Abstract
Innate immunity provides the first line of response to invading pathogens and a variety of environmental insults. Recent studies identified novel subsets of innate lymphoid cells that are capable of mediating immune responses in mucosal organs. Here we describe a subset of lymphoid cells that is involved in innate type-2 immunity in the lungs. Airway exposure of naïve BALB/c or C57BL mice to IL-33 results in a rapid (< 12 h) production of IL-5 and IL-13 and marked airway eosinophilia independently of adaptive immunity. In the lungs of non-sensitized naïve mice, IL-33-responsive cells were identified that have a lymphoid morphology, lack lineage markers, highly express CD25, CD44, Thy1.2, ICOS, Sca-1 and IL-7Rα (i.e. Lin−CD25+CD44hi lymphoid cells), and require IL-7Rα for their development. Airway exposure of naïve mice to a clinically relevant ubiquitous fungal allergen, Alternaria alternata, increases bronchoalveolar lavage levels of IL-33, followed by IL-5 and IL-13 production and airway eosinophilia without T or B cells. This innate type-2 response to the allergen is nearly abolished in mice deficient in IL-33 receptor (i.e. ST2), and the Lin−CD25+CD44hi lymphoid cells in the lungs are required and sufficient to mediate the response. Thus, a subset of innate immune cells that responds to IL-33 and vigorously produces Th2-type cytokines is present in mouse lungs. These cells may provide a novel mechanism for type-2 immunity in the airways and induction of allergic airway diseases such as asthma.
Introduction
Asthma, in particular allergic asthma, is characterized by inflammation of the airways involving CD4 T helper 2 (Th2) cells, Th2-associated cytokines and eosinophils (1). Airway inflammation is often induced by immune responses to environmental allergens, such as molds and excreta from insects, particularly house dust mite and cockroaches. The dysregulated interactions between mucosal epithelia and innate and adaptive immune cells are recently implicated as the underlining causes of asthma and allergic airway inflammation (2). Airway epithelial cells express multiple pattern-recognition receptors that mediate the responses to respiratory pathogens and airborne particles through the production of cytokines and other immunological molecules (3). Recently, three epithelium-derived cytokines, thymic stromal lymphopoietin (TSLP), IL-25 and IL-33, have emerged as potential links between the immune recognition of pathogens and Th2-type immune responses in various mucosal organs (4-7).
IL-33 is a member of the IL-1 cytokine family, along with IL-1α, IL-1β and IL-18 (8). A number of previous studies have shown a role for IL-33 in initiating and promoting Th2-type immune responses in the airways. For example, intraperitoneal injection of IL-33 in mice increases serum levels of IgE and Th2 cytokines and promotes airway eosinophilia, epithelial cell hyperplasia, and mucus production (8, 9). Antibody blockade of IL-33 or ST2 or IL-33 gene deficiency ameliorates airway eosinophilia and airway hyperreactivity in mice sensitized and challenged with OVA (10-13). In spite of this accumulating evidence linking IL-33 and Th2-type airway immunity, the cellular targets for IL-33 in such immune responses in vivo are not fully understood. ST2 is primarily expressed on differentiated CD4+ Th2-type T cells, but also on mast cells, eosinophils, basophils and dendritic cells (14-18). Indeed, IL-33 induces and enhances the production of IL-5 and IL-13 by fully-differentiated CD4+ Th2-type T cells in vitro (9, 19-22). In contrast, airway administration of IL-33 induces Th2 cytokine production and airway eosinophilia, even in mice deficient in T cells (9).
Recently, novel innate lymphoid cells that produce large quantities of Th2-type cytokines in response to IL-25 or IL-33 have been identified in gastrointestinal organs (23-26). Specifically, intraperitoneal injection of IL-25 or IL-33 into mice led to the expansion of cells that produce IL-4 and/or IL-13 in GALT (25) and in mesenteric lymph nodes (LNs) and spleen (24). Likewise, an IL-33-responsive cell type with a lymphoid morphology was identified in fat-associated lymphoid clusters (FALCs) in the mesentery layer (23). These innate lymphoid cells likely play important roles in host immunity against intestinal helminth infection (23-26). However, little information is currently available on whether these type-2 innate lymphoid cells are involved in other immune responses. Little is also known as to whether these cell type(s) exist in organs other than the digestive system.
