Abstract
Ets-2 is a ubiquitous transcription factor activated after phosphorylation at threonine-72. Previous studies highlighted the importance of phosphorylated ets-2 in lung inflammation and extracellular matrix remodeling, two pathways involved in pulmonary fibrosis. We hypothesized that phosphorylated ets-2 played an important role in pulmonary fibrosis, and we sought to determine the role of ets-2 in its pathogenesis. We challenged ets-2 (A72/A72) transgenic mice (harboring a mutated form of ets-2 at phosphorylation site threonine-72) and ets-2 (wild-type/wild-type [WT/WT]) control mice with sequential intraperitoneal injections of bleomycin, followed by quantitative measurements of lung fibrosis and inflammation and primary cell in vitro assays. Concentrations of phosphorylated ets-2 were detected via the single and dual immunohistochemical staining of murine lungs and lung sections from patients with idiopathic pulmonary fibrosis. Ets-2 (A72/A72) mice were protected from bleomycin-induced pulmonary fibrosis, compared with ets-2 (WT/WT) mice. This protection was characterized by decreased lung pathological abnormalities and the fibrotic gene expression of Type I collagen, Type III collagen, α–smooth muscle actin, and connective tissue growth factor. Immunohistochemical staining of lung sections from bleomycin-treated ets-2 (WT/WT) mice and from patients with idiopathic pulmonary fibrosis demonstrated increased staining of phosphorylated ets-2 that colocalized with Type I collagen expression and to fibroblastic foci. Lastly, primary lung fibroblasts from ets-2 (A72/A72) mice exhibited decreased expression of Type I collagen in response to stimulation with TGF-β, compared with fibroblasts from ets-2 (WT/WT) mice. These data indicate the importance of phosphorylated ets-2 in the pathogenesis of pulmonary fibrosis through the expression of Type I collagen and (myo)fibroblast activation.
Keywords: ets-2, Type I collagen, pulmonary fibrosis, bleomycin, fibroblast
Clinical Relevance
This study highlights the importance of transcription factor ets-2 in the pathogenesis of pulmonary fibrosis in mice and humans through the regulation of Type I collagen expression. Our data present the opportunity for scientists to target ets-2 pharmaceutically for the treatment of pulmonary fibrosis in humans.
Interstitial lung diseases are a broad set of diseases that perturb lung function by affecting the space between endothelial cells of the vascular bed and alveolar epithelial cells. In normal conditions, this interstitial space consists of a minimal amount of matrix, allowing the efficient transport of oxygen and carbon dioxide. The interruption of this normal lung architecture can alter lung function. One set of lung diseases characterized by interstitial matrix deposition, the destruction of alveolar–capillary units, and functional impairment is termed idiopathic interstitial pneumonias (IIPs).
The most prevalent form of IIP is idiopathic pulmonary fibrosis (IPF). Despite exhaustive research into underlying mechanisms, patients with IPF have a median survival of 3–5 years after diagnosis (1). From 1992–2003, the mortality rates for patients with IPF significantly increased, despite ongoing investigation into the molecular mechanisms of the disease (2). The only consistent treatment option is lung transplantation, although more than 30% of patients die on the waiting list (3). Thus, it is imperative to delineate the underlying disease physiology to identify treatment opportunities and disease biomarkers.
The roles of transcription factors, such as Smad3 (4), Nrf2 (5), CEBPβ (6), GATA-3 (7), PPARs (8, 9), Fra-2 (10), and p53 (11), are implicated in the pathogenesis of pulmonary fibrosis. Furthermore, the E26 transformation-specific sequence (ets) family of transcription factors is linked to extracellular matrix remodeling (12, 13), a process that is conserved in IPF. Our laboratory demonstrated that the hematopoietic growth factor macrophage colony–stimulating factor (M-CSF) is important in the development of pulmonary fibrosis (14), and M-CSF induces the activation of transcription factor ets-2 via phosphorylation at threonine-72 (15, 16). A transgenic mouse containing hypomorphic ets-2 alleles, where the critical threonine-72 residue is mutated to alanine (ets-2 A72/A72), exhibits decreased expression of matrix metalloproteinase (MMP)-3 and MMP-9 in cells stimulated with M-CSF (17). Importantly, when ets-2 (A72/A72) mice are crossed with motheaten-viable mice (hcph [me-v/me-v]), the double mutant mice (ets-2 [A72/A72]/hcph [me-v/me-v]) exhibit significantly less lung inflammation, compared with the single mutant motheaten viable mice (ets-2 [wild-type/wild-type]/hcph [me-v/me-v]) (18). Although ets-2 (A72/A72]/hcph (me-v/me-v) mice exhibit less lung inflammation than motheaten-viable mice (hcph [me-v/me-v]), no determination for the role of phosphorylated ets-2 in the pathogenesis of pulmonary fibrosis has been performed.
In this study, we found that phosphorylated ets-2 (threonine-72) is important in murine pulmonary fibrosis, and is preferentially found in the lungs of humans with IPF. Using sequential intraperitoneal injections of bleomycin in a murine model of pulmonary fibrosis, we discovered that ets-2 (A72/A72) mice were protected from pulmonary fibrosis, compared with ets-2 (wild-type/wild-type [WT/WT]) animals. This protection was characterized by decreased lung pathological changes, the decreased expansion of myofibroblasts, and decreased fibrotic protein and gene expression profiles. In lung sections from ets-2 (WT/WT) mice after treatment with bleomycin and from patients with IPF, phosphorylated ets-2 was increased and colocalized with Type I collagen. These data indicate that phosphorylated ets-2 plays an important role in the pathogenesis of pulmonary fibrosis.
