Abstract
BACKGROUND
In the PEPI-Malawi trial, infants received up to 14 weeks of extended nevirapine (NVP) or extended NVP plus zidovudine (NVP+ZDV) to prevent postnatal HIV transmission. We examined emergence and persistence of NVP resistance in HIV-infected infants who received these regimens prior to HIV diagnosis.
METHODS
Infant plasma samples collected at 14 weeks of age were tested using the ViroSeq HIV Genotyping System and a sensitive point-mutation assay, LigAmp (for K103N and Y181C). Samples collected at 6 and 12 months of age were analyzed using LigAmp.
RESULTS
At 14 weeks of age, NVP resistance was detected in samples from 82 (75.9%) of 108 HIV-infected infants. While the frequency of NVP resistance detected by ViroSeq was lower in the extended NVP+ZDV arm than in the extended NVP arm, the difference was not statistically significant (38/55=69.1% vs. 44/53=83.0%, P=0.12). Similar results were obtained using LigAmp. Using LigAmp, the proportion of infants who still had detectable NVP resistance at 6 and 12 months was similar among infants in the two study arms (at 6 months: 17/20=85.0% for extended NVP vs. 21/26=80.8% for extended NVP+ZDV, P=1.00; at 12 months: 9/16=56.3% for extended NVP vs.10/13=76.9% for extended NVP+ZDV, P=0.43).
CONCLUSIONS
Infants exposed to extended NVP or extended NVP+ZDV had high rates of NVP resistance at 14 weeks of age, and resistant variants frequently persisted for 6–12 months. Frequency and persistence of NVP resistance did not differ significantly among infants who received extended NVP only vs. extended NVP+ZDV prophylaxis.
Keywords: HIV, nevirapine, resistance, infants, Malawi
INTRODUCTION
Strategies to prevent postnatal HIV transmission are important when breastfeeding is critical to infant health and survival. In these settings, extended daily infant nevirapine (NVP) reduces the risk of postnatal mother-to-child transmission of HIV compared to single dose NVP (sdNVP) alone [1] or sdNVP plus a short tail of other antiretroviral (ARV) drugs [2, 3], and is one option recommended by the World Health Organization (WHO) for prevention of postnatal HIV transmission [4]. Unfortunately, NVP-resistant HIV frequently emerges in HIV-infected infants who receive sdNVP prior to HIV diagnosis [5–8]. NVP resistance emerges more frequently after exposure to extended NVP prophylaxis [9–12]. Prior exposure to sdNVP can compromise the response of HIV-infected infants to non-nucleoside reverse transcriptase inhibitor (NNRTI)-containing treatment regimens [13, 14]. For this reason, the WHO currently recommends use of protease inhibitor (PI)-based regimens rather than NNRTI-based regimens to treat NVP-exposed, HIV-infected infants [15]. Unfortunately, PIs are not widely available in many resource-limited settings and are more costly than NNRTIs. One approach to address the limited availability and higher cost of PI-containing regimens is to initiate treatment of NVP-exposed, HIV-infected infants with PI-containing regimens, and subsequently switch them to NNRTI-containing regimens [16]. It is not known whether this strategy would also be effective in HIV-infected infants who were exposed to extended NVP prophylaxis prior to HIV diagnosis.
Studies from our group and others have shown that NVP-resistant HIV can persist for a year or more in infants after sdNVP exposure [5, 6, 17]. Persistence of NVP-resistant HIV appears to be more pronounced after exposure to extended NVP prophylaxis [9–11]. In the Six-Week Extended Nevirapine (SWEN) trial, infants in Uganda, India, and Ethiopia received either sdNVP or extended NVP prophylaxis up to 6 weeks of age [1]. Among HIV-infected Ugandan infants who had NVP resistance at 6 weeks of age, NVP-resistant HIV variants were still detected at 6 months in all seven evaluable infants who received the extended NVP regimen, compared to only one of six infants who received sdNVP alone [9]. NVP-resistant strains were also detected in Indian infants [10], and in Ethiopian infants in the SWEN trial [11] at 6–7 months of age.
