Abstract
Endothelin-1 is a potent vasoactive peptide that occurs in chronically high levels in humans with pulmonary hypertension and in animal models of the disease. Recently, the unfolded protein response was implicated in a variety of diseases, including pulmonary hypertension. In addition, evidence is increasing for pathological, persistent inflammation in the pathobiology of this disease. We investigated whether endothelin-1 might engage the unfolded protein response and thus link inflammation and the production of hyaluronic acid by pulmonary artery smooth muscle cells. Using immunoblot, real-time PCR, immunofluorescence, and luciferase assays, we found that endothelin-1 induces both a transcriptional and posttranslational activation of the three major arms of the unfolded protein response. The pharmacologic blockade of endothelin A receptors, but not endothelin B receptors, attenuated the observed release, as did a pharmacologic blockade of extracellular signal–regulated kinases 1 and 2 (ERK-1/2) signaling. Using short hairpin RNA and ELISA, we observed that the release by pulmonary artery smooth muscle cells of inflammatory modulators, including hyaluronic acid, is associated with endothelin-1–induced ERK-1/2 phosphorylation and the unfolded protein response. Furthermore, the synthesis of hyaluronic acid induced by endothelin-1 is permissive for persistent THP-1 monocyte binding. These results suggest that endothelin-1, in part because it induces the unfolded protein response in pulmonary artery smooth muscle cells, triggers proinflammatory processes that likely contribute to vascular remodeling in pulmonary hypertension.
Keywords: unfolded protein response, endothelin, pulmonary artery smooth muscle, hyaluronic acid, inflammation
Clinical Relevance
Our data illustrate that, as a consequence of endothelin-1 signaling in rat pulmonary artery smooth muscle cells, the unfolded protein response is activated and proinflammatory biomolecules are produced. These observations indicate the importance of pulmonary artery smooth muscle cells in the inflammatory processes that underpin the development of pulmonary hypertension, particularly in the recruitment and retention of immune cells. Our data support the emerging concept that the therapeutic control of inflammation in patients with pulmonary hypertension may lead to improved outcomes.
Pulmonary hypertension (PH) is a disease characterized by extensive pulmonary vascular remodeling, present along the entire length of the vascular tree (1). Eventually, the increased impedance to flow causes right heart failure and death (2). Evidence is mounting that the recruitment of inflammatory cells and the development of persistent inflammation are key components of the pathological vascular remodeling observed in PH (3). In patients with idiopathic PH, macrophages and lymphocytes are found in bronchovascular locations and in occlusive neointimal lesions, and express a panoply of chemokines including CCL2, CCL5, and CX3CL1 (4–7). Concentrations of proinflammatory cytokines, including IL-1 and IL-6, are elevated in both human idiopathic pulmonary artery hypertension (PAH) (8) and in the monocrotaline (MCT) model of PH (9, 10). Depletion of the circulating pool of macrophages is sufficient to prevent hypoxia-driven PH (11), and dexamethasone treatment reverses MCT PH (12). Taken together, these studies suggest that PH is a syndrome characterized by the complex disturbance of innate and acquired immune cells, and of mediators and effectors of inflammation.
Building on studies that pointed to the importance of extracellular matrix metabolism in pulmonary vessel homeostasis (13, 14), recent work implicated hyaluronan and its degradative byproducts as key regulators of inflammation at vascular sites. The hyaluronic acid content is increased in idiopathic PH (iPAH), and is associated with decreases in the hyaluronan synthase–1 (HAS1) and in hyaluronidase-1 (HYAL1) (15, 16). Previous authors suggested that the increased HA content in lung was attributable to the decreased degradation of HA. In addition, pulmonary artery smooth muscle cells (PASMCs) isolated from patients with iPAH elaborated cable-like structures of hyaluronic acid (HA), to which monocytic-like cells preferentially attached (16). In the monocrotaline rat model of PH, increased activity of HYAL1 is evident early in the disease course, whereas increases in degradation and synthesis occur during later stages of PH (17). Those authors concluded that an early burst of proinflammatory HA fragments may herald the onset and progression of PH. These data collectively lead to the impression that hyaluronan fragments, produced largely by PASMCs, are inflammatory and promote leukocyte adhesion.
Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) were shown to induce the deposition of hyauronan and leukocyte adhesion in both human mucosal and rabbit aorta smooth muscle cells (18). The UPR is a highly conserved cell stress program initiated by a number of events, including viral infection, genetic mutation, hypoxia, and metabolic/oxidative stress (19). When cells engage the UPR, gene expression is modulated to promote either cell survival or apoptosis, depending on the nature, extent, and duration of the stress (20). Imbalances between a cell's capacity to process or clear unfolded proteins and the cellular load are sensed and relayed through three key pathways linking the UPR to autophagy, apoptosis, and inflammation (21). We observed that the UPR is robustly active in and around bronchovascular structures in humans with PH, as well as in a monocrotaline rat model of PH (M. Yeager, unpublished data). These regions tend to colocalize with areas of inflammation, HA, and vascular remodeling. Pharmacologic modulation of the UPR prevented and partly reversed PH in this model. In addition, defects in protein folding and Golgi trafficking are evident in the context of PH (22, 23).
We hypothesized that endothelin-1 (ET-1), a powerful vasoactive peptide present at high concentrations in patients with PH (24), induces the UPR in PASMCs. Once activated, the UPR might, in turn, cause PASMCs to release proinflammatory mediators, such as cytokines and HA. Using rat primary PASMCs, we investigated the effects of ET-1 with regard to activation of the UPR, the release of HA, and the production of inflammatory cytokines, as well as the dependence of PASMCs on the activation of the UPR. We found that ET-1 causes a rapid nuclear accumulation of activating transcription factor 6 (ATF6) and activates an ATF6–luciferase reporter, in an endothelin-A receptor–dependent and ERK/1–2–dependent manner. ET-1 induced PASMCs to produce inflammatory cytokines and HA. These effects were partly abrogated by endothelin receptor blockade, by ATF6 knockdown, and by modulation of the UPR via salubrinal. In addition, we found that THP-1 monocytes adhered to rat PASMCs stimulated by ET-1 in an HA-dependent manner. This adhesion persisted for 3 days after stimulation by ET-1. Similarly, THP-1 monocytes adhered to medial and adventitial vessel compartments rich in HA in tissue sections from MCT-treated rats compared with control rats. We conclude that (1) PASMCs can produce inflammatory biomolecules secondary to stimulation by ET-1 through an ERK/1–2 pathway associated with activation of the UPR, and (2) the HA produced induces persistent monocyte adhesion.