Materials And Methods
Materials
Information on materials and reagents is available in the online supplement.
Mice
Transgenic mice harboring a point mutation in ets-2 (ets-2tmA72Osh, referred to as ets-2 [A72/A72]), resulting in a threonine-to-alanine conversion at position 72, were provided by Michael Ostrowski, Ph.D. (Ohio State University, Columbus, OH). The development of this mouse is described elsewhere (17, 18). WT mice (FVB/N background) were used as controls.
Bleomycin Studies
Male mice, aged 6–12 weeks, underwent intraperitoneal injections as previously described (14). Briefly, mice were injected with 0.035 U bleomycin/g or PBS (vehicle control) on Days 1, 4, 8, 11, 15, 18, 22, and 25. Either 1 week after the final injection (Day 33), or on Days 11 or 22 after the initiation of the bleomycin time-course, the mice were killed. Before their removal, lungs were lavaged with 1.0 ml PBS. Lungs were then removed and inflated at 20-cm pressure. The left lobe of each lung was placed in 10% formalin and prepared for immunohistochemical processing (Histotechniques, Powell, OH), and the right lobes were snap-frozen in liquid nitrogen for RNA, collagen, and protein analyses.
Real-Time PCR, Sircol Assay, and Bronchoalveolar Lavage Analysis
Details on these methods are provided in the online supplement.
Immunohistochemical Staining and Quantification
A specific antibody to phosphorylated ets-2 was provided by Michael Ostrowski, Ph.D. Hematoxylin-and-eosin (H&E), trichrome, α–smooth muscle actin (α-SMA), and Type I collagen staining of murine lung sections was performed by the Pathology Core Facility at the Ohio State University College of Medicine (Columbus, OH). The expression of α-SMA and Type I collagen in the lungs was quantified using a histogram analysis in Adobe Photoshop CS2 (Adobe Systems, Inc., San Jose, CA), as previously detailed (19). The two stains were analyzed on separate slides for the same lung. The resultant values represent the percentages of SMA+ or collagen Type I+ cells per high-power field from at least 10 digital images per lung per mouse. Human IPF lung sections were analyzed by Drs. Charles Hitchcock and Gerard Nuovo (board-certified pathologists at the Ohio State University Medical Center, Columbus, OH). Co-staining was performed by Dr. Gerard Nuovo, using an automated immunohistochemistry benchmark according to the manufacturer's recommendations (Ventana Medical Systems, Tucson, AZ). Fluorescent images were derived using the Nuance Imaging System (CRi, Woburn, MA). Lung sections with no primary antibody and the addition of chromogen only were used as control samples.
Statistical Analysis
A Student t test was used for single comparisons, with P ≤ 0.05 considered significant. ANOVA was used for multiple comparisons. ANOVA requires that the cell counts be normally distributed, with stable variance across groups. Thus, cell counts were log-transformed to meet these assumptions. If the overall ANOVA F-test was significant, indicating that differences existed in the data, individual groups were tested to determine if these differences were statistically significant. P values were adjusted using the Holm's procedure to control Type I error at 5% (20).
Results
Ets-2 (A72/A72) Mice Are Protected from Bleomycin-Induced Pulmonary Fibrosis
The importance of the ets family of transcription factors is apparent in extracellular matrix remodeling (12, 13) and lung inflammation (18). Therefore, we examined the role of ets-2 by using a transgenic mouse that expresses a mutated form of ets-2 at threonine-72 (ets-2 [A72/A72]) in a model of bleomycin-induced pulmonary fibrosis.
As shown in Figure 1A, pathological analyses of the lungs revealed that ets-2 (A72/A72) mice demonstrated reduced lung injury and subpleural deposition of collagen after the administration of bleomycin, compared with ets-2 (WT/WT) mice at day 33, as evidenced by H&E staining (Figure 1A, top) and Masson's trichrome staining (Figure 1A, bottom). Although small, patchy areas of lung inflammation and fibrosis were evident in the ets-2 (A72/A72) mice, this remodeling appeared less extensive than in ets-2 (WT/WT) mice. We performed inflammatory cell differentials on bronchoalveolar lavage (BAL) fluid from ets-2 (WT/WT) and ets-2 (A72/A72) mice after the administration of bleomycin. As shown in Table E1 in the online supplement, no significant differences in total cells, alveolar macrophages, neutrophils, or lymphocytes were evident between the ets-2 (WT/WT) and ets-2 (A72/A72) mice. The quantitation of lung collagen content, using the Sircol collagen assay (Biocolor Ltd., Carrickfergus, UK), revealed that ets-2 (A72/A72) mice exhibited significantly less total lung collagen compared with ets-2 (WT/WT) mice after treatment with bleomycin on Day 33 (Figure 1B). As expected, a significant increase in total lung collagen occurred between PBS-treated ets-2 (WT/WT) mice and bleomycin-treated ets-2 (WT/WT) mice. We conclude that ets-2 (A72/A72) mice were protected from bleomycin-induced pulmonary fibrosis.