In this report, we analyzed emergence and persistence of NVP resistance among infants who received up to 14 weeks of extended NVP prophylaxis in the PEPI-Malawi trial (#NCT00115648) [2]. In PEPI-Malawi, women who presented early in labor received sdNVP; those who presented <4 hours prior to anticipated delivery (too late for HIV testing, counseling, and sdNVP administration prior to delivery) did not receive sdNVP. Infants were randomized at birth to one of three study arms: (1) sdNVP plus 1 week of daily zidovudine (ZDV) [control], (2) control + daily NVP up to 14 weeks of age [extended NVP], or (3) control + daily NVP and ZDV up to 14 weeks of age [extended NVP+ZDV]. We previously analyzed NVP resistance in infants in the extended study arms of PEPI-Malawi who were HIV-infected in utero [12]. In those infants the addition of extended ZDV to extended NVP prophylaxis significantly reduced the frequency of NVP resistance at 14 weeks of age from 86.0% (37/43) to 62.2% (28/45) P=0.015 [12]. This report extends our previous report by analyzing HIV drug resistance in all infants in the extended study arms who were diagnosed with HIV infection by 14 weeks of age. This report also includes analysis of the persistence of NVP-resistant HIV in infants up to 1 year old.
METHODS
Samples used for analysis
Samples and data were obtained from the PEPI-Malawi trial [2]. Prophylaxis was stopped in HIV infected infants following confirmation of an initial positive HIV DNA PCR test. Infant plasma was stored at birth, 3, 6, 9, and 14 weeks, and 6, 9, 12, 18, and 24 months of age. In November 2008, 220 infants in the extended study arms were identified who were HIV-infected by 14 weeks of age (106 in the extended NVP arm, 114 in the extended NVP+ZDV arm); this included infants who were HIV-infected in utero, who were not included in the primary analysis of the PEPI-Malawi trial [2]. Infants were excluded from analysis if their mothers started ARV treatment (all women who were excluded because of ARV treatment initiation received NVP/lamivudine/stavudine). The 108 infants included in the analysis in this report included 79 infants described in a previous study [12]; that study was limited to analysis samples collected at 14-weeks of age and used a single method for analysis of NVP resistance (the ViroSeq system, see below).
Laboratory methods
Infant plasma samples collected at 14 weeks of age were analyzed using the ViroSeq HIV Genotyping System (ViroSeq, Celera, Alameda, CA); 25–100 μL was used for analysis. The ViroSeq system is a population-sequencing based assay that provides information about mutations in HIV protease (amino acids 1–99) and HIV reverse transcriptase (amino acids 1–335). Samples collected at 14 weeks, 6 months, and 12 months of age were analyzed using the LigAmp assay [18] to detect the K103N mutation (assay cutoff: 0.5% K103N) and the Y181C mutation (assay cut-off: 1% Y181C). The LigAmp assay is a sensitive, quantitative point-mutation assay that can be used to detect specific drug-resistance mutations in HIV. LigAmp was performed as described [9] using primers designed for subtype C HIV.
Statistical Analysis
Fisher's exact tests compared proportions and Wilcoxon rank tests compared continuous variables between the two extended study arms. McNemars discordance tests were used to compare results of different laboratory tests for resistance in the same infants. Statistical significance was considered for two-sided P≤0.05. All analyses were performed using SAS Software Version 9.1 (Cary, North Carolina).
Ethical considerations
Written informed consent for participation in the PEPI-Malawi trial was obtained from all women. The study was approved by Institutional Review Boards (IRB) in Malawi and the U.S. as described elsewhere [2], including the U.S. Centers for Disease Control and Prevention IRB.
RESULTS
Plasma samples collected at 14 weeks of age were available for 151 (68.6%) of the 220 HIV-infected infants who received either extended NVP or extended NVP+ZDV prophylaxis in PEPI-Malawi. Sixty-nine infants did not have a 14-week sample available for testing (36 samples were used up in previous testing, the parents of 16 infants refused to participate in the trial after enrollment, 10 infants died, four infants were lost to follow-up, one infant missed the 14-week study visit, one infant relocated, and one infant's sample could not be located); four of the 151 available 14-week samples were not tested because the mother initiated ARV treatment prior to the 14-week study visit. Resistance test results (ViroSeq and LigAmp, see Methods) were obtained for 108 (73.5%) of the 147 samples that were tested (53 in the extended NVP arm, 55 in the extended NVP+ZDV arm); 39 samples failed analysis (no amplification).