Materials and Methods
Isolation of PASMCs
Rat PASMCs were cultured from explants as described elsewhere (25). Cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) with 10% FBS, penicillin (100 units/ml), streptomycin (100 units/ml), and fungizone (1.25 μg/ml). Cells from passages 3–8 at 70% confluence were used in the experiments.
Cell Culture, Cytokines, and Inhibitors
PASMCs were starved overnight in 0.1% FCS in DMEM. ET-1 (Sigma, St. Louis, MO) was applied at 100 nM. Endothelin A (ETA) and endothelin B (ETB) receptor inhibitors BQ123 and BQ788 (Calbiochem, Darmstadt, Germany), respectively, were applied at 5 μM in DMSO (Sigma) for 30 minutes before the application of ET-1. Salubrinal (Tocris Bioscience, Ellsville, MO) was applied to cells at 5 mg/ml in DMSO for the indicated times. Tunicamycin (Sigma) was applied at 10 μg/ml in DMSO for 24 hours. The selective noncompetitive mitogen-activated protein kinase (MEK) inhibitor PD98059 (Calbiochem) was applied to PASMCs at a final concentration of 20 μM.
Immunofluorescence
Immunofluorescent staining was performed as described elsewhere (11). The antibodies used are listed in Table 1. For HA binding protein (HABP) studies, we applied bitoinylated HABP (Calbiochem) at 20 ng/ml in PBS with 0.1% BSA for 1 hour at 30°C. Samples were then incubated with streptavidin–Alexa 488 (Invitrogen, Carlsbad, CA) in PBS/BSA for 30 minutes. Sections were mounted in VectaShield with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA), and images were acquired at room temperature using a Zeiss Axiovert S100 (Zeiss, Jena, Germany) fitted with Zeiss 20× 0.4 numerical aperture and 10× 0.3 numerical aperture objectives and an Axiocam camera (Zeiss).
TABLE 1.
ANTIBODIES AND PRIMERS USED IN THE STUDY
| Antigen | Source | Concentration Used | Species, Isotype |
| Calponin | Abcam Ab700 | 1:400 | Mouse IgG |
| Smooth muscle actin | Sigma 2547 | 1:1,000 | Mouse IgG |
| pERK/1-2 | Cell Signaling 4377S | 1:00 IF, 1:1,000 blot | Rabbit IgG |
| ERK/1-2 | Cell Signaling 4695 | 1:1,000 | Rabbot IgG |
| ATF6 | Imgenex 273 | 1:100 IF, 1:250 blot | Mouse IgG |
| Beta-action | Sigma A5316 | 1:1,000 | Mouse IgG |
| peIF2alpha | Cell Signaling 3597 | 1:50 IF, 1:500 blot | Rabbit IgG |
| Total eIF2alpha | Cell Signaling 2103 | 1:1,000 | Mouse IgG |
| Hyaluronic acide–binding protein, biotinylated | Seigaku | 20 ng/ml | N/A |
| Streptavidin–Alexa 488 | Invitrogen S11223 | 1:500 | N/A |
| Anti-mouse Cy3 | Chemicon AP124C | 1:500 | Goat |
| Anti-rabbit Alexa 488 | Invitrogen A11034 | 1:500 | Goat |
| Gene | Forward Primer | Reverse Primer | |
| sXBP-1 | GACTCCGCAGCAGGTG | GCGTCAGAATCCATGGGA | |
| XBP-1 | CAGACTACGTGCGCCTCTGC | CTTCTGGGTAGACCTCTGGG | |
| HPRT | AAGCTTGCTGGTGAAAAGGA | CAAGGGCATATCCAACAACA | |
| Hyal2 | ACAGAACTTAGCCAGATGGA | GGTGAGAGTCATAGCTACCA | |
| HAS2 | AGGGCCTGCCAGTCTTATTT | CCCTGTTGGTAAGGTGCCTA |
Immunoblotting
The immunoblotting of lysates from rat PASMCs was performed as previously described (26).
ELISAs
We used a commercially available kit for HA ELISA experiments (HA ELISA Kit, catalogue number 029-001; Corgenix, Broomfield, CO). We used a commercially available multiplex kit for cytokine ELISA experiments (Rat Inflammatory Cytokines Multi-Analyte ELISArray Kit, catalogue number MER-004A; SABiosciences, Frederick, MD).
Luciferase Assay
We used a commercially available kit for ATF6 reporter experiments, according to the manufacturer's instructions (Cignal ATF6 Reporter (luc) Kit; SABiosciences). Cells were lysed and processed for firefly luciferase activity, which was normalized to baseline renilla luciferase activity. We confirmed these data using reporter plasmids for ATF6 and mutant ATF6, kindly provided by Kazutoshi Mori (27).
Real-Time PCR
RNA was isolated and PCR was performed as previously described (15). Primer sequences are provided in Table 1.
Short Hairpin RNA Transfections
We used a commercially available kit for the knockdown of ATF6, according to the manufacturer's instructions (KR51427N for neomycin resistance; SABiosciences).