Figure 1.
Ets-2 (A72/A72) mice are protected from bleomycin-induced pulmonary fibrosis. Ets-2 (wild-type/wild-type [WT/WT]) and ets-2 (A72/A72) mice were treated with bleomycin (0.035 U/g) or PBS (vehicle control) for 33 days, as described in Materials and Methods. (A) After the mice had been killed, the lungs from bleomycin-treated mice were formalin-fixed, sectioned, and stained with hematoxylin and eosin (H&E) (top) or Masson's trichrome (bottom). Data represent the mean ± SEM of at least three mice per condition. (B) Murine lungs were used to quantitate collagen content, using the Sircol collagen assay. Data represent the mean ± SEM of at least three mice per condition. (C) After the mice were killed, their bronchoalveolar lavage fluid was analyzed for active TGF-β via ELISA. Data represent the mean ± SEM; n = 6 for ets-2 (WT/WT) + bleomycin; n = 8 for ets-2 (A72/A72) + bleomycin. A post hoc power analysis on TGF-β indicated that the power to detect this difference was 23%. (D–G) RNA from murine lungs was isolated, and mRNA was amplified and used for real-time PCR for Type I collagen (D), Type III collagen (E), connective tissue growth factor (CTGF) (F), and α–smooth muscle actin (α-SMA) (G). Data represent the mean ± SEM. n = 2 for ets-2 (WT/WT) + PBS; n = 5 for ets-2 (A72/A72) + PBS; n = 5 for ets-2 (WT/WT) + bleomycin; n = 7 for ets-2 (A72/A72) + bleomycin.
One of the primary growth factors involved in the pulmonary fibrotic response is active TGF-β. Therefore, we determined the amount of active TGF-β present in BAL fluid via ELISA. Interestingly, no significant difference was evident in the concentrations of active TGF-β (Figure 1C) or total TGF-β (Figure E1) between the ets-2 (WT/WT) and ets-2 (A72/A72) mice after treatment with bleomycin on Day 33. However, the expression of other prominent fibrotic genes, including Type I collagen, Type III collagen, connective tissue growth factor (CTGF), and α-SMA, were decreased in ets-2 (A72/A72) mice after treatment with bleomycin on Day 33 (Figures 1D–1G).
Similar to active TGF-β, myofibroblasts are important mediators of fibrosis in response to bleomycin (21–23) and in patients with IPF (24). These cells are characterized by the expression of various factors, including α-SMA. As shown in Figure 2, lungs from bleomycin-treated ets-2 (A72/A72) mice contained significantly fewer α-SMA–positive cells and Type I collagen–positive cells, compared with lungs from ets-2 (WT/WT) mice on Day 33 after the initiation of bleomycin (Figures 2A and 2C, respectively). The quantification of these data is shown in Figures 2B and 2D, respectively. These data are consistent with the differences in the expression of α-SMA and Type I collagen mRNA shown earlier (Figures 1F and 1G, respectively). Therefore, we conclude that although levels of active TGF-β did not significantly differ in the lungs of ets-2 (WT/WT) and ets-2 (A72/A72) mice after treatment with bleomycin on Day 33, the gene expression of Type I collagen, Type III collagen, CTGF, and α-SMA and numbers of myofibroblasts in the lungs were reduced in ets-2 (A72/A72) mice after bleomycin challenge.
Figure 2.
Ets-2 (A72/A72) mice exhibit decreased expression of Type I collagen and α-SMA in the lungs after treatment with bleomycin, compared with ets-2 (WT/WT) mice, indicative of decreased myofibroblasts. After treatment with bleomycin for 33 days, lungs from bleomycin-treated mice underwent immunohistochemical staining for α-SMA (A) or Type I collagen (C). The quantification of these results is shown in B and D, respectively. Data represent the mean ± SEM of at least three mice per condition.
Ets-2 (A72/A72) Mice Exhibit an Altered Time-Course Response to Bleomycin
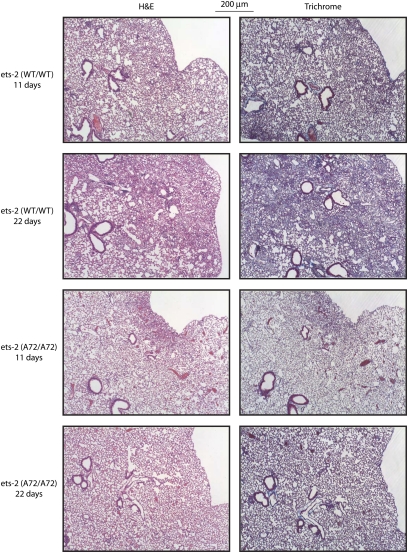
To understand the temporal events underlying the protection found in ets-2 (A72/A72) mice in response to bleomycin, we performed a bleomycin time-course experiment. In addition to the 33-day analyses described in Figures 1 and 2, we also evaluated lung tissue at 11 days (injury) and 22 days (inflammation and fibrosis) after the initiation of bleomycin. Whereas pathological analyses revealed injury in ets-2 (WT/WT) and ets-2 (A72/A72) mice on Day 11 (Figure 3), less anatomic lung distortion was evident in ets-2 (A72/A72) mice on Day 22, compared with ets-2 (WT/WT) mice. The quantitation of total lung collagen, the mRNA expression of Type I collagen, CTGF, and α-SMA, and ELISA measurements of active TGF-β revealed no significant differences between ets-2 (WT/WT) and ets-2 (A72/A72) mice on Days 11 or 22 (data not shown). We conclude that the pathological protection observed in ets-2 (A72/A72) mice after treatment with bleomycin occurred at all time-points measured, whereas changes in gene expression were primarily limited to the repair/remodeling phase of pulmonary fibrosis (22- to 33-d time period), and involved alterations in (myo)fibroblast trafficking and the production of collagen.
