed Mc4r−/− PF (n = 4, n = 6) and DKO PF (n = 4, n = 5) mice are shown of the PVN, x10; scale bar, 50 µm. B. Quantification of Trh expression. Data are presented as mean pixel density ± SEM. Significance was measured with 2-way ANOVA with Bonferroni post hoc test. * = p < 0.05. See also Figure S4.
Hepatic metabolism of thyroid hormone during fasting requires the MC4R and NPY
To explain the adaptive response of TH levels in Npy−/− mice during a fast despite the absence of Trh repression, we hypothesized that fasting-induced peripheral hepatic metabolism of TH was operative in Npy−/− mice, but not in pair-fed DKO mice (de Herder et al., 1988). This circuit would allow TH levels to fall independent of TRH and would require the MC4R. Previous studies have shown that the orphan nuclear receptor constitutive androstane receptor (CAR), or nuclear receptor subfamily 1, group I, member 3 (Nr1i3), and its target genes, sulfotransferases (Sults) and UDP-glucuronosyltransferases (Ugts), are pivotal to hepatic TH metabolism as they accelerate deiodination and clearance of TH (Maglich et al., 2004; Mol and Visser, 1985; Vansell and Klaassen, 2002; Visser, 1996). Furthermore, Nr1i3 and several of its target genes are up regulated in the liver during fasting to increase TH metabolism (Maglich et al., 2004). Indeed, fasting-induced repression of TH levels is impaired in CAR−/− mice (Maglich et al., 2004).
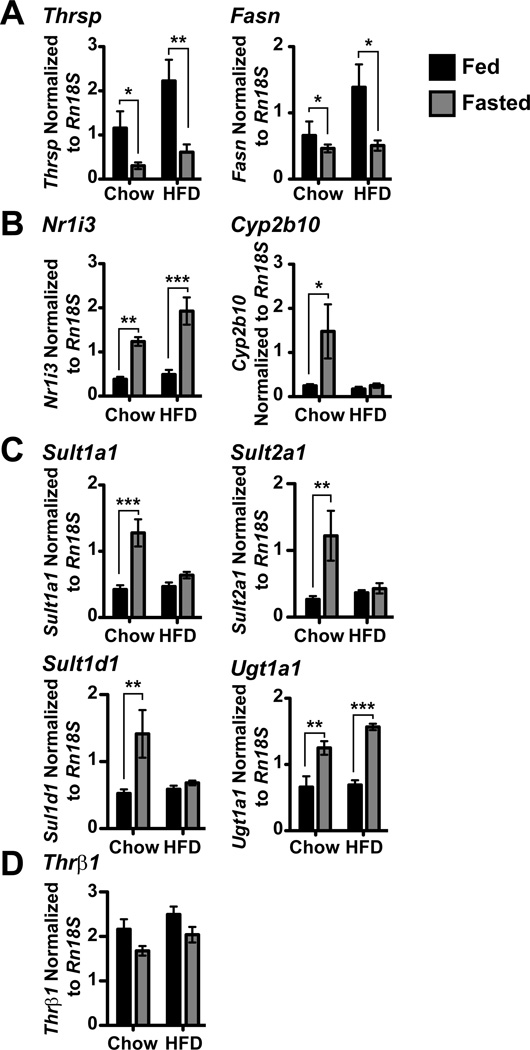
First, to establish the sensitivity of the livers of Npy−/− and pair-fed Mc4r−/− and DKO mice to fasting signals, we measured expression of lipogenic genes that decrease during fasting via QPCR (Carr et al., 1984; Clarke et al., 1990). Thyroid hormone responsive SPOT14 (Thrsp) mRNA was significantly repressed with fasting in all genotypes (Figure 4A) while fatty acid synthase (Fasn) mRNA levels also decreased with fasting in WT (2.6 fold) and Npy−/− (3.9 fold) mice and trended toward reduced levels in pair-fed Mc4r−/− (2.1 fold, p = 0.055) and DKO (2.6 fold, p = 0.051) mice.
Figure 4. MC4R and NPY are necessary for up regulation of Nr1i3 and its target genes during fasting.
Expression of various genes in the liver was measured via QPCR and normalized to Rn18S expression in WT (n = 17, n = 10), Npy−/− (n = 10, n = 7) and pair-fed Mc4r−/− PF (n = 7, n = 7) and DKO PF (n = 9, n = 7) mice. A, Expression of Thrsp and Fasn. B, Expression of Nr1i3 and its classical target gene, Cyp2b10. C, Expression of the Nr1i3-target genes involved in hepatic TH metabolism: Sult1a1, Sult2a1, Sult1d1 and Ugt1a1. D, Expression of Thrβ1. In all panels, data are presented as mean ± SEM. Significance was measured with 2-way ANOVA with Bonferroni post hoc test. * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
We next assessed the roles of MC4R and NPY signaling pathways in the regulation of Nr1i3 and its target genes in the liver. Nr1i3 expression was up regulated during fasting (1.8 fold, Figure 4B) in WT mice, which has been demonstrated previously (Maglich et al., 2004). Additionally, Nr1i3 expression increased with fasting in Npy−/− mice (2.1 fold), however expression of Nr1i3 remained unchanged in pair-fed Mc4r−/− and DKO mice. To understand the impact of impaired Nr1i3 regulation during fasting, we measured the expression of the classic Nr1i3-target gene, cytochrome p450, family 2, subfamily b, polypeptide 10 (Cyp2b10) (Honkakoski et al., 1998). Expression of Cyp2b10 has been shown to increase with fasting and our results agree as WT (8.4 fold) and Npy−/− (18.8 fold) mice both up regulate hepatic Cyp2b10 expression following a 36-hr fast (Figure 4B) (Brown et al., 1995). Cyp2b10 expression in pair-fed DKO mice showed no response to fasting, whereas pair-fed Mc4r−/− mice trended toward an up regulation (5.7 fold, p = 0.062). These results implicate a role for both MC4R and NPY in Nr1i3-mediated regulation of hepatic TH metabolism as Cyp2b10 may respond to fasting in pair-fed Mc4r−/− mice, but not in DKO mice.
