Abstract
Wyosine la, one of the fluorescent hypermodified Y nucleosides found in tRNAsPhe, was synthesized chemically from its biogenetic precursor guanosine 2. The route involved transformation of 2 into the tricyclic structure 3a and subsequent methylation at N-4. The major products of various methylation procedures were isomers of wyosine, methylated at N-5 (3b) or at N-1 (4). Mesoionic compound 4 is a new analogue of 7-methylguanosine 5, modified nucleoside occurring in the unique positions in transfer, messenger and ribosomal RNAs. The chromatographic and spectral characteristics of wyosine and its isomers is given.
Full text
PDF
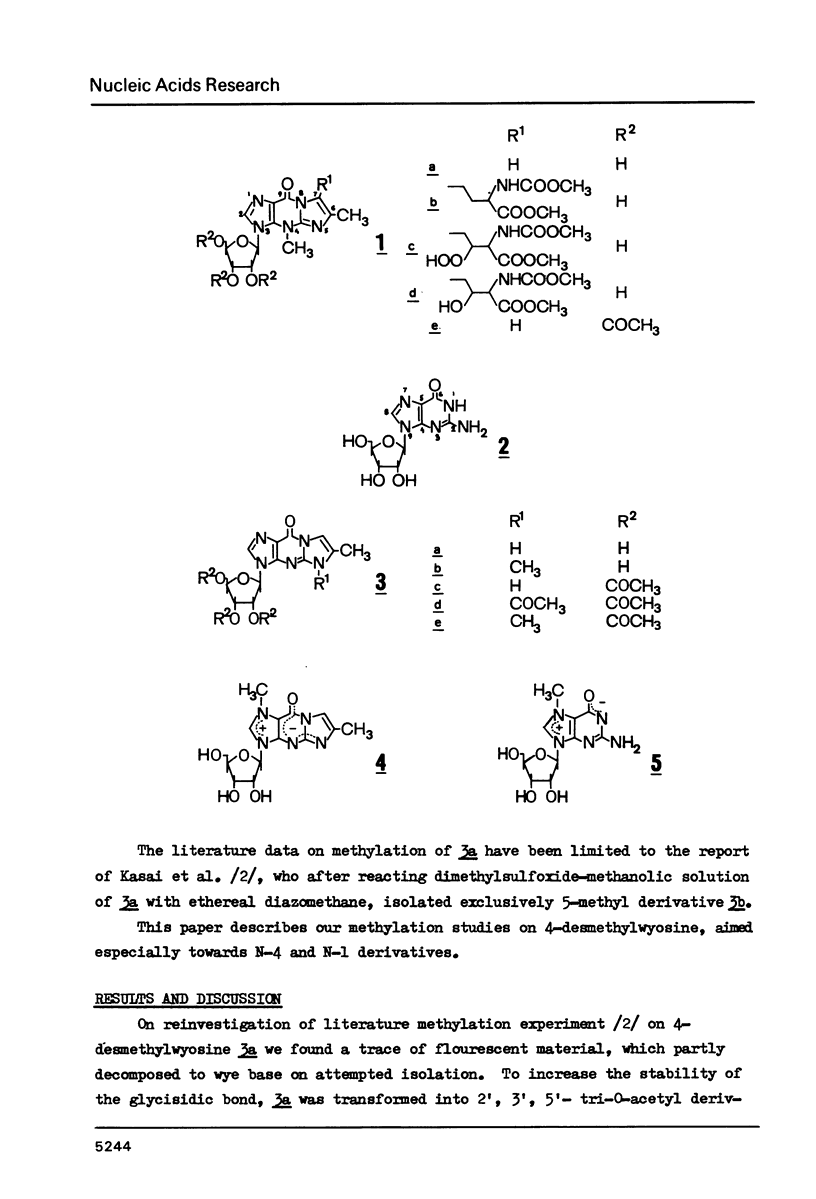

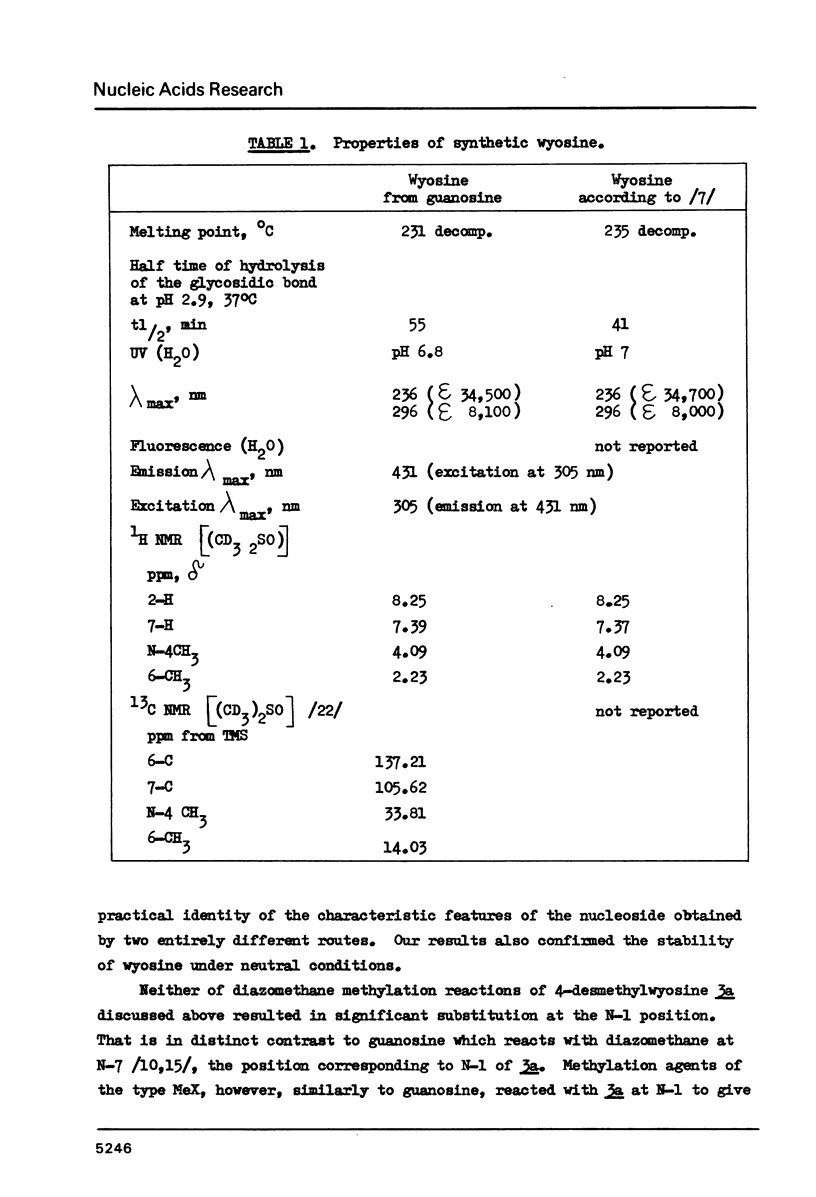
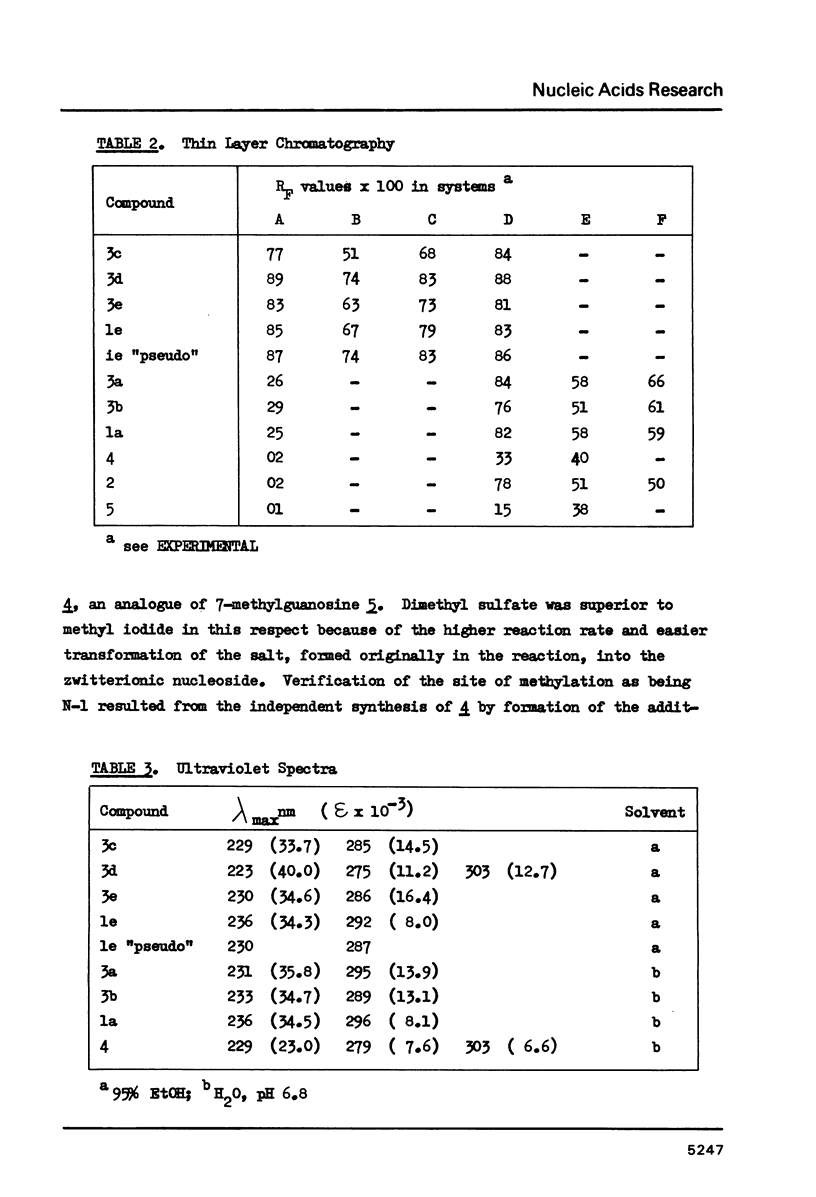
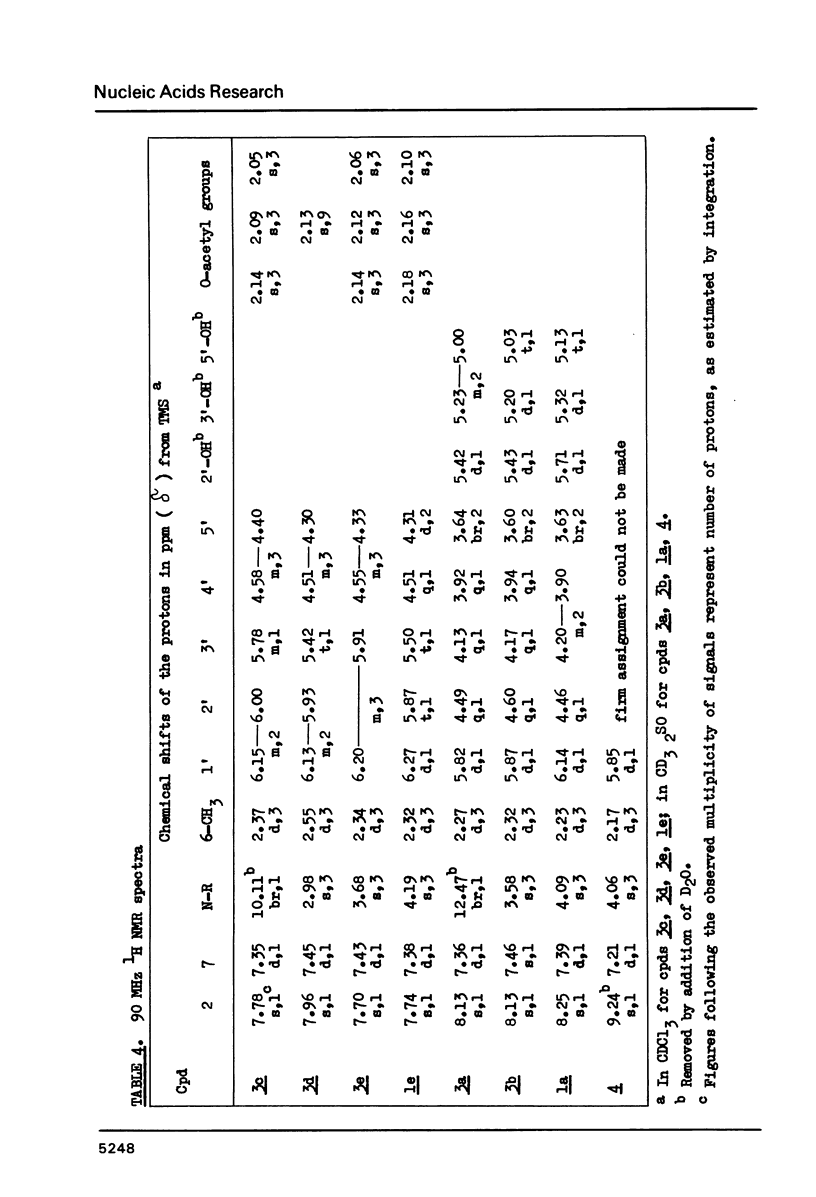