Figure 3.
Ets-2 (A72/A72) mice exhibit an altered time-course response to bleomycin. Ets-2 (WT/WT) and ets-2 (A72/A72) mice were treated with bleomycin (0.035 U/g) for 11 days or 22 days. After the mice were killed, the lungs from bleomycin-treated mice were formalin-fixed, sectioned, and stained with H&E (left) or Masson's trichrome (right).
Lung Sections from Patients with IPF and Mice Treated with Bleomycin Exhibit Positive Staining for Phosphorylated ets-2 that Colocalizes with Type I Collagen
The ets family of transcription factors is linked to extracellular matrix remodeling (12), a conserved process in the pathogenesis of pulmonary fibrosis. Trimboli and colleagues discerned the importance of phosphorylated ets-2 in extracellular matrix remodeling in mammary tumors (13). The transcription factor ets-2 becomes phosphorylated at threonine-72, accumulates in the nucleus, and mediates downstream signaling events and gene transcription (15, 16, 25). Because of the protection from bleomycin-induced pulmonary fibrosis found in ets-2 (A72/A72) mice, we hypothesized that concentrations of phosphorylated ets-2 (threonine-72) are increased in the lungs of patients with IPF.
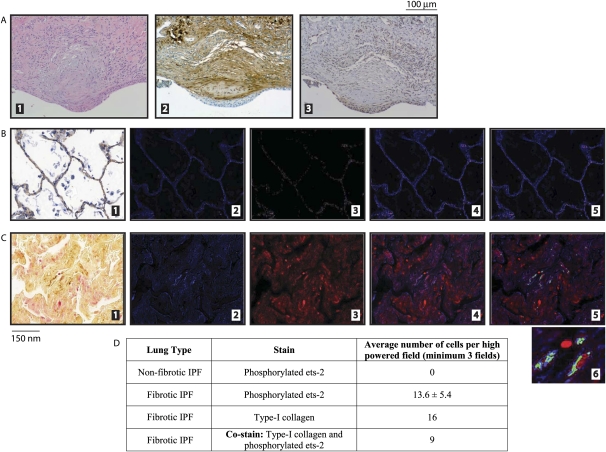
We obtained lung-tissue blocks from the tissue collection repository at the Department of Pathology at Ohio State University Medical Center, and used an antibody that specifically targets phosphorylated ets-2 at threonine-72 via immunohistochemistry (13). H&E staining of serial lung sections from patients with IPF contained fibroblastic foci, the pathological hallmark of IPF (Figure 4A, part 1). Cells within the fibroblastic foci also stained positive for Type I collagen (Figure 4A, part 2) and phosphorylated ets-2 (Figure 4A, part 3). These data indicate that lung tissue from patients with IPF stain for phosphorylated ets-2.
Figure 4.
Lung sections from patients with idiopathic pulmonary fibrosis (IPF) exhibit increased concentrations of phosphorylated ets-2 that colocalizes with Type I collagen. (A) Lung sections from patients with IPF were obtained from the storage bank at the Department of Pathology of Ohio State University Medical Center. Patients were selected according to their clinical diagnosis of interstitial lung disease and IPF. Embedded slides were first stained with H&E (part 1) to verify the pathological appearance of fibroblastic foci, indicative of usual interstitial pneumonia. Serial sections were then stained with Type I collagen (part 2) and phosphorylated ets-2 (part 3). (B and C) Lung tissues were obtained and classified as described in Materials and Methods. Nonfibrotic areas of IPF lungs (B) and fibrotic areas of IPF lungs (C) were stained for Type I collagen (brown signal) and phosphorylated ets-2 (red signal) (part 1). The Nuance imaging system then converted the Type I collagen signal to blue (part 2) and the phosphorylated ets-2 signal to red (part 3). The merging of parts 2 and 3 is shown in part 4. The Nuance imaging system then overlaid the images and converted those cells that stained positive for both Type I collagen and phosphorylated ets-2 to green (part 5). A magnification of the colocalization of Type I collagen and phosphorylated ets-2 in part 5 is shown in part 6. (D) Quantitation of images from B and C.
Ets-2 (A72/A72) mice expressed significantly less Type I collagen mRNA on Days 22 and 33, compared with ets-2 (WT/WT) mice, suggesting that the phosphorylation of ets-2 at threonine-72 affected fibrotic gene expression. Therefore, we hypothesized that the expression of Type I collagen would colocalize with phosphorylated ets-2 at threonine-72 in patients with IPF.
