Abstract
Objective
The purpose of this study was to characterize the relationship between adipose tissue phenotype and depot-specific microvascular function in fat.
Methods and Results
In 30 obese subjects (age 42±11 yr, BMI 46±11 kg/m2) undergoing bariatric surgery, we intra-operatively collected visceral and subcutaneous adipose tissue and characterized depot-specific adipose phenotypes. We assessed vasomotor function of the adipose microvasculature using videomicroscopy of small arterioles (75–250 μm) isolated from different fat compartments. Endothelium-dependent, acetylcholine-mediated vasodilation was severely impaired in visceral arterioles, compared to the subcutaneous depot (P<0.001 by ANOVA). Non-endothelium dependent responses to papaverine and nitroprusside were similar. Endothelial nitric oxide synthase (eNOS) inhibition with Nω-nitro-L-arginine methyl ester (L-NAME) reduced subcutaneous vasodilation but had no effect on severely blunted visceral arteriolar responses. Visceral fat exhibited greater expression of proinflammatory, oxidative stress-related, hypoxia-induced, and proangiogenic genes; increased activated macrophage populations; and higher capacity for cytokine production ex vivo.
Conclusions
Our findings provide clinical evidence that the visceral microenvironment may be intrinsically toxic to arterial health providing a potential mechanism by which visceral adiposity burden is linked to atherosclerotic vascular disease. Our findings also support the evolving concept that both adipose tissue quality and quantity may play significant roles in shaping cardiovascular phenotypes in human obesity.
Keywords: adiposity, endothelium, vasodilation, arteries, inflammation
The global obesity epidemic is expanding at an alarming rate producing a major health care burden.1, 2 Obesity is associated with endothelial dysfunction and early atherosclerosis,3, 4 and recent data highlight obesity as a major cause of premature death from ischemic heart disease and stroke.5 Clinical studies link the accumulation of intra-abdominal visceral fat and central adiposity to coronary heart disease, metabolic syndrome, and type 2 diabetes risk.6–8 As such, there is mounting evidence that intrinsic properties of visceral adipose tissue may be deleterious to the cardiovascular system owing to its high capacity for toxic lipolysis and increased production of adipokines and inflammatory cytokines.7, 9–11 We recently demonstrated that adipose tissue inflammation is linked to whole body metabolic dysregulation and systemic vascular dysfunction, providing evidence that qualitative features of fat may shape clinical phenotypes.12, 13
While experimental studies infer a pathogenic connection between visceral fat and cardiovascular risk, mechanisms are largely unknown. We hypothesized that if adipose tissue is a regulator of vascular function and the visceral milieu more pathogenic, depot-specific differences would be manifest in arterioles intrinsic to different adipose domains. The goal of the present study was to determine whether arterioles isolated from visceral compared to subcutaneous regions exhibit a more diseased profile manifest by endothelial vasomotor dysfunction, and to determine whether differences in inflammatory characteristics exist between the two different compartments.
Methods
Study subjects
Obese men and women (BMI≥30 kg/m2, age ≥18) were enrolled in the Boston Medical Center Bariatric Surgery Program. Subjects with unstable medical conditions such as active coronary syndromes, congestive heart failure, systemic infection, acute illness, malignancy or pregnancy were excluded. The study was approved by Boston Medical Center Institutional Review Board and all subjects gave written informed consent.
Anthropometric and metabolic measures
Clinical characteristics including blood pressure, heart rate, height, weight, BMI, and waist circumference were recorded. Biochemical analyses including lipids, glucose, insulin, homeostasis model assessment of insulin resistance (HOMA), glycosylated hemoglobin (HbA1c), high-sensitivity C-reactive protein (hs-CRP) were quantified from blood samples collected in a fasting state.
Adipose tissue collection and vessel preparation
Subcutaneous and visceral adipose tissue biopsies were collected intra-operatively during planned bariatric surgery. Subcutaneous adipose tissue was harvested from the lower abdominal wall and visceral tissue secured from the greater omentum, respectively. Specimens were placed immediately in cold HEPES buffer solution, pH 7.51 (American Bioanalytical, Natick, MA). Small adipose arterioles (75–250 μm internal diameter) were carefully removed of surrounding fat and connective tissue and suspended in an organ chamber containing Krebs solution, cannulated securely with glass micropipettes.14 The organ chamber was then mounted onto a stage with inverted microscope (magnification ×200) and video camera monitor (model VIA-100; Boeckler Instruments, Inc, Tuscon, AZ) for vascular diameter measurements using videomicroscopy. Arterioles were pressure equilibrated and continuously perfused with Krebs buffer aerated with a gas mixture of 5% O2, 21% CO2 and 74% N2.