Table 1 shows characteristics of the 108 infants who had resistance test results (included in the sub-study) and of the 112 infants who did not. There were no statistically significant differences between infants in these two groups of infants for the following variables: the proportion of infants in the extended NVP vs. extended NVP+ZDV study arms, the duration of prophylaxis received prior to HIV diagnosis, the median maternal CD4 cell count at study enrollment, the proportion of infants whose mothers received sdNVP, or the proportion of infants with in utero HIV infection. The median maternal HIV log10 viral load at enrollment was lower for infants included in this sub-study (Table 1).
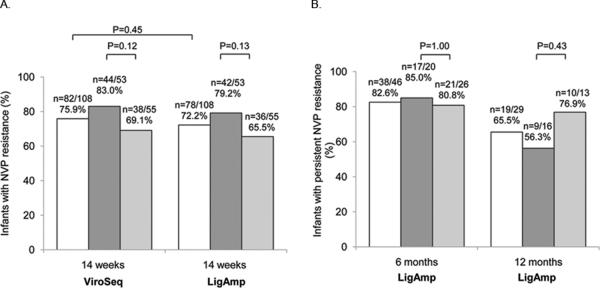
At 14 weeks of age, 82 (75.9%) of 108 infants had NVP resistance detected with the ViroSeq assay and 78 (72.2%) had K103N and/or Y181C detected with the LigAmp assay (P=0.45, McNemar's test, Figure 1A). None of the infants had ZDV resistance detected with the ViroSeq assay. There was no significant difference in the proportion of infants in the two study arms who had NVP resistance detected with the ViroSeq or LigAmp assay at 14 weeks of age (P=0.12 for ViroSeq, P=0.13 for LigAmp, Fisher's exact test, Figure 1A). We also did not find a significant association between detection of NVP resistance at 14 weeks of age using the LigAmp assay and any of the clinical or laboratory variables described above (Table 1).
Figure 1.
Resistance test results are shown for all infants tested (no shading), infants in the extended NVP arm (dark shading), and infants in the extended NVP+ZDV arm (light shading). (A) Panel A shows results obtained for samples collected at 14 weeks of age using the ViroSeq assay (detection of any NVP resistance mutation) and the LigAmp assay (detection of K103N and/or Y181C). The proportion of infants with NVP resistance detected with ViroSeq vs. LigAmp at 14 weeks was compared using McNemar's test. The proportion of infants with NVP resistance at 14 weeks in the two study arms was compared using Fisher's exact test. In 10 infants, NVP resistance was detected with ViroSeq only. Five of the 10 infants had NVP resistance mutations that were not probed by the LigAmp assay (V106A, Y188C/L, G190A). In four of the 10 infants, K103N or Y181C was encoded by a codon that was not probed with the LigAmp assay (K103N was encoded by AAT rather than AAC, Y181C was encoded by TGC rather than TGT). In six infants, NVP resistance was detected by LigAmp only. In those cases, the level of the relevant mutation (K103N or Y181C) was <20% of the viral population. (B) Panel B shows results obtained for samples collected at 6 and 12 months of age using the LigAmp assay (detection of K103N and/or Y181C). Samples collected at 6 months of age were analyzed if the infant had K103N and/or Y181C detected at 14 weeks. Samples collected at 12 months of age were analyzed if the infant either had K103N and/or Y181C detected at 6 months of age, or had K103N and/or Y181C detected at 14 weeks of age and did not have a 6-month sample available for analysis. The proportions of infants with NVP resistance in the two study arms at 6 and 12 months were compared using Fisher's exact test.