Here we describe a distinct population of innate lymphoid cells that mediates type-2 inflammation in the respiratory mucosa. We identified a subset of IL-33-responsive innate lymphoid cells that are resident in the lungs of non-treated naïve BALB/c and C57BL/6 mice, as well as Rag1−/− mice. These lung lymphoid cells highly expressed CD25, CD44, Thy1.2, IL-7Rα, Sca-1 and inducible T-cell co-stimulator (ICOS), but lacked expression of conventional lineage (Lin) markers or c-Kit. Importantly, they produced a large quantity of IL-5 and IL-13, and mediated allergic airway inflammation when mice were exposed to a clinically relevant fungal allergen, Alternaria alternata (27, 28). These findings suggest that innate type-2 lymphoid cells may play an important role in the mucosal immunity to respiratory pathogens and other airborne environmental factors. Further studies are warranted to elucidate the roles for this novel lung innate lymphoid cell population in human airway diseases such as asthma.
Materials and Methods
Mice
BALB/cJ (BALB/c), C57BL/6J (C57BL), Il7r−/− and Rag1−/− (BALB/c and C57BL background) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Rag1−/− (BALB/c background) mice were bred in-house from mice purchased from The Jackson Laboratory. ST2−/− mice (BALB/c background) were kindly provided by Dr. Andrew McKenzie (MRC Laboratory of Molecular Biology, Cambridge, UK). Male and female mice ages 8-12 weeks were used in all of the experiments. All the animal experiments and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee and performed according to the guidelines.
Reagents
Fluorescently-labeled antibodies to CD3 (145-2C11), CD25 (PC61; 7D4), CD44 (IM7), IL-5 (TRFK5), CD16/CD32 (2.4G2), CD14 (rmC5-3), CD11b (M1/70), CD4 (RM4-5), CD8α (53-6.7), c-Kit (ACK45), NK1.1 (PK136), Thy1.2 (53-2.1), ICOS (7E.17G9), TCRβ (H57-597), Sca-1 (D7), CD69 (H1.2F3), CD38 (90), MHC II (AMS-32.1), CD62L (MEL-14), CD27 (LG.3A10) and NKp46 (29A1.4) were purchased from BD Biosciences (San Jose, CA). Fluorescently-labeled antibodies to FcεRI (MAR-1), F4/80 (BM8), Gr-1 (RB6-8C5), CD45R/B220 (RA3-6B2), TCRγ/δ (UC7-13D5), and CD9 (eBioKMC8) were purchased from eBioscience (San Diego, CA). Anti-CD49b (DX5) was from SouthernBiotech (Birmingham, AL). Anti-T1/ST2 (DJ8) was from MD Biosciences (St. Paul, MN). PE-conjugated-goat anti-mouse CD127 (IL-7Rα), biotinylated goat anti-mouse IL-17Rb (IL-25 receptor), and recombinant mouse proteins, including IL-2, IL-7, IL-25, and IL-33, were from R&D Systems (Minneapolis, MN) and eBioscience. The culture filtrate extract of Alternaria alternata was from Greer Laboratories (Lenoir, NC); it contained detectable but minimal amounts of endotoxin (i.e. 3 ng endotoxin/mg extract).
Airway administration of cytokines and Alternaria extract
To examine the airway immune responses, IL-33 (100 ng/dose in 50 μl PBS), Alternaria extract (50 μg/dose in 50 μl PBS) or PBS were administered intranasally (i.n.) once or three times (days 0, 3 and 6) to naïve mice anesthetized with tribromoethanol (Avertin®); approximately 70% of the solutions administered i.n. reached the lungs. In the blocking studies, mice were injected i.p. with 40 μg anti-IL-5 (TRFK5) or isotype control antibody 24 h prior to the first administration of IL-33. At the indicated time points, mice were killed by an overdose of pentobarbital. The trachea was cannulated and the lungs were lavaged three times with HBSS (0.5, 0.25 and 0.25 ml). The cell number in the BAL fluids was counted with a hemocytometer and differentials were determined in cytospin preparations stained with Wright-Giemsa; ≥ 200 cells were analysed using conventional morphologic criteria. The BAL fluid supernatant was collected and stored at −20°C for cytokine assays. Lungs were also collected and homogenized in 1.0 ml of PBS for cytokine analyses or fixed with formaldehyde for histological analyses. The homogenates were centrifuged at 10,000 × g at 4 °C for 15 minutes, and the protein concentrations in the supernatant were quantified with the DC Protein Assay kit (Bio-Rad, Hercules, CA). Sections of fixed lung tissues were stained with H&E stain and periodic acid-Schiff (PAS) stain or with anti-mouse eosinophil major basic protein (MBP) (a gift from Dr. Lames J. Lee, Mayo Clinic Scottsdale) using an immunofluorescence staining protocol (29). A portion of the lungs was processed to obtain lung single cell suspensions and to analyze the expression of cell surface molecules by FACS, as described below.