We used nonfibrotic (Figure 4B) and fibrotic (Figure 4C) regions from lung sections of patients with IPF. No phosphorylated ets-2 staining (red signal) or Type I collagen (brown signal) was evident in nonfibrotic lung tissue adjacent to areas of fibrosis in IPF lung sections (Figure 4B, part 1), compared with fibrotic regions (Figure 4C, part 1). Subsequently, these lung sections from patients with IPF were photographed using the Nuance System, and the colors for phosphorylated ets-2 (red) and Type I collagen (brown) were separated and analyzed via fluorescence. Figures 4B and 4C (parts 2) represent the fluorescent image of Type I collagen (blue), whereas Figures 4B and 4C (parts 3) represent phosphorylated ets-2 (red). These images were then merged, as shown in Figures 4B and 4C (parts 4), and then overlaid to allow the Nuance system to capture those cells that stained positive for both Type I collagen and phosphorylated ets-2. Cells that stained positive for both are shown in green (Figures 4B and 4C, parts 5). The data from these images are quantitated in Figure 4D. The fibrotic regions of lung sections from patients with IPF contain cells that stained positive for both Type I collagen and phosphorylated ets-2, whereas nonfibrotic regions of lung sections from patients with IPF did not, indicating that phosphorylated ets-2 may regulate the expression of Type I collagen in the lung.
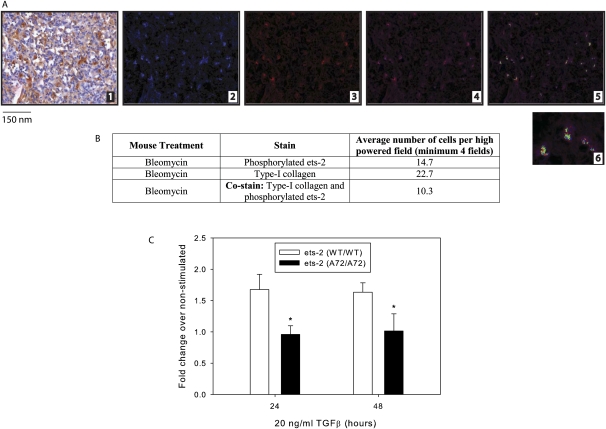
To corroborate these findings in our murine model of pulmonary fibrosis, we also stained lung sections from ets-2 (WT/WT) mice after treatment with bleomycin for phosphorylated ets-2 and Type I collagen. Type I collagen (Figure 5A, part 2) and phosphorylated ets-2 (Figure 5A, part 3) immunohistochemical staining were visible in fibrotic regions after treatment with bleomycin on Day 33. Merging these images demonstrated the co-staining of phosphorylated ets-2 and Type I collagen, as evidenced by a green signal (Figure 5A, part 5). The data from these images are quantitated in Figure 5B. We also analyzed lungs from Days 11 and 22, but observed minimal to absent staining for phosphorylated ets-2 (Figure E2). In summary, phosphorylated ets-2 colocalized with Type I collagen in lung sections from patients with IPF and in lung sections from ets-2 (WT/WT) mice treated with bleomycin.
Figure 5.
Lung sections from ets-2 (WT/WT) mice also exhibit co-staining of phosphorylated ets-2 and Type I collagen, and primary fibroblasts from ets-2 (A72/A72) mice express less Type I collagen in response to stimulation with TGF-β. (A) After treatment with bleomycin for 33 days, murine lung sections from ets-2 (WT/WT) mice were stained for Type I collagen (brown signal) and phosphorylated ets-2 (red signal) (part 1). The Nuance imaging system then converted the Type I collagen signal to blue (part 2) and the phosphorylated ets-2 signal to red (part 3). The merging of parts 2 and 3 is shown in part 4. The Nuance imaging system then overlaid the images and converted those cells that stained positive for both Type I collagen and phosphorylated ets-2 to green (part 5). The magnification of colocalization of Type I collagen and phosphorylated ets-2 in part 5 is shown in part 6. (B) Quantitation of images from A. (C) Lung fibroblasts were isolated as described in Materials and Methods. Cells were serum-starved in DMEM and then stimulated with or without 20 ng/ml of TGF-β for the indicated time points. RNA was isolated, and mRNA was amplified and used for real-time PCR for Type I collagen. Data represent the mean ± SEM of cells from four mice. *P = 0.05, versus ets-2 (WT/WT).
Primary Lung Fibroblasts from ets-2 (A72/A72) Mice Express Less Type I Collagen in Response to Stimulation with TGF-β
TGF-β is one of the primary growth factors involved in the development of pulmonary fibrosis. TGF-β is highly expressed during the pathogenesis of the disease (26), and it helps direct collagen synthesis, the proliferation of fibroblasts, and myofibroblast differentiation (24). In Figure 1C, we detected no significant difference in concentrations of TGF-β in the lungs of ets-2 (WT/WT) and ets-2 (A72/A72) mice after treatment with bleomycin for 33 days. However, we observed a significantly decreased expression of Type I collagen in the lungs of ets-2 (A72/A72) mice (Figure 1D), and a colocalization of Type I collagen and expression of phosphorylated ets-2 in lung sections from patients with IPF (Figure 4C) and from mice treated with bleomycin (Figure 5A). Therefore, to establish a causal relationship between ets-2 and Type I collagen, we performed in vitro experiments, using primary lung fibroblasts from ets-2 (WT/WT) and ets-2 (A72/A72) mice. After simulation with TGF-β, the mRNA expression of Type I collagen was assessed by real-time PCR. As shown in Figure 5C, primary lung fibroblasts from ets-2 (A72/A72) mice expressed significantly less Type I collagen compared with primary lung fibroblasts from ets-2 (WT/WT) mice. As a control, we also analyzed the expression of α-SMA, and observed no significant difference between ets-2 (WT/WT) and ets-2 (A72/A72) cells (data not shown). In summary, the phosphorylation of ets-2 at threonine-72 is important in the pulmonary fibrotic response through the involvement of Type I collagen expression.