Adhesion Assays
We used a modified Stamper–Woodruff Assay for the adhesion of THP-1 cells to either cultured PASMCs or frozen, unfixed rat lung tissue sections (28). We labeled 1 × 106 THP-1 cells with PKH26 dye (Sigma), and then applied them to PASMCs stimulated with 100 nM ET-1 or 10 μg/ml tunicamycin for 24 hours in media with 1% FCS. THP-1 was added to PASMCs incubated with hyaluronidase (50 U/ml in 500 μl at 37°C for 2 hours; Seikagaku, Tokyo, Japan) or salubrinal (5 mg/ml), or transfected with ATF6 short hairpin RNA plasmid. Replicate dishes were washed three times with phosphate buffered saline tween-20 (PBST), and incubated with VectaShield with DAPI (Vector Laboratories). Adherent PKH26+ cells were counted under a Zeiss fluorescent microscope (Zeiss). Alternatively, 1 × 106 THP-1 cells were added to frozen, unfixed tissue sections, washed three times in PBST, counterstained, and imaged as already described.
Statistical Analyses
All experiments were performed in triplicate. Data are expressed as means ± SE, and were analyzed by unpaired Student t tests for comparisons between two groups, or by one-way ANOVA with post hoc analysis for multiple comparisons. P < 0.05 was considered significant.
Results
ET-1 Induces the UPR in PASMCs, and Signals through Extracellular Signal–Regulated Kinases 1 and 2
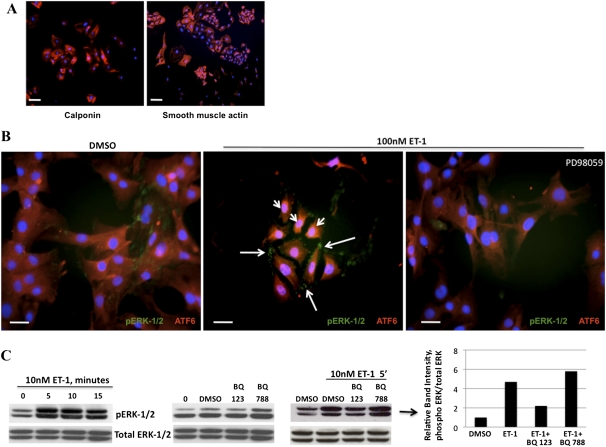
Concentrations of plasma and lung ET-1 are high in patients with PH and in animal models of PH (24, 29). In addition, increased vascular permeability is evident in the pulmonary vascular tree in PH (30), which may place PASMCs in increased contact with ET-1. Several studies established that increased signaling and cellular activity activate the UPR in a variety of cellular contexts (31–33). Furthermore, human aortic smooth muscle cells are known to process ET-1 signals through the extracellular signal–regulated kinase 1 and 2 (ERK-1/2) pathway (34). To determine if exposing PASMCs to ET-1 at disease levels activates the UPR and the ERK pathway, we performed immunofluorescence and immunoblotting. We noted that after a brief exposure of PASMCs to ET-1, after as early as 5 minutes, ATF6 nuclear translocation became apparent (Figure 1B). Moreover, ET-1 rapidly induced the phosphorylation of ERK-1/2, with similar kinetics (Figures 1B and 1C). The phosphorylation of ERK-1/2 could be blocked by the addition of the selective noncompetitive MEK inhibitor PD98059. The antagonism of the ETA receptor by BQ123, but not the antagonism of the ETB receptor by BQ788 (or using PASMCs lacking ETB; Figure E1A in the online supplement), ameliorated the accumulation of phosphorylated (p) ERK-1/2. These results suggest that in rat PASMCs, ET-1 specifically induces ATF6 nuclear translocation and the phosphorylation of ERK-1/2 in an ETA-dependent manner.
Figure 1.
Endothelin-1 (ET-1) induces the phosphorylation of extracellular signal–regulated kinase 1 and 2 (ERK-1/2) by pulmonary artery smooth muscle cells (PASMCs) and activation of the activating transcription factor 6 (ATF6) arm of the unfolded protein response (UPR). (A) Fluorescence microscopy of isolated PASMCs (calponin or smooth muscle actin = Cy3, 4',6-diamidino-2-phenylindole [DAPI] nuclei). (B) Within 5 minutes, ET-1 induced the rapid nuclear localization of the UPR protein ATF6 (arrowheads, Cy3), and also caused the phosphorylation (p) of ERK-1/2 (arrows, Alexa 488), both of which could be blocked by the mitogen activated protein kinase inhibitor PD98059. (C) Immunoblot time-course analysis of the phosphorylation of ERK-1/2 induced by ET-1, which peaked at 5–10 minutes. The phosphorylation of ERK-1/2 was blocked by the preincubation of PASMCs with the endothelin A receptor (ETA) receptor antagonist BQ123, but not the endothelin B receptor (ETB) receptor antagonist BQ788. Bar = 10 μm.
ET-1 Activates the Transcription of ATF6 and Also Activates the X-Box Binding Protein 1 Arm of the UPR
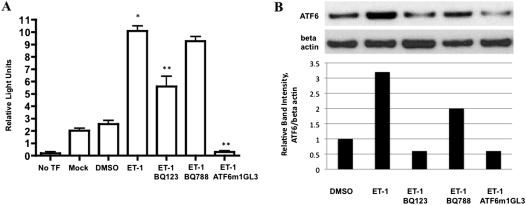
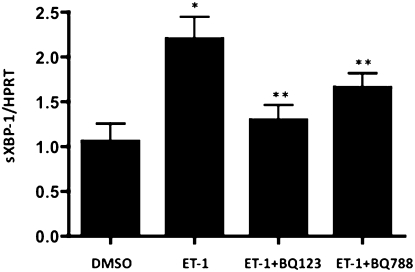
To examine whether ET-1 induced downstream transcriptional effects on the UPR, we transfected PASMC with a dual reporter system and used either an ATF6 reporter plasmid or a mutant (noninducible) ATF6 reporter plasmid. Rat PASMC treated for 24 hours with 100 nM ET-1 robustly activated the ATF6 reporter compared with any of three controls: no plasmid transfection, empty vector transfection, noninducible ATF6 (mutant), and ATF6 reporter transfection without stimulation by ET-1 (Figure 2A). BQ123, but not BQ788, prevented activation of the ATF6 reporter. We confirmed the increase in ATF6 at the protein level under the same conditions (Figure 2B). To test whether ET-1 activated the Inositol-requiring kinase 1/X-box binding protein 1 (IRE-1/XBP-1) UPR transcriptional pathway, we performed quantitative PCR for spliced XBP-1. IRE-1 is an ER transmembrane protein that, when phosphorylated under ER stress, cleaves XBP-1 into an active transcription factor (sXBP-1) through endoRNase activity (35). We found increased sXBP-1 in PASMCs exposed to ET-1 compared with DMSO alone (Figure 3). The increased sXBP-1 could be attenuated by BQ123, and less so by BQ788, and was increased in ETB-deficient PASMCs (Figure E1B). These results suggest that ET-1 activates both the ATF6 and IRE-1/XBP-1 transcriptional pathways of the UPR in a largely ETA-dependent manner.