Cytokine production by lung cells and cells from other organs
Lungs, thymi, spleens, and mediastinal and mesenteric LNs were harvested from naïve, unsensitized mice. To obtain single cell suspensions of lung cells, lungs were minced and incubated in digestion medium with a cocktail of collagenases (Roche Diagnostics, Indianapolis, IN) at 0.2 Wünsch units/lung for 60 minutes at 37 °C with gentle shaking. In some experiments, the digestion medium included 25 μg/ml DNase I (StemCell Technologies, Vancouver, Canada) and shaking was omitted. RBCs were lysed with ammonium chloride/potassium (ACK) lysing buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) and cells were resuspended in RPMI 1640 medium supplemented with 50 μM β-mercaptoethanol, 100 units/ml penicillin, 100 μg/ml streptomycin and 10% FBS (RPMI). Cells isolated from the spleen were treated with ACK to lyse RBCs, and the recovered splenocytes were resuspended in RPMI; cells from various LNs and thymi were not treated with ACK. To examine the cytokine production in response to IL-33, single cell suspensions from these organs were cultured at 1.5-2.5 × 106 cells/ml in RPMI with medium alone or with 10 ng/ml IL-33 for 2 or 4 days at 37 °C and 5% CO2. Supernatants were collected and analyzed for IL-4, IL-5, IL-13, IL-17 and IFN-γ.
In some experiments, the sorted fractions of lung cells were analyzed for their ability to produce cytokines. Single cell suspensions of lung cells were stained with fluorescently-labeled antibodies to lineage markers (CD3, CD11b, CD14, CD16/CD32, and B220), CD25 and CD44, and sorted on a fluorescence-activated cell sorter (FACS, BD FACSAria®) into Lin− and Lin+ populations. The Lin− cells were further sorted into 3 populations based on CD25 and CD44 expression, resulting in the Lin−CD25−CD44−, Lin−CD25−CD44+, and Lin−CD25+CD44hi populations. In some experiments, prior to FACS sorting, Lin− lung cells were isolated by magnetically depleting Lin+ cells using PE-conjugated antibodies to CD3, CD11b, CD14, CD16/CD32 and B220 along with EasySep® magnetic particles (StemCell Technologies). Unsorted lung cells, Lin+ cells and three populations of Lin− cells were cultured at 0.5-4 × 105 cells/ml with medium alone or IL-33 (10 ng/ml) for 96 h. For kinetic studies, IL-2 (50 ng/ml), IL-7 (10 ng/ml) or IL-25 (10 ng/ml) were also added to the culture. The supernatant was collected, stored at −20 °C and assayed for cytokine production by ELISA. Cells examined by electron microscopy were fixed with Trump’s solution after culture.
Analyses of cell surface markers and intracellular cytokines
After single cell suspensions of lung cells were cultured with 10 ng/ml IL-33 for 48 h, they were treated with brefeldin A for 10-12 h and stained for flow cytometry. Briefly, cells were preincubated with an FcR blocker (anti-CD16/CD32 clone 2.4G2, BD Biosciences) for 15 minutes at 4 °C, followed by staining with FITC-conjugated antibodies to cell surface markers (e.g. CD3). Cells were fixed and permeabilized using the BD Cytofix/Cytoperm Fixation/Permeabilization Kit as per the manufacturer’s instructions and subsequently stained intracellularly with PE-conjugated anti-mouse IL-5. The cells were analyzed immediately by flow cytometry. In some experiments, the cell surface marker expression by lung Lin−CD25+CD25hi cells was characterized using a panel of antibodies. Immediately after obtaining the single cell suspensions of lung cells, they were stained with a cocktail of -conjugated antibodies to Lin markers, allophycocyanin (APC)-conjugated anti-CD25, PerCP-conjugated anti-CD44 and FITC-conjugated antibodies to various cell surface molecules (e.g. ST2). In some experiments, the combinations of fluorochromes were modified based on the availability of labeled antibodies.