Discussion
This study supports a novel and important role for the phosphorylation of threonine-72 of ets-2 in human and murine pulmonary fibrosis. These studies were performed in response to observations by Trimboli and colleagues (13) and Wei and colleagues (18), who found that mutating the threonine-72 to alanine-72 of ets-2 in mice altered lung inflammation and extracellular matrix remodeling, two important mechanisms in the pathogenesis of pulmonary fibrosis.
The consensus core binding sequence for ets-2 is 5′-GGAA/T-3′ (27). Upon binding this core sequence, ets-2 supports a diversity of cellular functions, such as cell proliferation, adhesion, migration, survival, and angiogenic processes (27). As such, ets-2 plays an important role in several human diseases, including mammary tumors (13), Down syndrome (28), and leukemia (29). Although ets-2 is central in a host of processes, pinpointing the sole genetic target responsible for disease manifestations as a result of dysregulated ets-2 function is difficult. For example, in the original study of lung inflammation using ets-2 (A72/A72) mice, Wei and colleagues found that 10 different genes were down-regulated in the lungs of ets-2 (A72/A72) mice compared with ets-2 (WT/WT) mice, but they did not identify a single dominant ets-2–responsive gene responsible for the decreased inflammation (18). The report by Wei and colleagues indicates that ets-2 contributes to decreased lung inflammation by affecting genes with ets-2 promoter sites, supporting the notion that ets-2 is a multitarget transcription factor involved in numerous biological processes.
The promoter-binding capabilities and functions of the mutant form of ets-2 (ets-2 [A72]) are noteworthy. Previous studies demonstrated that the ets-2 (A72) protein translocates to the nucleus and binds a rat sarcoma (Ras)-responsive enhancer without activating the gene (16). As such, the mutant form of ets-2 may serve as a co-repressor. For example, the BS69 protein functions as a transcriptional co-repressor when associated with E1A and ets-2 (30, 31). In the absence of ets-2, BS69 may not function as a co-repressor of fibrotic gene expression. Interestingly, the association of BS69 and ets-2 is decreased when ets-2 is phosphorylated at the pointed domain, which contains the threonine-72 residue (31). Although several other co-repressors are thought to function coordinately with ets-2, including brahma-related gene 1 and the switch/sucrose nonfermentable (SWI/SNF) complexes (32), the mechanisms by which altered genetic co-repression contributes to the pathogenesis of pulmonary fibrosis require further study.
Three additional findings in this study concern the involvement of ets-2 in the expression of C-C chemokine ligand 12 (CCL12) and differences in lung inflammatory cell differentials and primary cell proliferative capacities between ets-2 (WT/WT) and ets-2 (A72/A72) mice. First, using bone marrow–derived macrophages stimulated with M-CSF, we observed that macrophages from ets-2 (A72/A72) mice expressed significantly less CCL12, as detected via ELISA, than macrophages from ets-2 (WT/WT) mice (Figure E3A). Furthermore, we observed that ets-2 (A72/A72) mice had significantly less CCL12 in the lungs after treatment with bleomycin, as detected via ELISA and real-time PCR (Figures E3B and E3C).
Second, as shown in Table E1, BAL fluid cell differentials revealed that ets-2 (A72/A72) mice manifested significantly fewer total cells and alveolar macrophages 11 days after the initiation of bleomycin, compared with ets-2 (WT/WT) mice. Interestingly, this trend reversed at 22 days after the initiation of bleomycin, insofar as ets-2 (A72/A72) mice exhibited a significantly increased number of total cells and alveolar macrophages compared with ets-2 (WT/WT) mice. By 33 days after the initiation of bleomycin, no significant difference in total cells or alveolar macrophages was evident between ets-2 (WT/WT) and ets-2 (A72/A72) mice after treatment with bleomycin.
Third, fibroblasts isolated from human fibrotic lungs exhibit variability in proliferative and apoptotic capacities that may contribute to the pathogenesis of the disease (33–35). Interestingly, primary lung fibroblasts (Figure E4) isolated from ets-2 (A72/A72) mice showed an increased proliferative capacity when compared with those cells isolated from ets-2 (WT/WT) mice. Although more studies are needed, our findings that ets-2 is a potential regulator of CCL12 expression, influences inflammatory cell profiles in the lungs in response to bleomycin, and exerts an effect on cell-proliferative capacities provide valuable insights into the importance of ets-2 in pulmonary fibrosis.
Recent studies highlighted the important role of transcription factors in the pathogenesis of pulmonary fibrosis by regulating fibrotic gene expression. For example, Lepparanta and colleagues demonstrated that the expression of GATA-6 in fibroblasts mediates the α-SMA–inducing signal of TGF-β, and that GATA-6 is overexpressed in the fibroblastic foci of lung sections from patients with IPF (36). Yasuoka and colleagues ascertained that the expression of early growth response 1 is up-regulated in lung sections from patients with IPF, and directs the expression of fibronectin in response to the stimulation of fibroblasts by (insulin-like growth factor binding protein 5) (37). Ponticos and colleagues highlighted the relevance of the mitogen-activated protein kinase (MAPK) pathway in the transcriptional activation of Type I collagen (38), to which ets-2 is implicitly related. Furthermore, Boon and colleagues demonstrated that genes in the extracellular signal-related kinase/MAPK pathway are up-regulated in patients with IPF, and these genes include a member of the SWI/SNF family and Ras (39).