As failure of hepatic Nr1i3 to respond to fasting signals in Mc4r−/− and DKO mice may explain the inability of DKO mice to suppress TH levels during a fast, we next measured the Nr1i3 targets responsible for hepatic thyroid hormone metabolism. Previous studies have demonstrated that Phase II sulfotransferases mediate hepatic metabolism of TH by sulfating the phenol group and marking TH for inactivation, which occurs via the type 1 deiodinase (Mol and Visser, 1985). Sulfotransferase 1A1 (Sult1a1), which sulfates TH in vitro and has a high affinity for T4 and T3, is up regulated during fasting (Kester et al., 1999; Li et al., 2001; Maglich et al., 2004). Sult1a1 expression was significantly increased with fasting in WT (2.3 fold) and Npy−/− (2.5 fold) mice compared to their fed counterparts (Figure 4C). Both pair-fed Mc4r−/− and DKO mice failed to up regulate Sult1a1 with fasting. Additionally, we examined two other sulfotransferases: Sult2a1 preferentially sulfates T3 and Sult1d1 has a very similar protein structure to Sult1a1 although its role in the liver is still unknown (Li and Anderson, 1999; Sakakibara et al., 1995). Both genes were significantly up regulated in WT (5.1 and 2.5 fold, respectively), Npy−/− (3.7 and 3.6 fold, respectively) and pair-fed Mc4r−/− (3.9 and 2.1 fold, respectively) mice, whereas pair-fed DKO mice did not regulate these genes during fasting (Figure 4C). Taken together, these data confirm that pair-fed DKO mice have impaired Nr1i3-mediated hepatic TH metabolism. To a lesser degree, pair-fed Mc4r−/− mice are also defective in Nr1i3 signaling as they fail to up regulate both Nr1i3 and Sult1a1 with fasting, but Cyp2b10, Sult2A1 and Sult1d1 are responsive. Removal of NPY signaling in addition to MC4R signaling ablates the liver’s ability to metabolize TH via sulfation.
Nr1i3 also regulates another pathway of phase II TH metabolism, glucuronidation. Conjugation of TH by Ugts increases the water solubility of TH, promoting biliary excretion (Burchell and Coughtrie, 1989). We measured the expression of the phenol/bilirubin UGT 1 family, polypeptide A1 (Ugt1a1) responsible for the glucuronidation of T4 (Beetstra et al., 1991; Findlay et al., 2000). Following a 36-hr fast, Ugt1a1 expression was up regulated in both WT (3.8 fold) and Npy−/− (3.5 fold) mouse livers compared to fed counterparts (Figure 4C) (Maglich et al., 2004). No change in Ugt1a1 expression was detected in pair-fed Mc4r−/− and DKO mice. These data further emphasize the role of MC4R and NPY in Nr1i3-mediated hepatic metabolism as both sulfation and glucuronidation of TH are impaired in pair-fed DKO mice. Furthermore, these results underline the mechanism by which Npy−/− mice and not DKO mice are able to repress TH levels during fasting despite impaired central HPT axis regulation. To ensure TH signaling was not impacted in fasting mouse livers, we measured the expression of thyroid hormone receptor β1 (Thrβ1 mRNA levels. Thrβ1 levels were not affected by fasting or by deletion of NPY, MC4R, or both (Figure 4D).
Taken together, these data demonstrate that signaling through NPY and the central melanocortin system regulates peripheral thyroid hormone metabolism in the liver and provides an additional role for this pathway in the regulation of energy expenditure.