Assessment of adipose arteriolar function
The internal arterial diameter of each vessel was initially measured at a steady state followed by administration of endothelin-1 (ET-1, 2×10−6 M, Sigma-Aldrich, St. Louis, MO) to preconstrict vessels to 50–70% of internal diameter. Endothelial nitric oxide synthase (eNOS)-dependent vasodilation was assessed by measuring arteriolar change in diameter to increasing doses of receptor mediated NO-agonist acetylcholine (Ach, 10−10 to 10−5 M, Sigma-Aldrich) in the presence or absence of eNOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 10−4 M, Sigma-Aldrich). Endothelium-independent vasodilation was determined using papaverine (Pap, 2×10−4 M, Sigma-Aldrich) and sodium nitroprusside (SNP, 10−10 to 10−4 M, Sigma-Aldrich). Pharmacological agents were added to the external bathing solution of the organ chamber, and the indicated concentration represents the final chamber molar concentration.
Adipose tissue gene expression
Immediately following biopsy collection, adipose tissue samples were stored in RNAlater (Sigma Aldrich) solution at −80°C. Total RNAs were isolated from homogenized whole adipose tissues using the miRNeasy kit (Qiagen, Germantown, MD). Quantitative real-time-PCR reactions were performed using a high throughput instrument (BioMark, Fluidigm, San Francisco, CA) by using Gene Expression Assays (Applied Biosystems, Foster City, CA) and DynamicArray chips (Fluidigm, San Francisco, CA). We additionally separated endothelial cells from non-vascular fractions in adipose tissue as previously described.15, 16 Briefly, fat samples were digested in a collagenase I solution (300 units/ml in PBS and 2%BSA; Worthington Biochemical Corp, Lakewood, NJ.), treated with erythrocyte-lysing buffer (R&D systems, Minneapolis, MN), and endothelial cells isolated using CD31 MACS MicroBead Kit (Miltenyi Biotec Inc, Auburn, CA). Cells were lysed with Qiazol lysis solution and kept at −80°C until RNA isolation. Total RNA isolation for vascular endothelial cells was performed as described above.
Histology
Blood vessels isolated from fat were fixed in 10% buffered formalin for analysis by the Pathology Department of Boston Medical Center. Paraffin embedded arterioles were stained with hematoxylin and eosin and also characterized by immunohistochemistry with endothelial cell-specific CD31 (BioGenex, Freemont, CA). Microvessels were stained for macrophages using a cell-specific target to CD68 (Ventana Medical Systems Inc, Tucson, AZ). Microvascular morphology was further assessed by elastica-van Gieson staining (Ventana Medical Systems Inc.) Media to lumen ratio was quantified using an established NIH Image J program as previously described.17
Flow cytometry and leukocyte isolation
Resident macrophages were isolated from fat as previously described.18 In brief, tissues were gently homogenized and filtered through 70 μm cell strainers (BD Falcon, Bedford, MA) for single cell suspension. Macrophages were isolated by dual density gradient (Histopaque-1077 and Histopaque-1119, Sigma Aldrich) to account for changes in cellular density of tissue. Recovered cells were assessed by flow cytometry to measure macrophage surface receptors CD14, CD209, and CD206 (BD Pharmingen, San Diego, CA) and TLR4 (eBioscience, San Diego, CA).
In a subset of specimens, isolated leukocytes were harvested and allowed to adhere to cell culture plates for 4 hours. Adherent cells comprised of 85–95% CD14+ macrophages, cultured overnight ±1 μg/ml of E.coli lipopolysaccharide (LPS, Alexis Corporation, Lausen, Switzerland), and supernatants assessed for secreted IL-6 and IL-8 concentrations using standard ELISA (R&D Systems, Minneapolis, MN).
Statistical Analysis
Group differences in clinical characteristics, gene expression, cytokine production, flow-cytometry measures, and baseline vascular parameters were examined by Mann–Whitney–Wilcoxon and Chi square tests for continuous and categorical variables, respectively. Repeated measures analysis of variance (ANOVA) was used to compare depot-specific vascular dose-response curves to pharmacological probes. Associations between vascular dilator responses (defined by dose-response area under the curve), adipose tissue gene expression, and clinical data were examined using Spearman's rank correlation analyses. Statistical significance was defined as p<0.05. All data are expressed as mean±SD, unless otherwise indicated. All data were analyzed using SPSS for Windows, version 13.1.