Ets-2 can be activated by multiple signaling pathways, including Ras–v-raf-1 murine leukemia viral oncogene homolog (Raf)–MAPK (16), PI3-kinase/mammalian target of rapamycin (40), and v-akt murine thymoma viral oncogene homolog/c-Jun N-terminal kinase (41). Raf is also activated via Ras-independent mechanisms (42), thereby creating a complex network of intracellular signaling mechanisms that facilitate the activation of ets-2. In the setting of IPF, broad pathway inhibitors have been used, such as cytotoxic agents and immune suppressants, but none were effective in reversing the course of the disease or slowing its progression. This raises the possibility of novel and specific therapeutic approaches for IPF, centering on the promoter-binding inhibitors of ets-2–mediated transcription or the direct targeting of phosphorylated ets-2. This more directed, specific therapeutic approach was successfully applied in other diseases, including breast cancer (tamoxifen) and rheumatoid arthritis (etanercept), and should be considered in the treatment of IPF. Ets-2 antagonists or inhibitors of phosphorylated ets-2 are presently unavailable; we are actively pursuing their development and use.
Supplementary Material
Acknowledgments
The authors thank Dr. Tim Eubank for generous help in quantifying the α-SMA and Type I collagen immunohistochemistry slides. The authors acknowledge the assistance of Dr. Carlo Croce and his use of the Nuance Microscopy System. The authors also acknowledge Susie Jones of the Ohio State University Medical Center Pathology Core Facility for assistance with murine immunohistochemistry sections.
Footnotes
This work was supported by National Institutes of Health grants R03 HL095431–01 (C.P.B.) and R01 HL067176 (C.B.M.).
C.P.B. and C.B.M. were responsible for the conception and design of this study. S.N.F., G.J.N., M.N.K., C.L.H., B.D.B., S.M., C.A.N., C.Z.C.-S., G.S.P., and M.C.O. were responsible for the analysis and interpretation of the data.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0490OC on May 11, 2011
Author Disclosure: C.L.H. served as expert witness for Freund, Freeze & Arnold, Bieser, Greer & Landis, Motely Rice LLC, Remlnger Co. PLA, and Bonezzi Switzer, Murphey Polito & Hupp; lectured at the University of Kansas for the Pathology Interest Group; and holds stock in Enlyton LLC, the Neoprobe Corp., and the Intact Medical Corp. None of the other authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement: American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664 [DOI] [PubMed] [Google Scholar]
- 2.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007;176:277–284 [DOI] [PubMed] [Google Scholar]
- 3.Nathan SD. Lung transplantation: disease-specific considerations for referral. Chest 2005;127:1006–1016 [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2002;282:L585–L593 [DOI] [PubMed] [Google Scholar]
- 5.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J 2004;18:1258–1260 [DOI] [PubMed] [Google Scholar]
- 6.Hu B, Ullenbruch MR, Jin H, Gharaee-Kermani M, Phan SH. An essential role for CCAAT/enhancer binding protein beta in bleomycin-induced pulmonary fibrosis. J Pathol 2007;211:455–462 [DOI] [PubMed] [Google Scholar]
- 7.Kimura T, Ishii Y, Yoh K, Morishima Y, Iizuka T, Kiwamoto T, Matsuno Y, Homma S, Nomura A, Sakamoto T, et al. Overexpression of the transcription factor GATA-3 enhances the development of pulmonary fibrosis. Am J Pathol 2006;169:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese T, Mazzon E, Di Paola R, Muia C, Crisafulli C, Caputi AP, Cuzzocrea S. Role of endogenous and exogenous ligands for the peroxisome proliferator–activated receptor alpha in the development of bleomycin-induced lung injury. Shock 2005;24:547–555 [DOI] [PubMed] [Google Scholar]
- 9.Lakatos HF, Thatcher TH, Kottmann RM, Garcia TM, Phipps RP, Sime PJ. The role of PPARs in lung fibrosis. PPAR Res 2007;2007:71323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eferl R, Hasselblatt P, Rath M, Popper H, Zenz R, Komnenovic V, Idarraga MH, Kenner L, Wagner EF. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc Natl Acad Sci USA 2008;105:10525–10530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis DW, Weidner DA, Holian A, McConkey DJ. Nitric oxide–dependent activation of p53 suppresses bleomycin-induced apoptosis in the lung. J Exp Med 2000;192:857–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trojanowska M. Ets factors and regulation of the extracellular matrix. Oncogene 2000;19:6464–6471 [DOI] [PubMed] [Google Scholar]
