Abstract
We have recently reported the sequence of a chicken Ig lambda light chain cDNA clone, isolated from a spleen partial cDNA library (1). In this paper, we describe the characterization of a cDNA clone coding for the chicken constant (C) region of the secreted mu chain. This is the first report on the nucleotide and amino acid sequence of a chicken Ig heavy chain constant region. It contains the 3' untranslated region of the mu mRNA up to the poly(A) tail, and, in comparison with the mouse Cmu sequence, displays the overall domain size and organization of a secreted mu chain, i.e.: a characteristic COOH-terminal region, a Cmu4, a Cmu3, a Cmu2, and part of a Cmu1 domain. The sequence homology between these two species ranges from 45% for the Cmu4 to 18% for the Cmu2. Thus, the Cmu sequence appears much less conserved between chicken and mouse than their respective lambda light chain constant regions (1). These results, together with some distinctive features of the Cmu2 domain, may be of evolutionary relevance and will be further discussed.
Full text
PDF

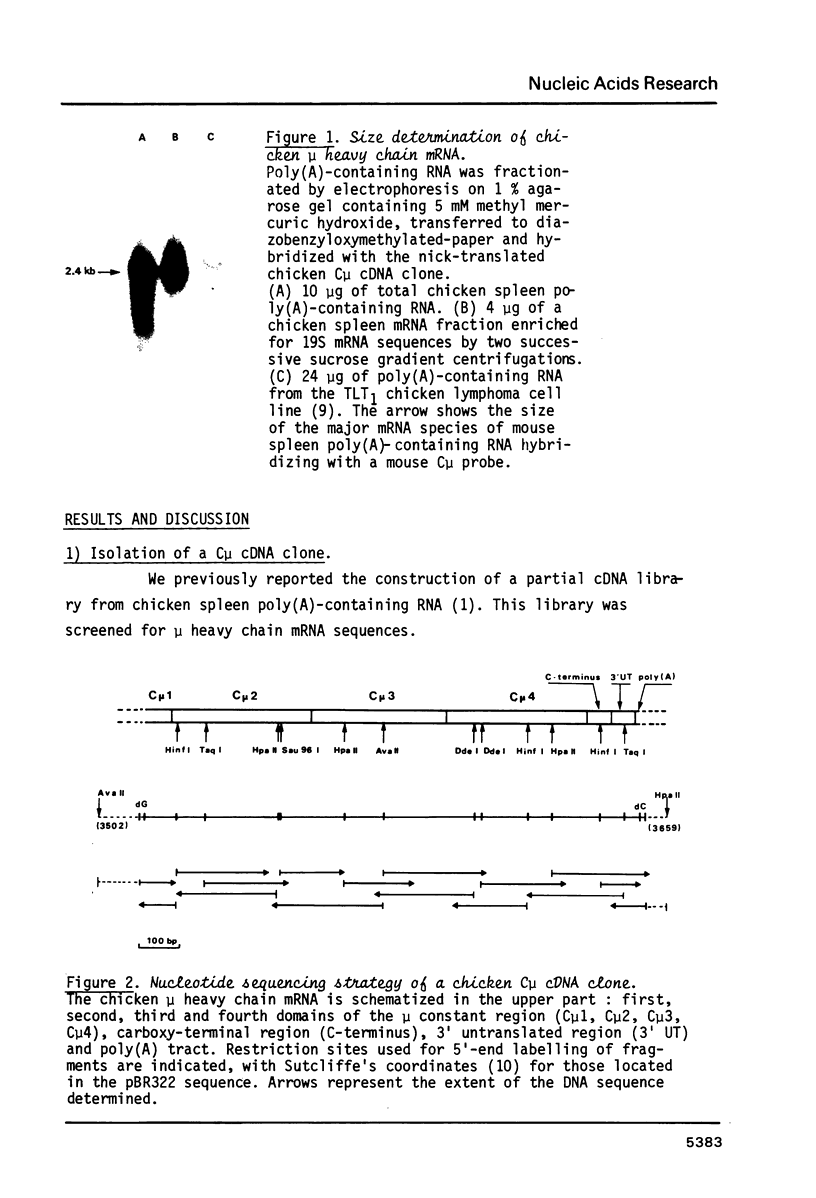



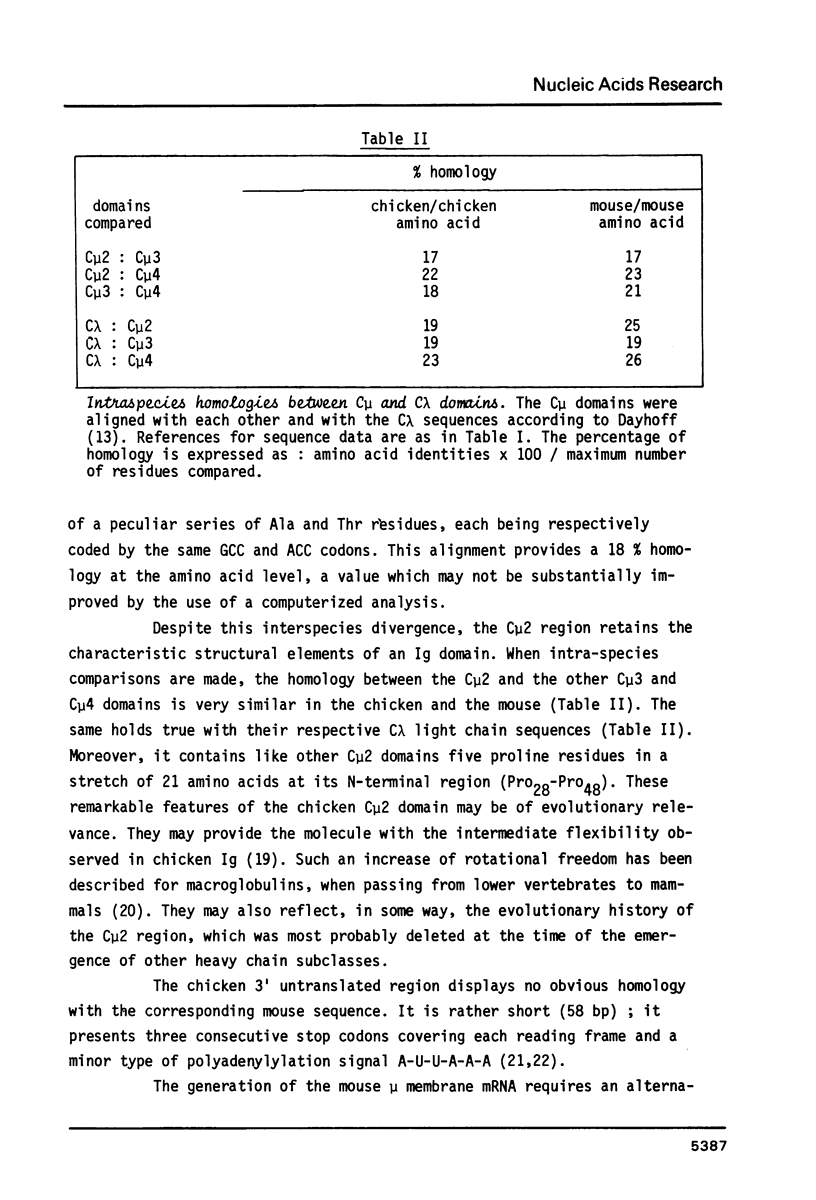


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Byers M. J., Primrose S. B., Lyons A. Rapid purification of plasmid DNAs by hydroxyapatite chromatography. Eur J Biochem. 1978 Nov 2;91(1):303–310. doi: 10.1111/j.1432-1033.1978.tb20966.x. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Munn E. A. Conformation of the free and antigen-bound IgM antibody molecules. Nature. 1969 Dec 27;224(5226):1307–1309. doi: 10.1038/2241307a0. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Vanin E. F., Zrolka A. M., Blattner F. R. Sequence of the gene for the constant region of the mu chain of Balb/c mouse immunoglobulin. Gene. 1981 Oct;15(1):33–42. doi: 10.1016/0378-1119(81)90102-5. [DOI] [PubMed] [Google Scholar]
- Grossi C. E., Lydyard P. M., Cooper M. D. Ontogeny of B cells in the chicken. II. Changing patterns of cytoplasmic IgM expression and of modulation requirements for surface IgM by anti-mu antibodies. J Immunol. 1977 Aug;119(2):749–756. [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hirai K., Calnek B. W. In vitro replication of infectious bursal disease virus in established lymphoid cell lines and chicken B lymphocytes. Infect Immun. 