Results
We harvested a total of 40 adipose arterioles from subcutaneous (n=17) and visceral (n=23) fat depots in 30 patients. Clinical characteristics of subjects that provided adipose samples during bariatric surgery are displayed in Table 1. There were no significant differences in clinical parameters or medication use listed between subjects that provided subcutaneous (sc) versus visceral arterioles.
Table 1.
Study population characteristics
| Clinical parameter | n=30 |
|---|---|
| Age (yrs) | 42±11 |
| Female (%) | 79% |
| BMI (kg/m2) | 46±11 |
| Waist circumference (cm) | 140±20 |
| Weight (kg) | 129±36 |
| Insulin (mU/ml) | 12±7 |
| Glucose (mg/dl) | 107±38 |
| HOMA | 3±2 |
| Triglycerides (mg/dl) | 123±61 |
| Total cholesterol (mg/dl) | 190±39 |
| HDL-C (mg/dl) | 47±10 |
| LDL-C (mg/dl) | 120±35 |
| hs-CRP (mg/dl) | 8.8±9.7 |
| HbA1c (%) | 6.0±0.9 |
| Duration of adulthood obesity (yrs) | 23±11 |
| Diabetes (%) | 44% |
| Hypertension (%) | 44% |
| Hypercholesterolemia (%) | 22% |
| ARB/ACE-inhibitor use (%) | 27% |
| DPP-4 inhibitor use (%) | 3% |
| Insulin use (%) | 3% |
| Metformin use (%) | 40% |
| Statin use (%) | 10% |
| Thiazolidinedione use (%) | 3% |
ARB = Angiotensin receptor blocker
ACE = Angiotensin converting enzyme
DPP-4 = Dipeptidyl peptidase-4
Depot-specific adipose arteriolar responses
Mean resting internal diameter of arterioles isolated from subcutaneous fat was 147±57 μm and in the visceral 171±50 μm (p=0.20). Additionally, the media/lumen ratio was not different between microvessels isolated from the sc (2.1) compared to visceral adipose depot (2.0, p=0.88). The degree of microvascular pre-constriction to ET-1 was also similar in both groups (57±12% and 56±12% of basal diameter in sc and visceral, respectively, p=0.87). Endothelium-dependent vasodilation was then assessed with dose-response relationship to increasing Ach concentrations (10−10–10−5 M). As shown in figure 1, Ach-mediated vasodilation was significantly impaired in visceral compared to subcutaneous adipose arterioles (p<0.001 by ANOVA), even exhibiting paradoxical vasoconstriction in some individuals by ≤5%. In contrast, responses to papaverine (Pap) as an endothelium-independent agonist were similar in both groups, indicating intact vascular smooth muscle function. In a subset of 10 patients that provided paired samples simultaneously from both visceral and sc depots, we again observed a profound decrement in endothelium-dependent vasorelaxation of visceral arterioles, similar in magnitude to results for the group as a whole (p<0.001, Supplemental figure I). These latter findings are particularly important since vessels for both depots originated from the same person, removing any potential confounding effect of unmeasured systemic parameters between individuals. Stratification of subjects based on presence or absence of diabetes mellitus (supplemental figure II) or hypertension (data not shown) did not alter the results.
Figure 1. Adipose tissues arteriolar responses.
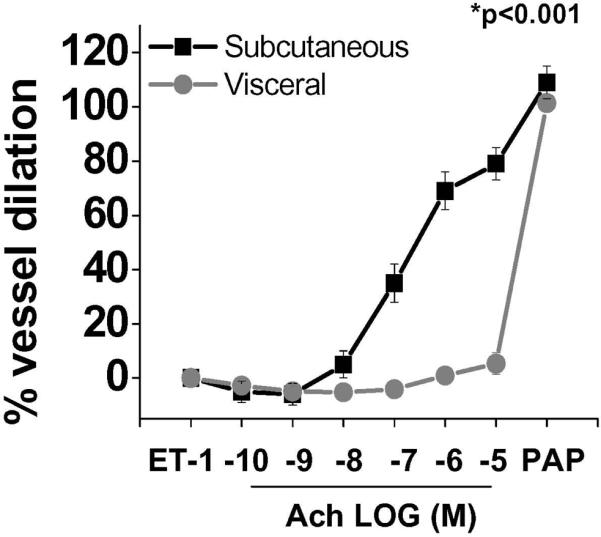
Endothelium-dependent, Ach-mediated vasodilation was severely impaired in visceral compared to sc adipose tissue arterioles (p<0.001 by ANOVA), while responses to papaverine were similar (n=40). Data presented as mean ± SEM.