Figure 2.
ET-1 increases the production of ATF6 in PASMCs. Rat PASMCs were transfected with 0.1 μg of one of two luciferase reporter genes: ATF6α binding-site reporter gene ATF6GL3, or the nonresponsive ATF6α mutant site reporter ATF6 m1GL3. PASMCs were exposed for 24 hours to 100 nM ET-1, and then the relative intensity of luciferase was measured (n = 3). ET-1 induced a significant increase in ATF6-driven luciferase activity compared with control samples, the mutant ATF6 reporter m1GL3, and DMSO alone (No TF, empty cells; Mock, transfection with empty vector; DMSO, reporter plasmid transfection without stimulation by ET-1). (B) Densitometry of ATF6 Western blot of protein lysates under identical conditions as in A. *P < 0.05 compared with no TF. **P < 0.05 compared with ET-1–induced activation of ATF6 reporter.
Figure 3.
ET-1 increases splicing of X-box binding protein 1 (XBP-1) in PASMCs. ET-1 activates the inositol requiring enzyme 1 (IRE-1)/XBP-1 arm of the UPR, as evidenced by a twofold increase in the spliced form of XBP-1 (sXBP-1), normalized to the housekeeping gene HPRT. This effect is inhibited by ETA antagonism (BQ123), and to a lesser extent, by ETB antagonism (BQ788). *P < 0.05, compared with DMSO. **P < 0.05, compared with ET-1–induced concentration of sXBP-1.
ET-1 Induces the Release by PASMCs of Inflammatory Cytokines through UPR Pathways
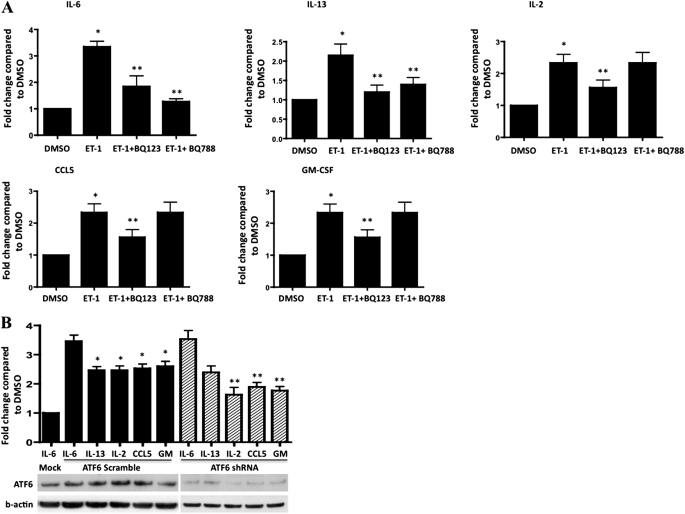
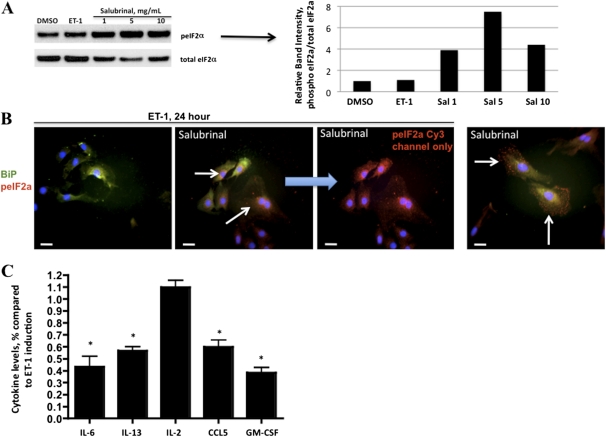
Activation of the UPR in endothelial cells by phospholipolyzed low density lipoprotein was previously shown to result in the elaboration of proinflammatory cytokines (36). To test whether a similar functional effect occurred downstream of the ATF6 transcriptional activation by ET-1 in PASMCs, we tested culture supernatants for the presence of proinflammatory cytokines, using a multicytokine ELISA. We found that ET-1 induced the production of IL-6, IL-13, IL-2, CCL5, and granulocyte macrophage colony stimulating factor (GM-CSF), compared with DMSO control samples (Figure 4A). For some cytokines, this effect was directly dependent on the activation by ET-1 of the ATF6 arm of the UPR, because the transfection of an ATF6 shRNA-expressing plasmid abolished the increases in IL-2, CCL5, and GM-CSF, but not IL-6 and IL-13 (Figure 4B). Interestingly, the production of IL-2, CCL5, and GM-CSF by ET-1 could be blocked with BQ123 but not with BQ788, suggesting an ETA-dependent mechanism. The production of IL-6 and IL-13 induced by ET-1 was blocked by both ETA and ETB antagonism. This result suggests that in rat PASMCs, ET-1 signaling through ETA and ETB receptors can induce the production of proinflammatory cytokines through pathways parallel to the UPR pathways investigated here. To test whether the PKR-like ER-localized eIF2α kinase/eukaryotic initiation factor α (PERK/eIF2α) pathway of the UPR is involved in the release induced by ET-1 of inflammatory cytokines in rat PASMCs, we treated cells with salubrinal. Salubrinal was shown in both cell culture (37) and animal (38) models to prevent the dephosphorylation of PeIF2α, which leads to selective protein translation and gene transcription for prosurvival responses. At three different doses, salubrinal caused an increased phosphorylation of eIF2α in cells treated with ET-1 for 24 hours (Figures 5A and 5B). With the exception of IL-2, with salubrinal prevented the increased release of inflammatory cytokines by ET-1 Figure 5C). Thus, the stimulation by ET-1 of rat PASMCs is associated with the release of proinflammatory cytokines, an effect largely preventable through the modulation of the PERK/eIF2α pathway by salubrinal.