ELISA
The levels of IL-4, IL-5, IL-13, IL-17, IFNγ, IL-25, IL-33 and TSLP in lung homogenates and BAL supernatants were measured by Quantikine ELISA kits (R&D Systems). Cytokine concentrations in the cell supernatants were measured by DuoSet ELISA kits (R&D Systems) for IL-4, IL-5, IL-13, IL-17 and GM-CSF, an Opt-EIA ELISA kit (BD Biosciences) for IFN-γ, and a Ready-Set-Go kit for IL-9 (eBioscience). All ELISAs were performed as per manufacturer’s instructions.
Adoptive transfer of Lin− or Lin−ICOS+ lung cells
To examine the roles of Lin−CD25+CD44hi lung cells, we adoptively transferred them to Il7r−/− mice, which are deficient in this cell type. Naïve C57BL mice were i.p. injected with a cocktail of IL-25 and IL-33 (400 ng/dose each), once daily for 3 days. Twenty-four h after the last injection, lungs and mediastinal LNs were collected, and Lin− cells were isolated by depleting Lin+ cells using PE-conjugated antibodies to CD3, CD11b, CD14, CD16/CD32 and B220 together with EasySep® magnetic particles (Stem Cell Technologies). Lung Lin− cells (1×106 cells per mouse) were adoptively transferred to recipient Il7r−/− mice by i.v. injection into a retroorbintal sinus. Alternatively, lung Lin−ICOS+ cells were isolated from the Lin− cells by FACS sorting. Lin−ICOS+ cells (1×105 cells per mouse) were adoptively transferred to recipient Il7r−/− mice by i.v. injection into a retroorbintal sinus. Control C57BL and Il7r−/− mice received PBS. Twenty-four h after the transfer, mice were exposed i.n. to Alternaria or PBS once or three times as described above.
Statistics
Data are presented as the mean ± SEM for the numbers of mice or experiments as indicated. Statistics were performed using paired and unpaired Student’s t test, ANOVA or repeated measures ANOVA as appropriate for each set of experimental conditions; p<0.05 was considered significant.
Results
IL-33 induces Th2-type cytokine production and eosinophilic airway inflammation in naïve mice independent of adaptive immunity
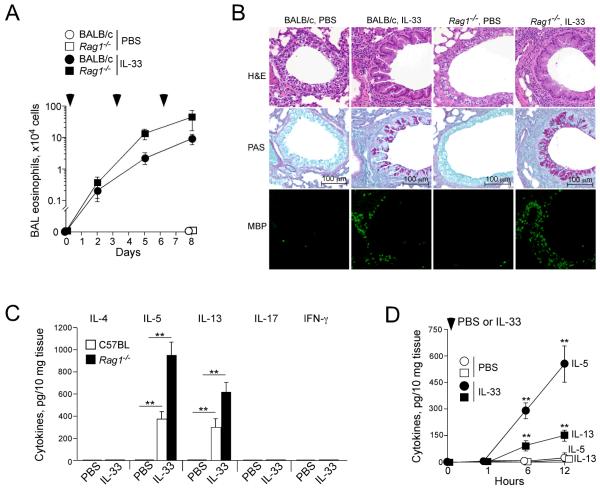
To examine the roles of IL-33 in type-2 immune response in the airway mucosa, we intranasally administered 100 ng/dose of IL-33 or PBS to the airways of naïve non-sensitized BALB/c wild type mice or Rag1−/− mice three times, 3 days apart. A kinetic study revealed increases in airway eosinophil number as early as 48 h after the first administration of IL-33, and the eosinophil number continued to rise after the subsequent administration of IL-33 (Figure 1A). Interestingly, comparable if not higher numbers of eosinophils were observed in the airways of Rag1−/− mice treated with IL-33; no airway eosinophilia was detected in wild type or Rag1−/− mice treated with PBS. Examination of lung tissues confirmed marked infiltration of inflammatory cells, in particular MBP-positive eosinophils, and mucus hyperplasia in both wild type and Rag1−/− mice treated with IL-33 (Figure 1B). Furthermore, airway eosinophilia was abolished in mice treated with neutralizing anti-IL-5 antibody (Supplemental Figure 1), suggesting that IL-5 is involved in airway eosinophilia in the IL-33-treated mice. These findings are consistent with previous observations by other investigators (9), and indicate that innate immunity, rather than adaptive immunity, mediates Th2-type cytokine production and airway inflammation in response to IL-33 in naïve mice.