1979 Sep;25(3):964–970. doi: 10.1128/iai.25.3.964-970.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D. A., Cathou R. E. Conformation of immunoglobulin M. 2. Nanosecond fluorescence depolarization analysis of segmental flexibility in anti-epsilon-l-dimethylamino-5-naphthalenesulfonyl-L-lysine anti-immunoglobulin from horse, pig, and shark. Biochemistry. 1976 Jul 27;15(15):3379–3390. doi: 10.1021/bi00660a033. [DOI] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehry M., Sibley C., Fuhrman J., Schilling J., Hood L. E. Amino acid sequence of a mouse immunoglobulin mu chain. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2932–2936. doi: 10.1073/pnas.76.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki R., Kearney J., Paige C., Tonegawa S. Immunoglobulin gene rearrangement in immature B cells. Science. 1980 Sep 19;209(4463):1366–1369. doi: 10.1126/science.6774416. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Milstein C., Clegg J. B., Jarvis J. M. Immunoglobulin lambda-chains. The complete amino acid sequence of a Bence-Jones protein. Biochem J. 1968 Dec;110(4):631–652. doi: 10.1042/bj1100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W., Florent G., Paul C., Shinoda T., Shimizu A. Complete amino acid sequence of the Mu heavy chain of a human IgM immunoglobulin. Science. 1973 Oct 19;182(4109):287–291. doi: 10.1126/science.182.4109.287. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Milstein C. P. Human immunoglobulin heavy chain genes: evolutionary comparisons of C mu, C delta and C gamma genes and associated switch sequences. Nucleic Acids Res. 1981 Sep 25;9(18):4509–4524. doi: 10.1093/nar/9.18.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman R. L., Capra J. D. Amino acid sequence of the Fc region of a canine immunoglobulin M: interspecies homology for the IgM class. Science. 1978 Jun 9;200(4346):1159–1161. doi: 10.1126/science.653360. [DOI] [PubMed] [Google Scholar]
- Zagyansky Y. A., Ivannikova E. I. The general structure of shark (Squalis acantias) and hen (Galus domesticus) immunoglobulins. Mol Biol Rep. 1974 Mar;1(6):301–304. doi: 10.1007/BF00309562. [DOI] [PubMed] [Google Scholar]