We next used the LigAmp assay to analyze persistence of K103N and Y181C in infants who had those mutations detected at 14 weeks of age. Sixty-eight (87.2%) of the 78 infants who had NVP resistance at 14 weeks of age were still alive at 6 months; 55 of those 68 infants had a 6-month sample available for analysis (13 samples were used up in other testing). Three of the 55 samples were not included in the analysis because the mother started ARV treatment before the 6-month visit and six samples failed analysis (no amplification). The remaining 46 samples were analyzed. Thirty-eight (82.6%) of the 46 infants still had K103N and/or Y181C detected at 6 months of age. There was no significant difference in detection of these mutations in the two study arms (P=1.0, Fisher's exact test, Figure 1B). Five infants had NVP resistance mutations other than K103N and Y181C detected by ViroSeq at 14 weeks (V106A, Y188C/L, G190A) in the absence of K103N or Y181C. Four of the five infants had a 6-month sample available for analysis with ViroSeq. Three of the four infants did not have any NVP resistance mutations detected in the 6-month sample; in one infant, G190A was detected.
A similar analysis was performed to analyze persistence of NVP resistance at 12 months of age. Twenty-six (68.4%) of the 38 infants who had NVP resistance at 6 months of age were still alive at 12 months; 21 of those 26 infants had a 12-month sample available for analysis (four samples were used up in previous testing, one infant relocated). Two of the 21 samples were not included in the analysis because ARV treatment was initiated before the 12-month visit in both of the mothers and in one of the infants. We also analyzed 12-month samples from infants who had NVP resistance detected at 14 weeks, but did not have a 6-month sample available for analysis. Among 19 infants in that group, 14 were still alive at 12 months and 10 of those were evaluable (one infant relocated, the parents of one infant refused further participation in the trial, one infant missed the 12-month study visit, and one mother started ARV treatment before the 12-month visit). Therefore, a total of 29 12-month samples were tested (19 samples from infants who had resistance detected at 6 months of age, and 10 samples from infants who had resistance detected at 14 weeks of age who did not have a resistance testing result from the 6-month visit). Nineteen (66.5%) of the 29 infants who were included in the analysis still had K103N and/or Y181C detected at 12 months of age (Figure 1B). There was no significant difference in the percentage of infants in the extended NVP vs. extended NVP+ZDV study arms who had detectable K103N or Y181C at either 6 or 12 months (P=1.0 at 6 months, P=0.43 at 12 months, Fisher's exact test, Figure 1B). The LigAmp assay provides a quantitative measure of the percentage of the viral population in a sample that has the mutation of interest. There was no significant difference in the level of K103N or Y181C detected in the infants in these two groups at the 6- or 12-month visits (data not shown).
DISCUSSION
This study first analyzed the frequency of NVP resistance in 14-week-old HIV-infected infants who received either extended NVP or extended NVP+ZDV prophylaxis prior to HIV diagnosis. Initiation of ARV treatment within the first three months of life is associated with a dramatic reduction in infant mortality [19]. The presence of detectable NVP resistance at 14 weeks of age may compromise the infant's response to ARV treatment initiated in the first 3 months of life. In PEPI-Malawi, most (82/108=75.9%) HIV-infected infants who received extended NVP or extended NVP+ZDV prophylaxis prior to HIV diagnosis (including those with in utero infection) had detectable NVP resistance at 14 weeks of age.
In our previous analysis of infants in PEPI-Malawi who had in utero HIV infection, NVP resistance was significantly less frequent at 14 weeks of age in the extended NVP+ZDV arm than in the extended NVP arm (62.2% vs. 86.0%, P=0.015) [12]. In this larger study, which included all evaluable infants who were diagnosed with HIV infection by 14-weeks of age, the same trend was observed (i.e., the frequency of NVP resistance was lower at 14 weeks in infants who received extended NVP+ZDV than among those who received extended NVP alone), but the difference was not statistically significant (69.1% vs. 83.0%, P=0.12). We did not identify any clinical or laboratory factors associated with emergence of NVP resistance at 14 weeks of age; factors analyzed included duration of prophylaxis (infant age when prophylaxis was stopped), maternal viral load or CD4 cell count at study enrollment, maternal sdNVP exposure, and timing of infant HIV infection (diagnosis of HIV infection (in utero infection) at vs. after delivery). Most often, infants who had NVP resistance at 14 weeks of age had at least one NVP resistance mutation that was present at a sufficient level for detection using a population-sequencing based genotyping assay (ViroSeq). However, there was no statistically significant difference in the frequency of NVP resistance mutations using ViroSeq or a sensitive point mutation assay (LigAmp) at that study visit.