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davanloo P., Sprinzl M., Cramer F. Proton nuclear magnetic resonance of minor nucleosides in yeast phenylalanine transfer ribonucleic acid. Conformational changes as a consequence of aminoacylation, removal of the Y base, and codon--anticodon interaction. Biochemistry. 1979 Jul 24;18(15):3189–3199. doi: 10.1021/bi00582a001. [DOI] [PubMed] [Google Scholar]
- Farmer P. B., Foster A. B., Jarman M., Tisdale M. J. The alkylation of 2'-deoxyguanosine and of thymidine with diazoalkanes. Some observations on o-alkylation. Biochem J. 1973 Sep;135(1):203–213. doi: 10.1042/bj1350203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. M., Adams B. L., Kozarich J. W. Chemical transformations of 7,9-disubstituted purines and related heterocycles. Selective reduction of imines and immonium salts. J Org Chem. 1976 Jun 25;41(13):2303–2311. doi: 10.1021/jo00875a019. [DOI] [PubMed] [Google Scholar]
- Kasai H., Goto M., Ikeda K., Zama M., Mizuno Y., Takemura S., Matsuura S., Sugimoto T., Goto T. Structure of wye (Yt base) and wyosine (Yt) from Torulopsis utilis phenylalanine transfer ribonucleic acid. Biochemistry. 1976 Feb 24;15(4):898–904. doi: 10.1021/bi00649a027. [DOI] [PubMed] [Google Scholar]
- Kasai H., Yamaizumi Z., Kuchino Y., Nishimura S. Isolation of hydroxy-Y base from rat liver tRNAPhe. Nucleic Acids Res. 1979 Mar;6(3):993–999. doi: 10.1093/nar/6.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi R. G., Engelhardt D. L., Grunberger D. Incorporation of lysine into Y base of phenylalanine tRNA in Vero cells. Nucleic Acids Res. 1979;6(6):2209–2216. doi: 10.1093/nar/6.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese C. B., Saffhill R. A 1 H nuclear magnetic resonance spectroscopic study of some N-methyl and N-acyl derivatives of guanosine. The structure of N,O(2'),O(3'),O(5')-tetra-acetylguanosine. J Chem Soc Perkin 1. 1972;23:2937–2940. doi: 10.1039/p19720002937. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Singer B. Reaction of guanosine with ethylating agents. Biochemistry. 1972 Oct 10;11(21):3939–3947. doi: 10.1021/bi00771a017. [DOI] [PubMed] [Google Scholar]
- Sullivan J. P., Wong J. L. Guanosine-methyldiazonium ion reaction. Variation of methylation product patterns with reaction variables. Biochim Biophys Acta. 1977 Nov 2;479(1):1–15. doi: 10.1016/0005-2787(77)90120-4. [DOI] [PubMed] [Google Scholar]
- Thiebe R., Poralla K. Origin of the nucleoside Y in yeast tRNAPhe. FEBS Lett. 1973 Dec 15;38(1):27–28. doi: 10.1016/0014-5793(73)80504-6. [DOI] [PubMed] [Google Scholar]
- Trempe M. R., Ohgi K., Glitz D. G. Ribosome structure. Localization of 7-methylguanosine in the small subunits of Escherichia coli and chloroplast ribosomes by immunoelectron microscopy. J Biol Chem. 1982 Aug 25;257(16):9822–9829. [PubMed] [Google Scholar]