Vessel viability was confirmed by preserved responses to ET-1 (pre-constriction) and papaverine (maximal vasodilation). We further excluded the possibility of endothelial denudation or anatomical damage to blood vessels by histological confirmation of structural integrity. Immunohistochemistry targeted to CD31 (A), and H&E staining (B) demonstrated intact nucleated vascular endothelial and smooth muscle layers in adipose vessels (Supplemental figure III). Additionally, elastica-van Gieson and CD68 stains showed no evidence of intimal thickening, atherosclerotic changes, or vascular macrophage infiltration (Supplemental Figure IV).
To determine the role of nitric oxide bioaction in endothelium-dependent responses of adipose microvessels, in a subset of individuals we examined vascular dilation to Ach ± L-NAME (10−4 M). As shown in figure 2, L-NAME significantly reduced Ach-mediated relaxation by 40% in subcutaneous vessels (n=6, p=0.005), whereas no significant effect was observed on visceral arterioles that already exhibited profound endothelial dysfunction (n=6, p=0.36). Additionally, we examined vasodilator responses to increasing doses of sodium nitroprusside which demonstrated no significant inter-depot differences in vasodilation (n=6, p=0.5; supplemental Figure V), further supporting the notion that depot-specific vasodilator defects are related to endothelial dysfunction.
Figure 2. Effect of L-NAME on adipose vasomotor responses.
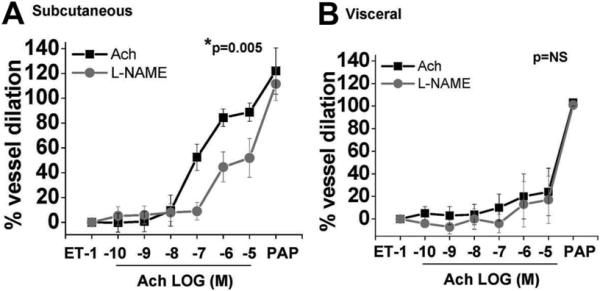
L-NAME (10−4 M) reduced vasodilator responses in subcutaneous microvessels indicating partial dependence on nitric oxide bioaction (n=6, P=0.005 by ANOVA). (B) In contrast, L-NAME had no effect on severely blunted visceral arteriolar responses (n=6, P=0.36 by ANOVA). Data are presented as mean ± SEM.
Adipose tissue characterization
As systemic clinical parameters failed to explain differences in depot-specific vascular responses, we sought to characterize disparities between fat depots focusing on inflammatory activation. As shown in table 2, using RT-PCR we demonstrated significantly higher expression of proinflammatory and oxidative stress related genes in visceral compared to the sc depot. Among the genes measured, the greatest difference was observed for interleukin-6 (IL-6), a cytokine upregulated in obesity and strongly implicated in the pathogenesis of diabetes and acute coronary syndromes.19, 20 Proangiogenic and hypoxia-related genes were also upregulated in visceral fat in line with recent data demonstrating depot-specific differences in vascularity and angiogenic potential.21 Results were directionally similar for subjects that provided paired sc and visceral fat, despite the smaller sample size (Supplemental Table I). In addition, gene expression analysis specifically performed in isolated endothelial cells for select cytokines yielded similar results (Supplemental Figure VI). Ach-mediated vasodilatation (AUC) correlated negatively with the following adipose tissue genes: IL-6, NF-κB, MYD88, CCL5, TGF-β, CD8, HIF1-α and VEGF (P<0.05 for all).
Table 2.
Gene expression in visceral compared to subcutaneous adipose tissue.