Figure 4.
ET-1 induces the release by PASMCs of IL-6, IL-13, IL-2, CCL5, and granulocyte macrophage colony stimulating factor (GM-CSF). (A) The blockade of ETA attenuates these cytokine increases. The blockade of ETB prevents the release of IL-6 and IL-13 but not of IL-2, CCL5, or GM-CSF (*P < 0.05, compared with DMSO; **P < 0.05, compared with ET-1–induced fold change). (B) ATF6 short hairpin RNA knockdown attenuates the ET-1–mediated production of IL-2, CCL5, and GM-CSF, but not of IL-6 or IL-13. Representative immunoblots of ATF6 knockdown in rat PASMC lysates correspond to cytokine supernatant analyses (Mock, PASMCs treated with transfection reagent alone and ET-1–induced IL-6 measured in medium; GM, GM-CSF). *P < 0.05, compared with Mock. **P < 0.05, compared with ET-1 treatment with ATF6 scramble transfection.
Figure 5.
ET-1 induces a release of inflammatory cytokine by PASMCs that is partly dependent on the dephosphorylation of eIF2α. (A) Immunoblot analysis of salubrinal dosing of PASMCs. Cells were pretreated overnight with salubrinal as indicated, and treated with 100 nM ET-1 and lysates taken after 24 hours. ET-1 did not increase the concentration of peIF2α, whereas salubrinal increased the concentration of peIF2α at all three doses. (B) Immunofluorescence of PASMCs treated with 100 nM ET-1 for 24 hours. With salubrinal, a pronounced increase in peIF2α is apparent (arrows, Cy3), regardless of ET-1 treatment. The localization of binding immunoglobulin protein (BiP) is not affected by either ET-1 or salubrinal. (C) Salubrinal causes decreased concentrations of IL-6, IL-13, CCL5, and GM-CSF, but not IL-2, in supernatants of PASMCs treated with ET-1. *P < 0.05, compared with induction of ET-1 as in Figure 4. Bar = 10 μm.
ET-1 Causes Increased Release of HA by PASMCs to Which Monocytes Persistently Bind
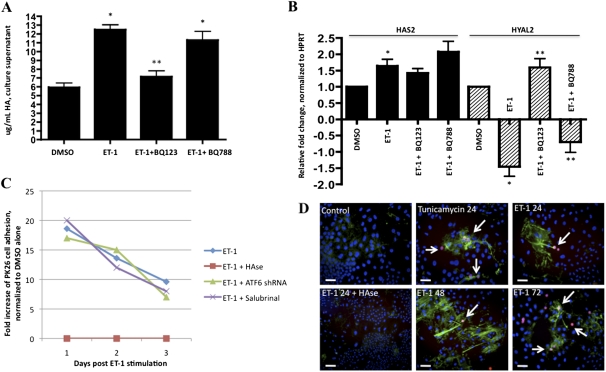
Recently, smooth muscle cells (airway, colonic, and aortic) were shown to produce HA in response to activation of the UPR (18, 39). Once produced, HA acts as a platform to which leukocytes adhere. The MCT rat model produces HA deposited in small to medium pulmonary vessels and in peribronchovascular regions (17). We therefore investigated whether ET-1 could induce the synthesis of HA by rat PASMCs. We found that PASMCs stimulated by ET-1 produced significantly larger amounts of HA compared with DMSO control samples (Figure 6A). This effect was blocked by BQ123 but not by BQ788, suggesting a requirement for the ETA receptor. The increased HA was likely not the result of increased activity of the HA synthase enzymes HAS1, HAS2, or HAS3, but may be the result of decreased HYAL2 activity (Figure 6B), consistent with previous reports (15–17). The HYAL2 activity stimulated by ET-1 was sensitive to BQ123, and to a lesser extent, BQ788. We next tested whether the HA produced by rat PASMCs in response to ET-1 could cause the binding of THP-1 monocytes. We found that THP-1 monocytes adhered to rat PASMCs stimulated by ET-1 for up to 3 days after the ET-1 stimulation was withdrawn (Figures 6C and 6D). THP-1 adhesion was prevented in PASMCs stimulated by ET-1 that were pretreated with Streptomyces hyaluronidase just before the addition of monocytes. Neither transfection with ATF6 shRNA nor the co-incubation of ET-1 with salubrinal prevented the observed THP-1 adhesion (Figure 6C). We conclude that ET-1–ETA signaling stimulates rat PASMCs to produce HA, to which THP-1 monocytes persistently adhere. In addition, although the production of proinflammatory cytokines required the UPR (Figures 4 and 5), the production of HA and the adhesion of monocytes apparently did not require the activation of ATF6 or PERK/eIF2α pathways in rat PASMCs.
Figure 6.