We also analyzed the persistence of NVP-resistant variants among infants who had NVP resistance detected at 14 weeks of age. The NVP resistance mutations, K103 and Y181C, were still detectable in 82.6% of evaluable infants at 6 months of age and in 65.5% of evaluable infants at 12 months of age. Five infants had other NVP resistance mutations detected at 14 weeks of age in the absence of K103N or Y181C (V106A, Y188C/L and G190A). Those mutations are associated with high-level NVP resistance, with variable levels of resistance to other NNRTIs [20]. We did not analyze persistence of those mutations in this study. However, a previous study suggests that some of those mutations (V106M, Y188C, G190A) may be less likely to persist at high levels in infants after NVP exposure than K103N and Y181C [11]. The frequent persistence of the K103N and Y181C mutations in infants after exposure to extended NVP prophylaxis prior to HIV diagnosis, with or without concomitant ZDV, suggests that exposure to these regimens may compromise an infant's subsequent response to an NNRTI-based treatment regimen, even if treatment initiation is delayed until 6–12 months of age. However, clinical trials are needed to evaluate the efficacy of different treatment strategies in infants who were exposed to these regimens prior to HIV diagnosis.
It is important to note that all of the infants described in this report had subtype C HIV infection. Our previous studies demonstrated that both women and infants with subtype C HIV are more likely to develop NVP resistance after sdNVP exposure than those infected with other HIV subtypes (A or D HIV) [7, 21]. In women, NVP resistance also tends to persist longer after sdNVP in some subtypes (e.g., longer in subtype D vs. A) [22]. Further studies are needed to evaluate persistence of NVP resistance in infants exposed to extended NVP prophylaxis who are infected with different HIV subtypes, and to evaluate whether HIV subtype influences the subsequent response of those infants to NNRTI-based treatment regimens, with or without initial treatment with a PI-based regimen.
ACKNOWLEDGEMENTS
We are indebted to Dr. George Kafulafula who died before this work was completed. Dr. Kafulafula was the Obstetrician/Gynecologist responsible for the clinical care of women and infants in the PEPI-Malawi trial. The authors thank the women and infants who participated in the PEPI-Malawi trial, the PEPI-Malawi study team, and laboratory staff at the College of Medicine, University of Malawi-Johns Hopkins University Research Project in Blantyre, Malawi for their assistance with sample processing and shipping.
Sources of funding This work was supported by (1) R03 HD061299, (2) R01 AI087139, (3) the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICH/HD), National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the NIH, DHHS (U01-AI-068613), (4) the International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) Network (U01-AI068633), and (5) Centers for Disease Control and Prevention Cooperative Agreement U50/CCU022061.
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention. Use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Centers for Disease Control and Prevention or the Department of Health and Human Services.
Note: This work was presented in part at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, February, 2010.
Conflict of Interest Statement None of the authors have a commercial or other association that might pose a conflict of interest with the following exception: Dr. Susan Eshleman is a co-inventor of the LigAmp assay and Johns Hopkins University has filed a patent application with the US-Patent and Trademark Office. The inventors may receive royalty payments if the patent is awarded and licensed.
CONTRIBUTION OF AUTHORS All authors contributed to preparation of the manuscript. In addition, individual authors had the following contributions to the study:
J Fogel: Performed resistance testing, analyzed resistance data and drafted the manuscript.
DR Hoover: Statistician for the PEPI-Malawi trial, responsible for statistical analysis.
J Sun: Data analyst for this sub-study and the PEPI-Malawi trial.
LM Mofenson: NICHD Medical Officer for the PEPI-Malawi trial, involved in the original design of the PEPI-Malawi trial and in monitoring adverse events during the trial.