| Gene | Fold difference in visceral compared to subcutaneous fat | p value | |
|---|---|---|---|
| Immune cell markers | CD3 | 3.89 | <0.001* |
| CD4 | 4.67 | 0.008* | |
| CD8 | 7.54 | <0.001* | |
| CD68 | 1.70 | 0.032* | |
| CD163 | 2.95 | 0.025* | |
| FOXP3 | 1.26 | 0.073 | |
| Inflammation and oxidative stress | Adiponectin | 1.90 | 0.095 |
| CCL2 | 1.03 | 0.929 | |
| CCL5 | 6.32 | <0.001* | |
| CCR2 | 4.27 | 0.004* | |
| FSTL1 | 3.42 | 0.009* | |
| IFN-γ | 2.20 | 0.831 | |
| ICAM-1 | 1.54 | 0.055 | |
| IL-1β | 4.23 | 0.077 | |
| IL-6 | 10.05 | <0.001* | |
| IL-10 | 3.93 | 0.059 | |
| MYD88 | 2.53 | 0.002* | |
| NF-κB | 2.06 | 0.001* | |
| NOX1 | 1.85 | 0.044* | |
| NOX2 | 4.41 | <0.001* | |
| NOX4 | 1.55 | 0.186 | |
| TGF-β | 5.58 | 0.001* | |
| TLR-4 | 2.80 | 0.003* | |
| TNF-α | 4.77 | 0.008* | |
| VCAM-1 | 1.54 | 0.180 | |
| eNOS | 2.55 | 0.149 | |
| iNOS | 5.50 | 0.05 | |
| Angiogenic and hypoxia-related genes | ANGPT2 | 4.55 | 0.004* |
| HIF1-α | 3.05 | 0.004* | |
| VEGF | 3.24 | <0.001* |
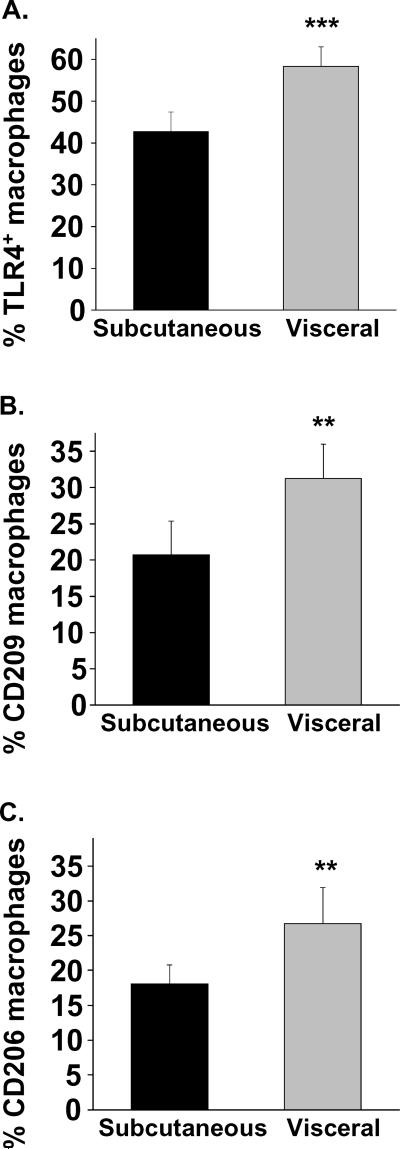
Infiltrating macrophages are thought to be the primary source of proinflammatory cytokine production in the adipose tissue.22 The notion that macrophages exist in proinflammatory (M1) and reparative (M2) states that may be relevant to disease mechanisms is an evolving concept.23 We identified a subset of activated adipose tissue macrophages (ATM) expressing pro-inflammatory cell surface toll-like receptor 4 (TLR4+) suggestive of M1 phenotype. As shown in figure 3, using flow cytometry we demonstrated higher population of TLR4+ macrophages in visceral vs. sc fat (58±20% vs. 43±20%, P<0.001, n=19). The same relationship was evident for macrophages expressing two surface markers traditionally associated with M2 populations: CD209 (31±18% vs. 21±17%, p<0.01, n=14) and CD206 (27±21% vs.18±11% p<0.01, n=17).
Figure 3. Depot-specific macrophage polarization in adipose tissue.
Adipose flow cytometry identified higher populations of (A) TLR4+ M1 polarized macrophages in visceral vs. sc adipose tissue. Similarly, macrophages expressing markers traditionally associated with M2 phenotypes (B) CD209 and (C) CD206 were also significantly higher in visceral fat. ***p<0.001; **p<0.01 compared to subcutaneous depot. Data presented as mean ± SEM.
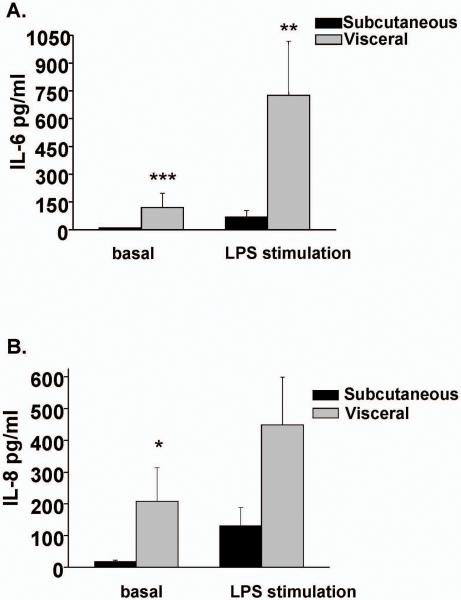
To further characterize functional aspects of adipose immune cells, leukocytes primarily comprising of macrophages, were isolated from different depots and cultured in the presence or absence of LPS. As shown in figure 4, leukocytes recovered from visceral tissue produced higher basal levels of IL-6 (p<0.001) and IL-8 (p<0.05) compared to sc fat. Further, LPS stimulated production of IL-6 was higher in visceral fat (p<0.01). The findings suggest that immune cells within visceral domains express higher basal and stimulated levels of cytokines that contribute to regional differences in proinflammatory profiles.