ET-1 affects biology of hyaluronic acid (HA) in rat PASMCs. (A) HA in PASMC supernatant increases in response to ET-1, and was inhibited by ETA antagonism but not by ETB antagonism. (B) Hyaluronan synthase (HAS)–2 transcripts slightly increase in response to ET-1, and this increase was independent of ETA or ETB. hyaluronidase (HYAL)–2 decreases in response to ET-1, and was reversed by the antagonism of ETA, but not of ETB. (C) Incubation for 24 hours of rat PASMCs with ET-1 induces prolonged HA-mediated THP-1 monocyte adhesion lasting 3 days. The treatment of PASMCs with ATF6 shRNA or salubrinal did not abrogate HA-mediated THP-1 adhesion. (D, from top left to right) PKH26-labeled THP-1 cells (arrows) do not adhere to untreated PASMCs, and HA is barely detectable. When PASMCs are treated with either tunicamycin or ET-1 for 24 hours, THP-1 cells adhere and HA can be detected, except if treated with hyaluronidase (HAse) before THP-1 cell incubation (bottom left). THP-1 cells persistently adhered, and HA remained detectable, at 48 and 72 hours after the stimulation by ET-1 of PASMCs (lower middle and right). *P < 0.05, compared with DMSO. **P < 0.05, compared with ET-1 alone. Bar = 10 μm.
Monocytes Bind HA-Rich Regions in MCT Rat Lung Tissue Sections
To corroborate our cell culture findings with respect to the production of HA and THP-1 adhesion, we performed a modified Stamper–Woodruff adhesion assay (28) on lung tissue sections from rats with monocrotaline-induced PH. We confirmed previous findings that HA is significantly up-regulated in MCT rat lung compared with control lungs, and we found that THP-1 cells adhere to peribronchovascular regions rich in HA (Figures 7A–7D). THP-1 adhesion was abrogated by treating lung sections with hyaluronidase before incubation with THP-1 cells (Figure 7E). We conclude that monocytes bind HA in the rat lung, localizing particularly to medial and adventitial vascular compartments, thus corroborating our cultured PASMC data.
Figure 7.
Leukocytes adhere to rat pulmonary vessels rich in HA. (A and B) Peri(broncho)vascular HA was significantly more abundant in rats (B, arrows) with monocrotaline (MCT)–induced pulmonary hypertension compared with (A) control rats (biotin–HA binding protein + streptavidin–Alexa 488, with DAPI counterstain). (C) PKH26-labeled THP-1 cells did not adhere to control rat lung sections. (D) THP-1 cells (PKH26 in red, indicated by arrows) adhered to HA-rich medial and adventitial regions in MCT rat lung sections. (E) HAse pretreatment abolished THP-1 adhesion. (F) THP-1 cell adhesion quantification per bronchovascular structure was counted in five fields from five sections each, with six rats per group. Bar = 20 μm.
Discussion
Persistent inflammation associated with vascular remodeling is a hallmark of PH, and constitutes an attractive therapeutic target. We assert that a more precise understanding of the contributions of lung resident cells and circulating cells (monocytes, macrophages, lymphocytes, and mast cells) to inflammatory processes will lead to new adjuvant therapies and improved outcomes for patients with PH. We demonstrate that ET-1 couples the synthesis by PASMCs of HA and the UPR-dependent production of inflammatory cytokines to persistent monocyte adhesion. Our study may also link previous observations of vascular leak in PH to an increased access of potent multifunctional soluble ligands (ET-1) to subendothelial compartments and downstream functional sequelae (e.g., inflammation). To facilitate this, ET-1 appears to engage a posttranslational and transcriptional UPR in PASMCs that results in an elaboration of inflammatory cytokines. Furthermore, in ET-1–treated PASMC culture and monocrotaline lung sections, monocytes readily bind HA. We conclude that ET-1, through ET receptor–dependent and independent mechanisms, induces a UPR-associated proinflammatory PASMC phenotype, and the UPR-independent production of HA capable of monocytic cell adhesion.
These data, considered in the context of studies demonstrating endoplasmic reticulum/Golgi dysfunction in PH (22, 23), compel us to propose that the UPR pathway, by virtue of interactions with other environmental and genetic factors, could be a major promoter of unresolved inflammation. For example, viral infection (particularly HIV) is associated with PH, and is an established ER stressor (19). Hypoxia, genetic mutations, and reactive oxygen species, as seen in the settings of PH and cancer, are also common ER stressors, and lead to inflammation (19). In any of these contexts, severe ER stress may promote perpetual inflammation by inducing apoptosis in resident vascular cells, and may also cause the sustained recruitment of inflammatory cells and the production of inflammatory mediators such as HA. The treatment with ET-1 of rat PASMCs at 100 nM (the highest dose we used) did not induce apoptosis in our study (Figure E1C), whereas tunicamycin did. Thus, high circulating concentrations of ET-1 may engage the UPR in vascular cell types, altering the transcriptional and translational phenotype, but without causing cell death.
In our experiments, we focused on the ERK-1/2 signaling downstream from the ET receptor level. ET-1 is known to induce this pathway in several types of smooth muscle cells (34, 40), but additional signaling cascades are concomitantly activated, such as PI3K, PKC-δ, and PKC-ε, and p38 mitogen-activated protein kinase (41). Therefore, although we found significant contributions of the ERK system to the production of HA (Figures 6A and 6B) and inflammatory cytokines (Figures 4 and 5), the impact of other pathways in PASMC should be evaluated. In addition, we did not investigate the disturbances in protein glycosylation likely present in our tunicamycin experiments, nor did we examine the role of intracellular calcium, which is sensitive to ET-1 and largely responsible for PASMC vasoconstriction. Nevertheless, our finding of ET-1 signaling, in part by activation of the UPR, is an important first mechanistic link between clinical observations (high concentrations of ET-1, inflammatory cytokines, and HA) with the functionally persistent activation of a proinflammatory smooth muscle cell phenotype in vitro (Figures 4–6) and in vivo (Figure 7). Future studies to clarify the ET-1–induced intracellular signaling pathways controlling this phenotype, using pathway-specific agonists and antagonists, will likely identify potential therapeutic targets.