Sulfotransferases become Nr1i3-resistant with HFD
To determine the impact of obesity and high leptin levels on thyroid hormone metabolism in the liver, we examined the expression of Nr1i3 mRNA and its target genes in the liver of mice on a chow diet or HFD. As before, we first measured Thrsp and Fasn expression to determine the sensitivity of livers to fasting in mice on a HFD. Both Thrsp and Fasn expression decreased with fasting in mice on a chow diet (3.8 and 1.4 fold, respectively) and HFD (3.6 and 2.7 fold, respectively, Figure 5A). Next we measured Nr1i3 expression during fasting in HFD-fed mice. Nr1i3 levels were up regulated during fasting in mice on a HFD (3.9 fold) compared to their fed controls, which was similar to mice on a chow diet (3.2 fold, Figure 5B). The classic Nr1i3 target, Cyp2b10, was increased in response to fasting in mice on a chow diet (6.0 fold, Figure 5B). However, there was no change in Cyp2b10 levels in fasted mice on a HFD. Additionally, HFD-fed mice failed to up regulate the expression of Sult1a1, Sult2a1 and Sult1d1 during fasting, whereas Ugt1a1 levels increased with fasting (2.3 fold, Figure 5C). Thrβ1 levels were unaffected by HFD (Figure 5D). Although Nr1i3 expression is responsive to fasting in mice on HFD, these data suggest that the expression of Cyp2b10 and sulfotransferases are resistant to Nr1i3-mediated induction during fasting. Resistance to Nr1i3 on a HFD does not permeate to all target genes since Ugt1a1 still increases during fasting in HFD-fed mice.
Figure 5. Genes enhancing hepatic thyroid hormone metabolism are Nr1i3-resistant in HFD-fed mice.
Expression of various genes in the liver was measured via QPCR and normalized to Rn18S expression in C57Bl/6 mice on chow (n = 4, n = 4) or HFD (n = 4, n = 4). A, Expression of the TH sensitive genes Thrsp and Fasn. B, Expression of Nr1i3 and Cyp2b10. C, Expression of the Nr1i3-target genes: Sult1a1, Sult2a1, Sult1d1 and Ugt1a1. D, Expression of Thrβ1. In all panels, data are presented as mean ± SEM. Significance was measured with 2-way ANOVA with Bonferroni post hoc test. * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
Discussion
Nutritional regulation of the HPT axis provides an important adaptive response to caloric restriction. Indeed, humans who undergo weight loss or controlled caloric restriction experience both reduced TSH and thyroid hormone levels (Rosenbaum et al., 2005; Rosenbaum et al., 2002). Previous studies in both humans and rodents have demonstrated that the fall in leptin levels during caloric restriction controls thyroid hormone levels (Ahima et al., 1996; Rosenbaum et al., 2002; Sanchez et al., 2004). Prevailing hypotheses suggest that leptin’s role is mediated by its ability to regulate Trh expression in hypophysiotropic neurons in the PVN, which can be either direct through leptin receptors or indirect via POMC and AgRP/NPY neurons in the arcuate nucleus (Fekete et al., 2001; Fekete et al., 2000a; Fekete et al., 2002b; Huo et al., 2004; Kim et al., 2000; Legradi et al., 1997; Legradi et al., 1998). While showing the importance of POMC and AgRP/NPY signaling in the regulation of Trh expression, we also demonstrate their effect on a key peripheral hepatic circuit that controls thyroid hormone levels in the setting of nutritional stress. Thus, leptin engages the HPT axis on multiple levels to control energy expenditure during nutritional stress.
Prior to this report, the specific roles of arcuate nucleus neuropeptides downstream of leptin in the regulation of Trh gene expression had not been determined. To assess these roles, we created mice that lack either MC4R signaling or NPY signaling or both. Although we have deleted MC4R and NPY signaling globally, reports to date implicate the arcuate nucleus as the source of NPY, AgRP and α-MSH to the PVN (Fekete et al., 2001; Fekete et al., 2000a; Legradi et al., 1998). Previous studies that modified NPY signaling in different areas of the hypothalamus did not report abnormalities in thyroid hormone levels (Chao et al., 2011; Gardiner et al., 2005; Tiesjema et al., 2007; Tiesjema et al., 2009; Yang et al., 2009). Importantly, we had to control for the obese phenotype in Mc4r−/− and DKO mice in order to use these models as the high leptin levels present in obesity preclude a fall in thyroid hormone levels during a fast. As suggested from earlier studies, input from the arcuate nucleus is not required for basal function of the HPT axis as Mc4r−/−, Npy−/− and DKO mice all have normal TH, serum TSH and Tshβ levels at baseline (Erickson et al., 1997; Fekete et al., 2004). In contrast, mice with leptin receptor mutations that impair signal transducer and activator of transcription 3 (STAT3) signaling have central hypothyroidism (Bates et al., 2004). Taken together, these contrasting data suggest that leptin acts independently of α-MSH, AgRP and NPY to establish the set point of the HPT axis in the basal fed state (Perello et al., 2011). Although this does suggest a direct action of leptin on TRH neurons, it remains possible that cocaine and amphetamine-regulated transcript (CART) production in the arcuate nucleus could play a role initiating the set point of the HPT axis (Broberger, 1999; Fekete et al., 2000b; Kadar et al., 2010; Raptis et al., 2004).
Unlike the basal state, the indirect pathway plays a critical role in fasting. The data presented herein demonstrate that NPY signaling is required for suppression of Trh in hypophysiotropic TRH neurons and that both the MC4R and NPY signaling pathways are required for the full suppression in TH levels seen during a fast (Figure 6). It is likely that NPY targets TRH neurons via the NPY Y1 or Y5 receptors and represses Trh expression by down regulation of the cAMP-PKA pathway, a known activator of Trh expression (Fekete et al., 2002a; Herzog et al., 1992; Sarkar and Lechan, 2003). The failure of Npy−/− mice to repress Tshβ during a fast suggests that TRH peptide levels in these animals are also unchanged and further establishes the role of NPY in controlling Trh expression in the PVN.