Figure 4. Cytokine production in cultured adipose tissue leukocytes.
Basal IL-6 (A) and IL-8 (B) secretion was higher in leukocytes isolated from visceral compared to sc adipose tissue. LPS stimulation significantly increased IL-6 production. ***p<0.001; **p<0.01; *p<0.05 versus subcutaneous depot. Data presented as mean ± SEM.
Discussion
In the present study of severely obese subjects, we show that human arterioles isolated from visceral omental fat exhibit severely impaired endothelium-dependent vasodilation compared to subcutaneous adipose microvessels. The degree of vascular impairment in the visceral compartment was profound with some arterial segments exhibiting paradoxical vasoconstriction suggesting a significant defect in endothelial vasodilator function.24 We observed greater expression of proinflammatory, oxidative stress-related, hypoxia-induced, and proangiogenic genes, and higher ambient and inducible production of inflammatory cytokines in visceral compared to subcutaneous depots. The present findings are clinically significant as they provide direct evidence that the visceral microenvironment is intrinsically more toxic to arterial health, lending support for a potential mechanism by which overall visceral adiposity burden may link to atherosclerotic vascular disease.
Obesity, as defined by elevated BMI, is associated with increased cardiovascular mortality, independent of associated traditional risk factors.1, 2 Clinical studies consistently demonstrate that degree of central abdominal obesity, measured by waist circumference or waist-to-hip ratio, more strongly links to cardiovascular death than general obesity measures such as BMI, even in normal weight patients.8–10 While central adiposity correlates closely with visceral fat load and metabolic dysfunction such as insulin resistance, dyslipidemia, and hypertension, this clinical phenotype is also coupled to a heightened state of chronic inflammation characterized by increased circulating levels of adhesion molecules, CRP, and IL-6 which predict diabetes and myocardial infarction risk.19, 20 A key mechanism, now recognized in both animal models and humans, is inflammation of hepatic and adipose stores, largely driven by infiltrating macrophages and cytokine overproduction that contribute to both local and whole body immune dysregulation in association with excess weight.13, 22, 25, 26 While inflammatory markers correlate well with the degree of fat burden in both subcutaneous and visceral compartments, the latter relationship appears to be stronger.27 We recently demonstrated that both central fat deposition and the state of inflammation in fat are linked to systemic vascular endothelial dysfunction in the obese, suggesting that both quantity and quality of fat, may be germane to cardiometabolic phenotypes.13
In the present study, we now have evidence that both these processes may be intertwined. In keeping with prior studies showing differing metabolic and secretory profiles between visceral and subcutaneous fat,28, 29 we demonstrate the adipose transcriptome and secretome to be markedly more proinflammatory in visceral depots. Specifically, we observed increased expression of T-lymphocyte and macrophage markers in the visceral compartment in conjunction with upregulated repertoire of cytokines and activation of the innate immune system. Inter-depot differences in gene expression were most pronounced for IL-6, a cytokine upregulated in obesity and closely associated with systemic endothelial dysfunction30 and myocardial infarction risk.20 Up to 35% of plasma IL-6 concentration is estimated to originate from adipose tissue, and blood levels of IL-10, IL-8, TNF-α, and CRP are all also reported to be elevated in obesity, and linked to a wide range of metabolic complications.31, 32 The findings are particularly important from a cardiovascular standpoint since inflammatory mechanisms play a key role not only in early stages of endothelial dysfunction but also destabilization of advanced atherosclerotic plaques that precipitate acute cardiovascular events as primary cause of mortality in obese individuals.
Our contention that severe impairment in vasomotor function in the visceral domain may result from local overproduction of cytokines that exert direct pathogenic effects on the vasculature is highly plausible. Paired experiments in both visceral and subcutaneous arterioles from the same individual strongly implicated that differences in vascular reactivity were attributable to properties specific to sampled adipose tissue. In this regard, a proinflammatory visceral transcriptome was associated with upregulation of several cytokines known to impair vasomotor function. For example, TNF-α activates NF-κB, stimulates NADPH oxidase, induces superoxide production, and abolishes vascular NO bioavailability and endothelium-dependent vasodilation.33, 34 Interleukin-1β and IL-6 disrupt arterial function via generation of reactive oxygen species,35 and altered eNOS bioactivity.36 In contrast, microvessels exposed to IL-6 and TNF-α antagonists rescue the abnormal phenotype.37, 38 Additional, experimental studies further support the growing notion that inflammatory topology of adipose tissue modulates vascular biology. For example, transplanting inflamed visceral fat accelerates aortic atherosclerosis in recipient Apo E−/− mice.11 Macrophage ablation of inflamed perivascular fat restores loss of anti-contractile properties in mesenteric vessels39 and supports a role of adipose inflammation in modulating vascular responses.