To determine precisely what each arm of the UPR may contribute to the proinflammatory phenotype of ET-1–stimulated PASMCs will be also interesting. For example, partial sequences of endoplasmic reticulum response elements 1 and 2 can be found in the promoter regions of rat HYAL 1, 2, and 3 and HAS 2 and 3 genes (M. Yeager, unpublished observations). Thus, the ATF6, IRE-1, and pPERK arms of the UPR may influence the transcriptional control of HA biology. On the other hand, the inhibition of protein synthesis by cycloheximide does not stop the production of HA, and the kinetics of HA production are rather fast (42), highlighting the importance of posttranslational control mechanisms. ER stress–mediated protein synthesis selection by the pPERK arm of the UPR may therefore be more important for PASMC HA biology than de novo transcription. Moreover, complementary factors contribute to leukocyte adhesion, such as the expression of vascular cell adhesion molecule–1 (VCAM-1), which was shown to be induced by polyinosinic:polycytidylic acid–treated murine airway smooth muscle cells (40). In that study, however, tunicamycin (a powerful inducer of the UPR) did not up-regulate VCAM-1, although it did induce the production of HA and leukocyte adhesion, consistent with our observations.
Beyond the increased production of HA, the mechanisms of HA clearance in the (diseased) lung and how the balance is shifted toward accumulation are yet to be fully described. This will likely comprise an important step toward a more complete understanding of the robust and sustained inflammation seen in PH. The clearance of HA, either through lymphatics or degraded on site by neighboring cells, is critical to organ homeostasis (43). PH is associated with the decreased degradation and increased synthesis of HA (particularly high molecular weight isoforms) (16, 17). In two similar studies, no differences were evident in the expression of HAS1 in PASMCs from control subjects or patients with PAH, yet contradictory results were obtained for Hyal1 (15, 16). With respect to our endpoints, we found no ET-1–inducible differences in HAS1, HAS3, HYAL1, or HYAL2 (data not shown), but we did for HAS2 and HYAL2 (Figure 6B). In whole-lung lysates from the monocrotaline rat, differences in mRNA expression for all of these enzymes progressed to a prosynthetic, less degradative state by 28 days after MCT (17). Further studies are needed to clarify HA metabolism more fully in the context of PH and isolated PASMCs. With regard to inflammation, we show that after activation by ET-1 of the UPR in PASMCs, HA cables are elaborated and are capable of supporting persistent THP-1 monocyte binding. Interestingly, HAS2, which we found to be induced by ET-1, is functionally required for HA-mediated leukocyte adhesion to endothelial cells, and is induced by inflammatory cytokines such as IL-1b and TNF-α (44). Furthermore, HA fragments cleaved by HYAL2 trigger the monocyte-mediated production of proinflammatory cytokines (45). These studies collectively suggest that the local “signature” of HA biology affects the recruited circulating cell phenotype, and that amplifying feedback loops may be operational with respect to adherent inflammatory cells. Although the exact consequences of persistent leukocyte adhesion at sites rich in inflammatory cytokines and HA remain undefined, our study highlights the potential for therapeutic interventions at multiple points in PASMC and leukocyte biology. ET-1 signaling antagonism, the modulation of the UPR, anti-inflammatory agents, and the control of hyaluronic acid metabolism represent putative strategies to break the persistent inflammation observed in PH.
In conclusion, our data illustrate that, as a consequence of ET-1 signaling in rat PASMCs, the UPR is activated and proinflammatory biomolecules are produced. These observations point to the importance of PASMCs in the inflammatory processes that underpin the development of PH, particularly in the recruitment and retention of immune cells. Our data support the emerging concept that the therapeutic control of inflammation in patients with PH may lead to improved outcomes.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Specialized Centers of Clinically Oriented Research Grant HL-084923–02 and National Institutes of Health Program Project Grant HL-014985–35 (K.R.S.), the Brigid Hope Research Fund, and the Leah Bult Pulmonary Hypertension Research Fund.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0506OC on July 21, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 2010;121:2045–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milan A, Magnino C, Veglio F. Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J Am Soc Echocardiogr 2010;23:225–239 [DOI] [PubMed] [Google Scholar]
- 3.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009;54:S10–S19 [DOI] [PubMed] [Google Scholar]
- 4.Dorfmuller P, Humbert M, Perros F, Sanchez O, Simonneau G, Muller KM, Capron F. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 2007;38:893–902 [DOI] [PubMed] [Google Scholar]
- 5.Dorfmuller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, Coulomb-Lhermine A, Marfaing-Koka A, Simonneau G, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002;165:534–539 [DOI] [PubMed] [Google Scholar]
- 6.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, Darteville P, Simonneau G, Adnot S, Eddahibi S. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2007;176:1041–1047 [DOI] [PubMed] [Google Scholar]
- 7.Balabanian K, Foussat A, Dorfmuller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, Marfaing-Koka A, Krzysiek R, Rimaniol AC, et al. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med 2002;165:1419–1425 [DOI] [PubMed] [Google Scholar]
- 8.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151:1628–1631 [DOI] [PubMed] [Google Scholar]
- 9.Bhargava A, Kumar A, Yuan N, Gewitz MH, Mathew R. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis 1999;1:126–132 [PubMed] [Google Scholar]
- 10.Gillespie MN, Goldblum SE, Cohen DA, McClain CJ. Interleukin 1 bioactivity in the lungs of rats with monocrotaline-induced pulmonary hypertension. Proc Soc Exp Biol Med 1988;187:26–32 [DOI] [PubMed] [Google Scholar]
- 11.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 2006;168:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price LC, Montani D, Tcherakian C, Dorfmüller P, Souza R, Gambaryan N, Chaumais MC, Shao DM, Simonneau G, Howard LS, et al. Dexamethasone reverses monocrotaline-induced pulmonary arterial hypertension in rats. Eur Respir J 2011;37:813–822 [DOI] [PubMed] [Google Scholar]