Figure 6. Model of NPY and MC4R signaling in T4 regulation during fasting.
A. During fasting, reduced leptin levels invoke an increase in Npy expression in the arcuate nucleus. NPY signals through Y1 and Y5 receptors in the PVN to repress Trh expression. Central repression of Trh leads to a decrease in Tshβ mRNA levels in the pituitary and thus, reduced T4 and T3 levels from the thyroid. Trh and serum TSH levels remain unchanged in fasting Npy−/− mice, yet T4 and T3 levels are decreased. Adapted from (Vella and Hollenberg, 2009), Copyright 2009, The Endocrine Society. B, Npy−/− mice are able to suppress TH levels during fasting through Nr1i3-mediated hepatic metabolism of TH, which requires both MC4R and NPY. The MC4R and NPY may act on the liver directly, through sympathetic outputs or a combination of both. The Nr1i3-targets SULT1A1, SULT2A1, SULT1D1 and UGT1A1 inactivate TH through sulfation (T4S) or glucuronidation (T4G). These genes are regulated during fasting in Npy−/− mice, but not in DKO mice. T4S is converted to reverse T3 sulfate (rT3S) by the type 1 deiodinase (Dio1) and T4G is cleared through biliary excretion. 3V = third ventricle. AgRP = agouti-related peptide. α-MSH = α-melanocyte stimulating hormone. Nr1i3 = constitutive androstane receptor. CNS = central nervous system. Dio1 = type 1 deiodinase. MC4R = melanocortin 4 receptor. Ob-Rb = leptin receptor, long form. NPY = neuropeptide Y. POMC = proopiomelanocortin. PVN = paraventricular nucleus. Sults = sulfotransferases. T3 = triiodothyronine. rT3S = reverse triiodothyronine sulfate. T4 = thyroxine. T4G = glucuronidated thyroxine. T4S = thyroxine sulfate. TSH = thyroid-stimulating hormone. Trh = thyrotropin-releasing hormone. Ugt1a1 = UDP-glucuronosyltransferase 1A1. Y1, Y5 = NPY receptors.
Despite the clear role of NPY in controlling fasting-induced suppression of Trh expression, TT4 and TT3 levels were still partially decreased in Npy−/− mice during a fast, which indicates that another pathway controlling TH levels must be operative. Strikingly, the HPT axis was entirely resistant to fasting in pair-fed DKO animals demonstrating that the separate pathway controlling TH levels may require the MC4R. While it is possible that changes in TSH bioactivity contribute to the fasting response of the HPT axis in general, this is unlikely in DKO mice. Here, serum TSH and TT4 levels are the same between fed and fasted mice, which is indicative of similar TSH bioactivity (Nikrodhanond et al., 2006). Likewise, serum TSH and TT4 levels are similar across all genotypes in the fed state suggesting that TSH bioactivity is not altered by MC4R and/or NPY deletion. Certainly, when Trh falls during a fast in WT and pair-fed Mc4r−/− mice, both total and biologically active serum TSH may fall. Given that the MC4R activates key hepatic pathways that control metabolism we focused on the hepatic metabolism of TH due to its important role in determining TH levels during acute illness (Nogueiras et al., 2007; Wiersinga, 2005). Notably, we have demonstrated that the MC4R and NPY are required for the fasting-induced up regulation of the nuclear receptor Nr1i3, which then controls the expression of key sulfotransferases and Ugt1A1 that are necessary for the metabolism and excretion of T4 and T3 (Maglich et al., 2004). Whereas pair-fed Mc4r−/− mice are partially deficient in the activation of this Nr1i3-mediated pathway; DKO mice are completely defective, further supporting the concept of a compound effect by NPY and MC4R signaling on TH levels (Lechan and Fekete, 2006). Further work will be required to delineate how the hypothalamic neurocircuitry, sympathetic output, and/or direct effects controlled by MC4R and NPY signaling regulate Nr1i3-driven metabolism of TH in the liver. Importantly, we have seen up regulation of Nr1i3 and its targets at least as early as 16 hrs of fasting (data not shown) consistent with both the measured 13–18 hr half-life of T4 in mice and the greater than 50% drop in TT4 levels during a 36 hr-fast, which supports the relevance of this pathway in vivo (van Buul-Offers et al., 1983).
In summary, the development of mice that lack key arcuate nucleus inputs to hypophysiotropic TRH neurons in the PVN has allowed us to establish the mechanism by which leptin regulates the HPT axis during fasting. While we demonstrate the unique requirement of NPY signaling for suppression of the central axis, we identify a fasting-induced hepatic pathway necessary for full suppression of TH levels that requires both MC4R and NPY signaling and is independent of TRH expression (Figure 6). These data suggest that the fall in TH levels seen during caloric restriction in humans could be targeted either centrally or peripherally and suggest that the fasting-induced activation of the MC4R and NPY controls not only food intake but also energy expenditure through the regulation of TH levels.