Human adipose tissue in massively obese humans is a major systemic source of adipocytokines of which >90% is derived from non-adipocyte fractions, primarily macrophages.32 While probably oversimplified, the notion that macrophages exist in proinflammatory (M1) and reparative (M2) states that may be relevant to disease mechanisms is an evolving concept.40 Although macrophages within the adipose tissue of obese mice exhibit M1 characteristics,41 immune dysregulation in human fat appears more complex and lacks a distinct phenotype at this time.42, 43 We identified a higher percentage of macrophages in visceral fat expressing cell surface TLR4, known to exhibit pro-inflammatory M1 characteristics in atherosclerotic plaques.44 Similarly, we observed parallel upregulation of macrophages expressing CD206 or CD209, traditionally associated with M2 subpopulations43 with no significant depot-specific difference in M1/M2 ratio. We speculate that adipose remodeling may not only be species-specific but also relate to obesity duration that have generally been short-term in animal studies, in contrast to our human cohort afflicted with an average of 20 years of obesity. Understanding mechanisms involved in resolution of adipose tissue inflammation may reveal novel therapeutic targets.45
What sets off the inflammatory cascade in adipose tissue is of great interest and likely to provide treatment clues for several obesity-related diseases that have inflammatory mechanistic basis including atherosclerosis, diabetes, and cancer. Experimental data suggest that adipose overexpansion, particularly in visceral fat, may suffer from capillary rarefaction and tissue hypoperfusion that locally triggers a vicious cycle of ischemia, hypoxia, necrosis, and inflammation within the adipose milieu.46 As such, our observation of significantly greater hypoxia-related and proangiogenic imprint in visceral fat prompts speculation that tissue-specific functional defects in vascularity and perfusion may have profound effects on the adipose microenvironment and the organism as a whole, and leaves room for additional studies.
Our present study has several limitations. First, we studied individuals with advanced class III–IV obesity referred for bariatric surgery which provided clinical feasibility to access multiple adipose depots simultaneously. We recognize that studying extreme weight categories may not be representative of the general population with milder degrees of obesity. However, recent data show that even modest visceral fat gain in normal weight subjects promotes vascular endothelial dysfunction, suggesting a biological connection.47 Second, most participants in the study were women, reflecting the female predominance of our bariatric service and clinical practice nationally.48 Third, we speculated that TLR4 expression signified M1 polarization, although the functional characterization of this specific macrophage phenotype in human fat remains unproven. Fourth, medications could not be entirely washed out owing to medical treatment indications and depot-specific effects in treated patients cannot be completely excluded. Lastly, we attributed the cytokine source from cultured leukocytes to primarily macrophages based on prior experimental data, but recognize that adipocytes and other immune cell populations including B and T lymphocytes, neutrophils, and mast cells likely contribute to chemokine and cytokine release in adipose tissue.32, 49
In conclusion, we provide direct human evidence that the visceral microenvironment is intrinsically toxic to arterial health lending support for a potential mechanism by which overall visceral adiposity burden may link to atherosclerotic vascular disease. Our data also support the growing paradigm that qualitative features of adipose tissue may play a role in shaping disease phenotypes in obese individuals.
Supplementary Material
Acknowledgements
We thank Jingli Wang for her assistance with technical aspects of the adipose tissue microvessel videomicroscopy methodology.
Sources of Funding: Dr. Gokce is supported by National Institutes of Health (NIH) grants R01 HL084213 and P01 HL081587. Dr. Vita is supported by NIH grants HL083269, HL083801, HL081587, and HL75795. Dr. Widlansky is supported by NIH grant K23 HL089326 and AHA Grant-in-Aid 10GRNT3880044.