MG Fowler: Former CDC Medical Officer for the PEPI-Malawi trial, involved in the original design of the PEPI-Malawi trial and in monitoring adverse events during the trial.
AW Taylor: CDC Medical Officer for the PEPI-Malawi trial and involved with monitoring of adverse events.
N Kumwenda: Local (Malawi) PI for the PEPI-Malawi trial, conceived the original PEPI trial, conducted and coordinated the PEPI trial, and analyzed and interpreted the overall PEPI trial results.
TE Taha: U.S. PI for the PEPI-Malawi trial, conceived the original PEPI trial, conducted and coordinated the PEPI trial, and analyzed and interpreted the overall PEPI trial results.
SH Eshleman: Conceived of the study, coordinated the study, responsible for study design, data interpretation, and manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Six Week Extended-Dose Nevirapine (SWEN) Study Team Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 2.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 3.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. [Accessed May 28, 2010];Rapid advice. 2009 Available at: http://www.who.int/hiv/pub/mtct/rapid_advice_mtct.pdf. [PubMed]
- 5.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15:1951–7. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 6.Martinson NA, Morris L, Gray G, et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007;44:148–53. doi: 10.1097/QAI.0b013e31802b920e. [DOI] [PubMed] [Google Scholar]
- 7.Eshleman SH, Hoover DR, Chen S, et al. Resistance after single-dose nevirapine prophylaxis emerges in a high proportion of Malawian newborns. AIDS. 2005;19:2167–9. doi: 10.1097/01.aids.0000194800.43799.94. [DOI] [PubMed] [Google Scholar]
- 8.Church JD, Mwatha A, Bagenda D, et al. In utero HIV infection is associated with an increased risk of nevirapine resistance in ugandan infants who were exposed to perinatal single dose nevirapine. AIDS Res Hum Retroviruses. 2009;25:673–7. doi: 10.1089/aid.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Church JD, Omer SB, Guay LA, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-Dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198:1075–82. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moorthy A, Gupta A, Bhosale R, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS One. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persaud D, Bedri A, Ziemniak C, et al. Slower Clearance of Nevirapine Resistant Virus in Infants Failing Extended Nevirapine Prophylaxis for Prevention of Mother-to Child HIV-Transmission. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2010.0346. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lidstrom J, Li Q, Hoover DR, et al. Addition of extended zidovudine to extended nevirapine prophylaxis reduces nevirapine resistance in infants who were HIV-infected in utero. AIDS. 2010;24:381–6. doi: 10.1097/QAD.0b013e3283352ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) Report of the WHO Technical Reference Group [Accessed: June 6, 2010];Paediatric HIV/ART Care Guideline Group Meeting. 2009 Available at: http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008pdf.
- 16.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–90. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flys T, Nissley DV, Claasen CW, et al. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J Infect Dis. 2005;192:24–9. doi: 10.1086/430742. [DOI] [PubMed] [Google Scholar]
- 18.Shi C, Eshleman SH, Jones D, et al. LigAmp for sensitive detection of single-nucleotide differences. Nat Methods. 2004;1:141–7. doi: 10.1038/nmeth713. [DOI] [PubMed] [Google Scholar]
- 19.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Accessed: January 20, 2011];Stanford University HIV Drug Resistance Database. Available at: http://hivdb.stanford.edu/.
- 21.Eshleman SH, Hoover DR, Chen S, et al. Nevirapine (NVP) resistance in women with HIV-1 subtype C, compared with subtypes A and D, after the administration of single-dose NVP. J Infect Dis. 2005;192:30–6. doi: 10.1086/430764. [DOI] [PubMed] [Google Scholar]
- 22.Eshleman SH, Guay LA, Wang J, et al. Distinct patterns of emergence and fading of K103N and Y181C in women with subtype A vs. D after single-dose nevirapine: HIVNET 012. J Acquir Immune Defic Syndr. 2005;40:24–9. doi: 10.1097/01.qai.0000174656.71276.d6. [DOI] [PubMed] [Google Scholar]