Experimental Procedures
Animals
All experiments were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. For high fat diet (HFD) experiments, male C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). NPY/MC4R mice were generated by crossing the previously described mice heterozygous for the loxTB Mc4r allele (Mc4r+/−) with mice heterozygous for a functional Npy allele (Npy+/−) (Balthasar et al., 2005; Erickson et al., 1996). Details of the cohorts in these studies are provided in the Supplementary Experimental Procedures. Collections of serum, flash frozen tissue, and formalin-fixed brains and storage have been previously described (Astapova et al., 2011; Sugrue et al., 2010). All mice were given free access to water and the appropriate diet. In fasting experiments, mice were fasted for 36 hr. In pair-feeding experiments, obesity-prone Mc4r−/− and DKO mice were fed the same amount of food consumed by WT mice on the previous day from weaning to the end of the experiment. Pair-fed Mc4r−/− and DKO mice with a body fat percentage of greater than 15% were excluded from the study.
CLAMS
NPY/MC4R mice from cohort 1 were placed in a Comprehensive Lab Animal Monitoring System apparatus (Columbus Instruments, Columbus OH) at 8–12 wk of age and monitored for oxygen consumption, using a previously described protocol (Segal-Lieberman et al., 2003a; Segal-Lieberman et al., 2003b). Mice were acclimated in CLAMS cages for 48 hr prior to measurement of metabolic parameters.
DEXA analysis
Body mass composition of male and female mice from NPY/MC4R cohort 1 was measured at 18–20 wk of age by dual energy X-ray absorptiometry using a densitometer for mice (Piximus Lunar PIXI 51048).
EchoMRI
At 9 wk of age, NPY/MC4R mice from cohorts 2 and 3 were subjected to magnetic resonance imaging (MRI) using EchoMRI (Echo Medical Systems, Houston, TX) to determine the body composition.
Serum Analysis
Total serum T4 (TT4) and T3 (TT3) were measured by solid-phase RIA (Coat-a-Count; Diagnostic Products Corp., Los Angeles, CA) in 25 and 50 µl of serum, respectively. Serum leptin concentration was measured via ELISA (Quantikine M Mouse Leptin Immunoassay, R & D Systems, Minneapolis, MN). Serum TSH was measured via IRMA (TSH IRMA, ALPCO, Salem, NH) in 100 µl of serum.
Real-time quantitative PCR
Total RNA was extracted from frozen tissues with STAT-60 reagent (Tel-Test, Friendswood, TX). Then 0.5 µg of total RNA was reverse transcribed using Advantage RT-for-PCR kit (CLONTECH, Mountain View, CA) with random hexamer primers. TaqMan gene expression assays for all mRNAs were purchased from Applied Biosystems (Carlsbad, CA). Quantitative PCR was performed in triplicate using the 800HT thermal cycler (Applied Biosystems, Foster City, CA). Relative mRNA levels were calculated using the standard curve method and normalized to peptidylprolyl isomerase A (cyclophilin, Ppia) (pituitary) or 18S ribosomal RNA (Rn18S, liver).
In Situ Hybridization
Trh expression was detected by ISH using 35S-radiolabeled antisense riboprobe on formalin-fixed brain slices as described previously (Sugrue et al., 2010). Dark-field digital images of the paraventricular nucleus (PVN), the anterior hypothalamic area (AHA), dorsomedial hypothalamus (DMH) and lateral hypothalamic area (LHA) were acquired with the same exposure time, brightness, and contrast on Zeiss Axioimager Z1 with Axiovision 4.5 software (Oberkochen, Germany). The 10X magnification images were quantified using ImageJ (Sugrue et al., 2010). To ensure that hypophysiotropic neurons in the PVN were the focus of Trh mRNA measurements, quantification of brain sections was limited to the medial portion of the PVN, which has been described in detail recently (Kadar et al., 2010). Positive pixels per unit area (pixel density) was calculated on both sides of the PVN by highlighting the medial portion of the PVN excluding the periventricular zone and subtracting background from areas not expressing Trh. Pixel density per mouse was averaged from a minimum of 3 sections of the medial portion of the PVN.
Statistical Analyses
Data are presented as sample means ± SEM. Sample numbers range from n = 3 to n = 40 and are stated specifically within the text or figure legends. The text or figure legends highlight how differences between groups were measured, which include unpaired t test, 1-way ANOVA with Tukey’s multiple comparison post hoc test, repeated-measures 2-way ANOVA with Bonferroni post hoc test, and 2-way ANOVA with Bonferroni post hoc test.
Highlights.