- 13.Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 2002;105:516–521 [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Wigle D, Hinek A, Kobayashi J, Ye C, Zuker M, Dodo H, Keeley FW, Rabinovitch M. The endogenous vascular elastase that governs development and progression of monocrotaline-induced pulmonary hypertension in rats is a novel enzyme related to the serine proteinase adipsin. J Clin Invest 1994;94:1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papakonstantinou E, Kouri FM, Karakiulakis G, Klagas I, Eickelberg O. Increased hyaluronic acid content in idiopathic pulmonary arterial hypertension. Eur Respir J 2008;32:1504–1512 [DOI] [PubMed] [Google Scholar]
- 16.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2008;295:L789–L799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ormiston ML, Slaughter GR, Deng Y, Stewart DJ, Courtman DW. The enzymatic degradation of hyaluronan is associated with disease progression in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2010;298:L148–L157 [DOI] [PubMed] [Google Scholar]
- 18.Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem 2003;278:47223–47231 [DOI] [PubMed] [Google Scholar]
- 19.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2008;7:1013–1030 [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008;454:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett 2010; Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 22.Sehgal PB, Mukhopadhyay S, Xu F, Patel K, Shah M. Dysfunction of Golgi tethers, SNAREs, and SNAPs in monocrotaline-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2007;292:L1526–L1542 [DOI] [PubMed] [Google Scholar]
- 23.Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Functional analysis of bone morphogenetic protein Type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 2002;11:1517–1525 [DOI] [PubMed] [Google Scholar]
- 24.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 1991;114:464–469 [DOI] [PubMed] [Google Scholar]
- 25.Archer SL, Huang JMC, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res 1996;78:431–442 [DOI] [PubMed] [Google Scholar]
- 26.Strassheim D, Riddle SR, Burke DL, Geraci MW, Stenmark KR. Prostacyclin inhibits IFN-gamma–stimulated cytokine expression by reduced recruitment of CBP/p300 to STAT1 in a SOCS-1–independent manner. J Immunol 2009;183:6981–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins: involvement of basic leucine zipper transcription factors. J Biol Chem 1998;273:33741–33749 [DOI] [PubMed] [Google Scholar]
- 28.Stamper HB, Woodruff JJ. An in vitro model of lymphocyte homing: I. Characterization of the interaction between thoracic duct lymphocytes and specialized high endothelial venules of lymph nodes. J Immunol 1977;119:772–780 [PubMed] [Google Scholar]
- 29.Miyauchi T, Yorikane R, Sakai S, Sakurai T, Okada M, Nishikibe M, Yano M, Yamaguchi I, Sugishita Y, Goto K. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary hypertension. Circ Res 1993;73:887–897 [DOI] [PubMed] [Google Scholar]
- 30.Carpenter TC, Schomberg S, Stenmark KR. Endothelin-mediated increases in lung VEGF content promote vascular leak in young rats exposed to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol 2005;289:L1075–L1082 [DOI] [PubMed] [Google Scholar]
- 31.Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the pERK–eIF2{alpha}–ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem 2011;286:4809–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lugea A, Tischler D, Nguyen J, Gong J, Gukovsky I, French SW, Gorelick FS, Pandol SJ. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 2011;140:987–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seimon TA, Kim MJ, Blumenthal A, Koo J, Ehrt S, Wainwright H, Bekker LG, Kaplan G, Nathan C, Tabas I, et al. Induction of ER stress in macrophages of tuberculosis granulomas. PLoS ONE 2010;5:e12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen QW, Edvinsson L, Xu CB. Role of ERK/MAPK in endothelin receptor signaling in human aortic smooth muscle cells. BMC Cell Biol 2009;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001;107:881–891 [DOI] [PubMed] [Google Scholar]
- 36.Gora S, Maouche S, Atout R, Wanherdrick K, Lambeau G, Cambien F, Ninio E, Karabina SA. Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB J 2010;24:3284–3297 [DOI] [PubMed] [Google Scholar]
- 37.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 2005;307:935–939 [DOI] [PubMed] [Google Scholar]
- 38.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 2007;27:901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauer ME, Mukhopadhyay D, Fulop C, de la Motte CA, Majors AK, Hascall VC. Primary murine airway smooth muscle cells exposed to poly(I,C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix. J Biol Chem 2009;284:5299–5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamanaka R, Otsuka F, Nakamura K, Yamashita M, Otani H, Takeda M, Matsumoto Y, Kusano KF, Ito H, Makino H. Involvement of the bone morphogenetic protein system in endothelin- and aldosterone-induced cell proliferation of pulmonary arterial smooth muscle cells isolated from human patients with pulmonary arterial hypertension. Hypertens Res 2010;33:435–445 [DOI] [PubMed] [Google Scholar]
- 41.Ivey ME, Osman N, Little PJ. Endothelin-1 signalling in vascular smooth muscle: pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis 2008;199:237–247 [DOI] [PubMed] [Google Scholar]
- 42.Rilla K, Siiskonen H, Spicer AP, Hyttinen JM, Tammi MI, Tammi RH. Plasma membrane residence of hyaluronan synthase is coupled to its enzymatic activity. J Biol Chem 2005;280:31890–31897 [DOI] [PubMed] [Google Scholar]
- 43.Fischer JW, Schrör K. Regulation of hyaluronan synthesis by vasodilatory prostaglandins: implications for atherosclerosis. Thromb Haemost 2007;98:287–295 [PubMed] [Google Scholar]
- 44.Vigetti D, Genasetti A, Karousou E, Viola M, Moretto P, Clerici M, Deleonibus S, De Luca G, Hascall VC, Passi A. Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor–kappaB (NF-kappaB) pathway. J Biol Chem 2010;285:24639–24645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de la Motte C, Nigro J, Vasanji A, Rho H, Kessler S, Bandyopadhyay S, Danese S, Fiocchi C, Stern R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am J Pathol 2009;174:2254–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.