Footnotes
Disclosures: Dr. Apovian has served on the advisory boards for Allergan, Amylin, Orexegin, Merck, Johnson and Johnson, Arena, and Sanofi-Aventis, and has received research funding from Lilly, Amylin, Pfizer, Sanofi-Aventis, Orexigen, MetaProteomics, and the Dr. Robert C. and Veronica Atkins Foundation. There are no conflicts of interest with the current manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Berrington de GA, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, nton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Arkin JM, Alsdorf R, Bigornia S, Palmisano J, Beal R, Istfan N, Hess D, Apovian CM, Gokce N. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. Am J Cardiol. 2008;101:98–101. doi: 10.1016/j.amjcard.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).McGill HC, Jr., McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- (5).Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de SG, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- (7).Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- (8).Coutinho T, Goel K, Correa de SD, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Central obesity and survival in subjects with coronary artery disease a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57:1877–1886. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- (9).Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, Buchan I, Day N, Khaw KT. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- (10).Lakka HM, Lakka TA, Tuomilehto J, Salonen JT. Abdominal obesity is associated with increased risk of acute coronary events in men. Eur Heart J. 2002;23:706–713. doi: 10.1053/euhj.2001.2889. [DOI] [PubMed] [Google Scholar]
- (11).Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Farb MG, Bigornia S, Mott M, Tanriverdi K, Morin KM, Freedman JE, Joseph L, Hess DT, Apovian CM, Vita JA, Gokce N. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–237. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol. 2007;292:H93–100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- (15).Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, Bouloumie A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- (16).Peinado JR, Jimenez-Gomez Y, Pulido MR, Ortega-Bellido M, az-Lopez C, Padillo FJ, Lopez-Miranda J, Vazquez-Martinez R, Malagon MM. The stromal-vascular fraction of adipose tissue contributes to major differences between subcutaneous and visceral fat depots. Proteomics. 2010;10:3356–3366. doi: 10.1002/pmic.201000350. [DOI] [PubMed] [Google Scholar]
- (17).Kalk P, Godes M, Relle K, Rothkegel C, Hucke A, Stasch JP, Hocher B. NO-independent activation of soluble guanylate cyclase prevents disease progression in rats with 5/6 nephrectomy. Br J Pharmacol. 2006;148:853–859. doi: 10.1038/sj.bjp.0706792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009;86:1007–1016. doi: 10.1189/jlb.0309203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- (20).Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- (21).Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- (24).Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- (25).Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr., Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- (28).Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 2010;18:879–883. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- (29).Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–889. doi: 10.1038/oby.2009.443. [DOI] [PubMed] [Google Scholar]
- (30).Esteve E, Castro A, Lopez-Bermejo A, Vendrell J, Ricart W, Fernandez-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30:939–945. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- (31).Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58:727–736. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- (32).Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- (33).Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- (34).Wimalasundera R, Fexby S, Regan L, Thom SA, Hughes AD. Effect of tumour necrosis factor-alpha and interleukin 1beta on endothelium-dependent relaxation in rat mesenteric resistance arteries in vitro. Br J Pharmacol. 2003;138:1285–1294. doi: 10.1038/sj.bjp.0705168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1016–H1021. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- (36).Hung MJ, Cherng WJ, Hung MY, Wu HT, Pang JH. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J Hypertens. 2010;28:940–951. doi: 10.1097/HJH.0b013e32833992ef. [DOI] [PubMed] [Google Scholar]
- (37).Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- (38).Virdis A, Santini F, Colucci R, Duranti E, Salvetti G, Rugani I, Segnani C, Anselmino M, Bernardini N, Blandizzi C, Salvetti A, Pinchera A, Taddei S. Vascular generation of tumor necrosis factor-alpha reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol. 2011;58:238–247. doi: 10.1016/j.jacc.2011.01.050. [DOI] [PubMed] [Google Scholar]
- (39).Withers SB, gabiti-Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA, Heagerty AM. Macrophage Activation Is Responsible for Loss of Anticontractile Function in Inflamed Perivascular Fat. Arterioscler Thromb Vasc Biol. 2011;31:908–913. doi: 10.1161/ATVBAHA.110.221705. [DOI] [PubMed] [Google Scholar]
- (40).Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- (41).Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).ron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619–4623. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- (43).Bourlier V, Zakaroff-Girard A, Miranville A, De BS, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumie A. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- (44).Cole JE, Georgiou E, Monaco C. The expression and functions of toll-like receptors in atherosclerosis. Mediators Inflamm. 2010;2010:393946. doi: 10.1155/2010/393946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- (46).Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, Orban M, Gami A, Davison D, Singh P, Pusalavidyasagar S, Huyber C, Votruba S, Lopez-Jimenez F, Jensen MD, Somers VK. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol. 2010;56:662–666. doi: 10.1016/j.jacc.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, Pories W, Courcoulas A, McCloskey C, Mitchell J, Patterson E, Pomp A, Staten MA, Yanovski SZ, Thirlby R, Wolfe B. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.