Fasted HFD mice are unable to suppress the HPT axis
Npy−/− mice fail to suppress Trh mRNA and TSH during a fast
Fasting mice that lack NPY and MC4R fail to suppress Trh, TSH and thyroid hormone
MC4R and NPY are required for activation of hepatic metabolism of T4
Supplementary Material
Acknowledgments
We thank Dr. Richard Palmiter for the Npy+/− mice, Dr. Brad Lowell for the Mc4r loxTB+/− mice, Dr. Roy Weiss of the University of Chicago for TT4 and TT3 measurements in the cohort 1 HFD mice, and Ms. Kaila Holtz for technical assistance. This work was supported by NIH grant DK-078090 and the Smith Family ADA Pinnacle Award to ANH and NIH grant T32 DK07516 to KRV. CLAMS is a part of the Physiological Core (Dr. Maratos-Flier, Beth Israel Deaconess Medical Center), supported by NIH Grant (NIH/5P01DK056116-10).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Astapova I, Vella KR, Ramadoss P, Holtz KA, Rodwin BA, Liao XH, Weiss RE, Rosenberg MA, Rosenzweig A, Hollenberg AN. The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic-pituitary-thyroid axis. Mol Endocrinol. 2011;25:212–224. doi: 10.1210/me.2010-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Barker SB. Mechanism of action of the thyroid hormone. Physiol Rev. 1951;31:205–243. doi: 10.1152/physrev.1951.31.3.205. [DOI] [PubMed] [Google Scholar]
- Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- Beetstra JB, van Engelen JG, Karels P, van der Hoek HJ, de Jong M, Docter R, Krenning EP, Hennemann G, Brouwer A, Visser TJ. Thyroxine and 3,3',5-triiodothyronine are glucuronidated in rat liver by different uridine diphosphate-glucuronyltransferases. Endocrinology. 1991;128:741–746. doi: 10.1210/endo-128-2-741. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Hollenberg AN. Leptin and melanocortin signaling in the hypothalamus. Vitam Horm. 2002;65:281–311. doi: 10.1016/s0083-6729(02)65068-x. [DOI] [PubMed] [Google Scholar]
- Blake NG, Eckland DJ, Foster OJ, Lightman SL. Inhibition of hypothalamic thyrotropin-releasing hormone messenger ribonucleic acid during food deprivation. Endocrinology. 1991;129:2714–2718. doi: 10.1210/endo-129-5-2714. [DOI] [PubMed] [Google Scholar]
- Blake NG, Johnson MR, Eckland DJ, Foster OJ, Lightman SL. Effect of food deprivation and altered thyroid status on the hypothalamic-pituitary-thyroid axis in the rat. J Endocrinol. 1992;133:183–188. doi: 10.1677/joe.0.1330183. [DOI] [PubMed] [Google Scholar]
- Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain research. 1999;848:101–113. doi: 10.1016/s0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- Brown BL, Allis JW, Simmons JE, House DE. Fasting for less than 24 h induces cytochrome P450 2E1 and 2B1/2 activities in rats. Toxicol Lett. 1995;81:39–44. doi: 10.1016/0378-4274(95)03407-2. [DOI] [PubMed] [Google Scholar]
- Burchell B, Coughtrie MW. UDP-glucuronosyltransferases. Pharmacology & therapeutics. 1989;43:261–289. doi: 10.1016/0163-7258(89)90122-8. [DOI] [PubMed] [Google Scholar]
- Carr FE, Bingham C, Oppenheimer JH, Kistner C, Mariash CN. Quantitative investigation of hepatic genomic response to hormonal and pathophysiological stimuli by multivariate analysis of two-dimensional mRNA activity profiles. Proc Natl Acad Sci U S A. 1984;81:974–978. doi: 10.1073/pnas.81.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY Expression in the Dorsomedial Hypothalamus Promotes Development of Brown Adipocytes and Prevents Diet-Induced Obesity. Cell Metab. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SD, Armstrong MK, Jump DB. Nutritional control of rat liver fatty acid synthase and S14 mRNA abundance. J Nutr. 1990;120:218–224. doi: 10.1093/jn/120.2.218. [DOI] [PubMed] [Google Scholar]
- Connors JM, DeVito WJ, Hedge GA. Effects of food deprivation on the feedback regulation of the hypothalamic-pituitary-thyroid axis of the rat. Endocrinology. 1985;117:900–906. doi: 10.1210/endo-117-3-900. [DOI] [PubMed] [Google Scholar]
- de Herder WW, Bonthuis F, Rutgers M, Otten MH, Hazenberg MP, Visser TJ. Effects of inhibition of type I iodothyronine deiodinase and phenol sulfotransferase on the biliary clearance of triiodothyronine in rats. Endocrinology. 1988;122:153–157. doi: 10.1210/endo-122-1-153. [DOI] [PubMed] [Google Scholar]
- Du Bois EF. Basal Metabolism in health and disease. Philadelphia: Lea & Febiger; 1936. [Google Scholar]
- Erickson JC, Ahima RS, Hollopeter G, Flier JS, Palmiter RD. Endocrine function of neuropeptide Y knockout mice. Regul Pept. 1997;70:199–202. doi: 10.1016/s0167-0115(97)01007-0. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Fekete C, Kelly J, Mihaly E, Sarkar S, Rand WM, Legradi G, Emerson CH, Lechan RM. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary- thyroid axis. Endocrinology. 2001;142:2606–2613. doi: 10.1210/endo.142.6.8207. [DOI] [PubMed] [Google Scholar]
- Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000a;20:1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Marks DL, Sarkar S, Emerson CH, Rand WM, Cone RD, Lechan RM. Effect of Agouti-related protein in regulation of the hypothalamic-pituitary-thyroid axis in the melanocortin 4 receptor knockout mouse. Endocrinology. 2004;145:4816–4821. doi: 10.1210/en.2004-0476. [DOI] [PubMed] [Google Scholar]
- Fekete C, Mihaly E, Luo LG, Kelly J, Clausen JT, Mao Q, Rand WM, Moss LG, Kuhar M, Emerson CH, Jackson IM, Lechan RM. Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J Neurosci. 2000b;20:9224–9234. doi: 10.1523/JNEUROSCI.20-24-09224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, Beck-Sickinger A, Lechan RM. Neuropeptide Y1 and Y5 receptors mediate the effects of neuropeptide Y on the hypothalamic-pituitary-thyroid axis. Endocrinology. 2002a;143:4513–4519. doi: 10.1210/en.2002-220574. [DOI] [PubMed] [Google Scholar]
- Fekete C, Sarkar S, Rand WM, Harney JW, Emerson CH, Bianco AC, Lechan RM. Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology. 2002b;143:3846–3853. doi: 10.1210/en.2002-220338. [DOI] [PubMed] [Google Scholar]
- Findlay KA, Kaptein E, Visser TJ, Burchell B. Characterization of the uridine diphosphate-glucuronosyltransferase-catalyzing thyroid hormone glucuronidation in man. The Journal of clinical endocrinology and metabolism. 2000;85:2879–2883. doi: 10.1210/jcem.85.8.6715. [DOI] [PubMed] [Google Scholar]
- Gardiner JV, Kong WM, Ward H, Murphy KG, Dhillo WS, Bloom SR. AAV mediated expression of anti-sense neuropeptide Y cRNA in the arcuate nucleus of rats results in decreased weight gain and food intake. Biochem Biophys Res Commun. 2005;327:1088–1093. doi: 10.1016/j.bbrc.2004.12.113. [DOI] [PubMed] [Google Scholar]
- Goodman MN, Larsen PR, Kaplan MM, Aoki TT, Young VR, Ruderman NB. Starvation in the rat. II. Effect of age and obesity on protein sparing and fuel metabolism. Am J Physiol. 1980;239:E277–E286. doi: 10.1152/ajpendo.1980.239.4.E277. [DOI] [PubMed] [Google Scholar]
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjorbaek C, Elmquist JK, Flier JS, Hollenberg AN. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001;107:111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog H, Hort YJ, Ball HJ, Hayes G, Shine J, Selbie LA. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Munzberg H, Nillni EA, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic trh gene expression by leptin. Endocrinology. 2004;145:2516–2523. doi: 10.1210/en.2003-1242. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Kadar A, Sanchez E, Wittmann G, Singru PS, Fuzesi T, Marsili A, Larsen PR, Liposits Z, Lechan RM, Fekete C. Distribution of hypophysiotropic thyrotropin-releasing hormone (TRH)-synthesizing neurons in the hypothalamic paraventricular nucleus of the mouse. The Journal of comparative neurology. 2010;518:3948–3961. doi: 10.1002/cne.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MH, Kaptein E, Roest TJ, van Dijk CH, Tibboel D, Meinl W, Glatt H, Coughtrie MW, Visser TJ. Characterization of human iodothyronine sulfotransferases. The Journal of clinical endocrinology and metabolism. 1999;84:1357–1364. doi: 10.1210/jcem.84.4.5590. [DOI] [PubMed] [Google Scholar]
- Kim MS, Small CJ, Stanley SA, Morgan DG, Seal LJ, Kong WM, Edwards CM, Abusnana S, Sunter D, Ghatei MA, Bloom SR. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest. 2000;105:1005–1011. doi: 10.1172/JCI8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–235. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- Legradi G, Emerson CH, Ahima RS, Rand WM, Flier JS, Lechan RM. Arcuate nucleus ablation prevents fasting-induced suppression of ProTRH mRNA in the hypothalamic paraventricular nucleus. Neuroendocrinology. 1998;68:89–97. doi: 10.1159/000054354. [DOI] [PubMed] [Google Scholar]
- Li X, Anderson RJ. Sulfation of iodothyronines by recombinant human liver steroid sulfotransferases. Biochemical and biophysical research communications. 1999;263:632–639. doi: 10.1006/bbrc.1999.1419. [DOI] [PubMed] [Google Scholar]
- Li X, Clemens DL, Cole JR, Anderson RJ. Characterization of human liver thermostable phenol sulfotransferase (SULT1A1) allozymes with 3,3',5-triiodothyronine as the substrate. The Journal of endocrinology. 2001;171:525–532. doi: 10.1677/joe.0.1710525. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem. 2004;279:19832–19838. doi: 10.1074/jbc.M313601200. [DOI] [PubMed] [Google Scholar]
- Mol JA, Visser TJ. Rapid and selective inner ring deiodination of thyroxine sulfate by rat liver deiodinase. Endocrinology. 1985;117:8–12. doi: 10.1210/endo-117-1-8. [DOI] [PubMed] [Google Scholar]
- Nikrodhanond AA, Ortiga-Carvalho TM, Shibusawa N, Hashimoto K, Liao XH, Refetoff S, Yamada M, Mori M, Wondisford FE. Dominant role of thyrotropin-releasing hormone in the hypothalamic-pituitary-thyroid axis. J Biol Chem. 2006;281:5000–5007. doi: 10.1074/jbc.M511530200. [DOI] [PubMed] [Google Scholar]