- 13.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, et al. PTEN in stromal fibroblasts suppresses mammary epithelial tumours. Nature 2009;461:1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baran CP, Opalek JM, McMaken S, Newland CA, O'Brien JM, Jr, Hunter MG, Bringardner BD, Monick MM, Brigstock DR, Stromberg PC, et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowles LF, Martin ML, Nelsen L, Stacey KJ, Redd D, Clark YM, Nagamine Y, McMahon M, Hume DA, Ostrowski MC. Persistent activation of mitogen-activated protein kinases p42 and p44 and ets-2 phosphorylation in response to colony-stimulating factor 1/C–FMS signaling. Mol Cell Biol 1998;18:5148–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang BS, Hauser CA, Henkel G, Colman MS, Van Beveren C, Stacey KJ, Hume DA, Maki RA, Ostrowski MC. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol 1996;16:538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Man AK, Young LJ, Tynan JA, Lesperance J, Egeblad M, Werb Z, Hauser CA, Muller WJ, Cardiff RD, Oshima RG. Ets2-dependent stromal regulation of mouse mammary tumors. Mol Cell Biol 2003;23:8614–8625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei G, Guo J, Doseff AI, Kusewitt DF, Man AK, Oshima RG, Ostrowski MC. Activated ets2 is required for persistent inflammatory responses in the motheaten viable model. J Immunol 2004;173:1374–1379 [DOI] [PubMed] [Google Scholar]
- 19.Eubank TD, Roberts RD, Khan M, Curry JM, Nuovo GJ, Kuppusamy P, Marsh CB. Granulocyte macrophage colony–stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res 2009;69:2133–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 1997;16:2529–2542 [DOI] [PubMed] [Google Scholar]
- 21.Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast alpha–smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol 1996;148:527–537 [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363 [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis: a combined immunohistochemical and in situ hybridization study. Am J Pathol 1994;145:114–125 [PMC free article] [PubMed] [Google Scholar]
- 24.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 2007;132:1311–1321 [DOI] [PubMed] [Google Scholar]
- 25.Yordy JS, Muise-Helmericks RC. Signal transduction and the ets family of transcription factors. Oncogene 2000;19:6503–6513 [DOI] [PubMed] [Google Scholar]
- 26.Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ. Transforming growth factors–beta 1, –beta 2, and –beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol 1997;150:981–991 [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem 2004;91:896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papas TS, Watson DK, Sacchi N, Fujiwara S, Seth AK, Fisher RJ, Bhat NK, Mavrothalassitis G, Koizumi S, Jorcyk CL. Ets family of genes in leukemia and Down syndrome. Am J Med Genet Suppl 1990;7:251–261 [DOI] [PubMed] [Google Scholar]
- 29.Sacchi N, Watson DK, Guerts van Kessel AH, Hagemeijer A, Kersey J, Drabkin HD, Patterson D, Papas TS. Hu–ets-1 and Hu–ets-2 genes are transposed in acute leukemias with (4;11) and (8;21) translocations. Science 1986;231:379–382 [DOI] [PubMed] [Google Scholar]
- 30.Hateboer G, Gennissen A, Ramos YF, Kerkhoven RM, Sonntag-Buck V, Stunnenberg HG, Bernards R. BS69, a novel adenovirus E1A-associated protein that inhibits E1A transactivation. EMBO J 1995;14:3159–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei G, Schaffner AE, Baker KM, Mansky KC, Ostrowski MC. Ets-2 interacts with co-repressor BS69 to repress target gene expression. Anticancer Res 2003;23:2173–2178 [PubMed] [Google Scholar]
- 32.Baker KM, Wei G, Schaffner AE, Ostrowski MC. Ets-2 and components of mammalian SWI/SNF form a repressor complex that negatively regulates the BRCA1 promoter. J Biol Chem 2003;278:17876–17884 [DOI] [PubMed] [Google Scholar]
- 33.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 2001;24:591–598 [DOI] [PubMed] [Google Scholar]
- 34.Horowitz JC, Thannickal VJ. Epithelial–mesenchymal interactions in pulmonary fibrosis. Semin Respir Crit Care Med 2006;27:600–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lepparanta O, Pulkkinen V, Koli K, Vahatalo R, Salmenkivi K, Kinnula VL, Heikinheimo M, Myllarniemi M. Transcription factor GATA-6 is expressed in quiescent myofibroblasts in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2010;42:626–632 [DOI] [PubMed] [Google Scholar]
- 37.Yasuoka H, Hsu E, Ruiz XD, Steinman RA, Choi AM, Feghali-Bostwick CA. The fibrotic phenotype induced by IGFBP-5 is regulated by MAPK activation and Egr-1–dependent and –independent mechanisms. Am J Pathol 2009;175:605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, Hoyles RK, Bou-Gharios G, Black CM, Denton CP, et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of Type I collagen. Arthritis Rheum 2009;60:2142–2155 [DOI] [PubMed] [Google Scholar]
- 39.Boon K, Bailey NW, Yang J, Steel MP, Groshong S, Kervitsky D, Brown KK, Schwarz MI, Schwartz DA. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF). PLoS ONE 2009;4:e5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res 2007;67:1988–1996 [DOI] [PubMed] [Google Scholar]
- 41.Smith JL, Schaffner AE, Hofmeister JK, Hartman M, Wei G, Forsthoefel D, Hume DA, Ostrowski MC. Ets-2 is a target for an Akt (protein kinase B)/Jun N-terminal kinase signaling pathway in macrophages of motheaten-viable mutant mice. Mol Cell Biol 2000;20:8026–8034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liebmann C. Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell Signal 2001;13:777–785 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.