- Nillni EA. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front Neuroendocrinol. 2010;31:134–156. doi: 10.1016/j.yfrne.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni EA, Vaslet C, Harris M, Hollenberg A, Bjorbak C, Flier JS. Leptin regulates prothyrotropin-releasing hormone biosynthesis. Evidence for direct and indirect pathways. J Biol Chem. 2000;275:36124–36133. doi: 10.1074/jbc.M003549200. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. The Journal of clinical investigation. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Cakir I, Cyr NE, Romero A, Stuart RC, Chiappini F, Hollenberg AN, Nillni EA. Maintenance of the thyroid axis during diet-induced obesity in rodents is controlled at the central level. Am J Physiol Endocrinol Metab. 2011;299:E976–E989. doi: 10.1152/ajpendo.00448.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Stuart RC, Nillni EA. The role of intracerebroventricular administration of leptin in the stimulation of prothyrotropin releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology. 2006;147:3296–3306. doi: 10.1210/en.2005-1533. [DOI] [PubMed] [Google Scholar]
- Raptis S, Fekete C, Sarkar S, Rand WM, Emerson CH, Nagy GM, Lechan RM. Cocaine- and amphetamine-regulated transcript co-contained in thyrotropin-releasing hormone (TRH) neurons of the hypothalamic paraventricular nucleus modulates TRH-induced prolactin secretion. Endocrinology. 2004;145:1695–1699. doi: 10.1210/en.2003-1576. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y, Takami Y, Zwieb C, Nakayama T, Suiko M, Nakajima H, Liu MC. Purification, characterization, and molecular cloning of a novel rat liver Dopa/tyrosine sulfotransferase. The Journal of biological chemistry. 1995;270:30470–30478. doi: 10.1074/jbc.270.51.30470. [DOI] [PubMed] [Google Scholar]
- Sanchez VC, Goldstein J, Stuart RC, Hovanesian V, Huo L, Munzberg H, Friedman TC, Bjorbaek C, Nillni EA. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest. 2004;114:357–369. doi: 10.1172/JCI21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Lechan RM. Central administration of neuropeptide Y reduces alpha-melanocyte-stimulating hormone-induced cyclic adenosine 5'-monophosphate response element binding protein (CREB) phosphorylation in pro-thyrotropin-releasing hormone neurons and increases CREB phosphorylation in corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology. 2003;144:281–291. doi: 10.1210/en.2002-220675. [DOI] [PubMed] [Google Scholar]
- Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, Bates S, Myers MG, Jr, Flier JS, Maratos-Flier E. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci U S A. 2003a;100:10085–10090. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal-Lieberman G, Trombly DJ, Juthani V, Wang X, Maratos-Flier E. NPY ablation in C57BL/6 mice leads to mild obesity and to an impaired refeeding response to fasting. Am J Physiol Endocrinol Metab. 2003b;284:E1131–E1139. doi: 10.1152/ajpendo.00491.2002. [DOI] [PubMed] [Google Scholar]
- Spencer CA, Lum SM, Wilber JF, Kaptein EM, Nicoloff JT. Dynamics of serum thyrotropin and thyroid hormone changes in fasting. J Clin Endocrinol Metab. 1983;56:883–888. doi: 10.1210/jcem-56-5-883. [DOI] [PubMed] [Google Scholar]
- Sugrue ML, Vella KR, Morales C, Lopez ME, Hollenberg AN. The thyrotropin-releasing hormone gene is regulated by thyroid hormone at the level of transcription in vivo. Endocrinology. 2010;151:793–801. doi: 10.1210/en.2009-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesjema B, Adan RA, Luijendijk MC, Kalsbeek A, la Fleur SE. Differential effects of recombinant adeno-associated virus-mediated neuropeptide Y overexpression in the hypothalamic paraventricular nucleus and lateral hypothalamus on feeding behavior. J Neurosci. 2007;27:14139–14146. doi: 10.1523/JNEUROSCI.3280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesjema B, la Fleur SE, Luijendijk MC, Adan RA. Sustained NPY overexpression in the PVN results in obesity via temporarily increasing food intake. Obesity (Silver Spring) 2009;17:1448–1450. doi: 10.1038/oby.2008.670. [DOI] [PubMed] [Google Scholar]
- van Buul-Offers S, Hackeng WH, Schopman W. Thyroxine and triiodothyronine levels in Snell mice. Acta Endocrinol (Copenh) 1983;102:396–409. doi: 10.1530/acta.0.1020396. [DOI] [PubMed] [Google Scholar]
- Vansell NR, Klaassen CD. Effect of microsomal enzyme inducers on the biliary excretion of triiodothyronine (T(3)) and its metabolites. Toxicol Sci. 2002;65:184–191. doi: 10.1093/toxsci/65.2.184. [DOI] [PubMed] [Google Scholar]
- Vella KR, Hollenberg AN. The ups and downs of thyrotropin-releasing hormone. Endocrinology. 2009;150:2021–2023. doi: 10.1210/en.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser TJ. Pathways of thyroid hormone metabolism. Acta Med Austriaca. 1996;23:10–16. [PubMed] [Google Scholar]
- Wiersinga WM. Nonthyroidal Illness. In: Braverman LE, Utiger RD, editors. Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 247–264. [Google Scholar]
- Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.