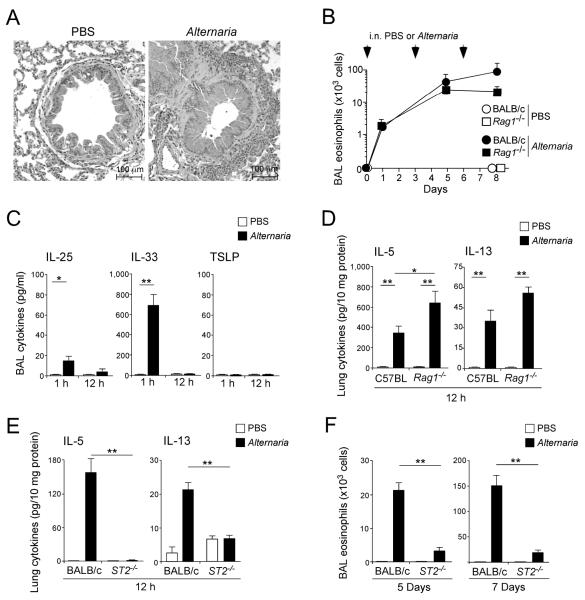
Figure 1.
IL-33 induces airway eosinophilia and Th2 cytokine production in naïve mice lacking adaptive immunity.
(A) IL-33 (100 ng/dose) or PBS were administered i.n. to naïve BALB/c and Rag1−/− mice three times, as indicated by the arrowheads. BAL fluid was collected 48 h after each administration, and the eosinophil number was counted. Data are means ± SEMs of 3-5 mice per group and representative of 2 individual experiments. (B) Lungs were collected 24 h after the third IL-33 administration as described in A, formalin fixed, paraffin embedded, and stained with H&E (top row), PAS (middle row) or anti-MBP (bottom row). (C) IL-33 (100 ng/dose) or PBS were administered i.n. to naïve C57BL and Rag1−/− mice once. After 12 h, lungs were collected, and the cytokine levels in the lung homogenates were measured. Data shown are means ± SEM of 5-8 mice per group and representative of 3 individual experiments. **, p<0.01 compared to PBS administration in each mouse strain. (D) IL-33 or PBS was administered i.n. to naïve BALB/c mice. Lungs were collected at the indicated time points, and the levels of IL-5 (circles) and IL-13 (squares) in the lung homogenates were measured. Data shown are means ± SEM of 4-8 mice per group and representative of 2 individual experiments. **, p<0.01 compared to PBS administration.
To examine this concept further, we administered IL-33 to naïve wild type or Rag1−/− mice just once and compared their airway cytokine responses 12 h later. Significantly increased levels of IL-5 and IL-13 were detected in lung tissues from wild type mice treated with IL-33 as compared to mice treated with PBS (Figure 1C, p<0.01). Importantly, the increases in IL-5 and IL-13 were also observed in naïve Rag1−/− mice treated with IL-33. No increases in IL-4, IL-17 or FN-γ were observed in wild type or Rag1−/− mice treated with IL-33. In addition, kinetic studies showed both IL-5 and IL-13 were detectable in the lungs within 6 h after the administration of IL-33 to naïve BALB/c mice (Figure 1D). Taken together, these findings suggest that an innate immune mechanism(s) operates via rapid and robust production of Th2-type cytokines in the lungs of mice treated with IL-33.
Lineage(Lin)-negative, CD25-positive and CD44-high (Lin−CD25+CD44hi) lymphoid cells in mouse lungs produce a large amount of IL-5 and IL-13 when cultured with IL-33 in vitro
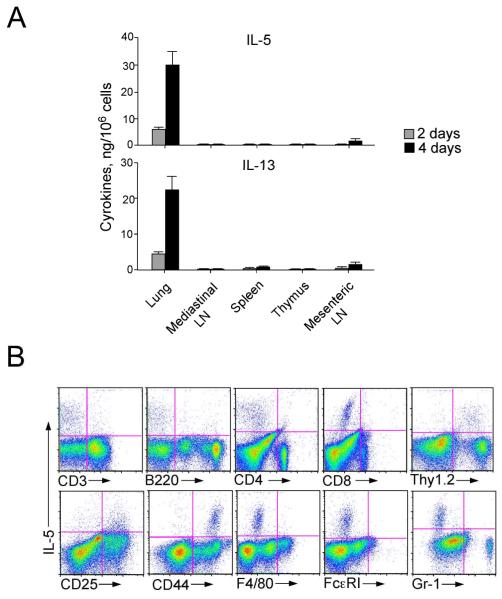
To identify the specific cell type(s) responsible for Th2-type cytokine production in response to IL-33, we turned to in vitro models. We obtained single cell suspensions of lungs, mediastinal LNs, spleen, thymus and mesenteric LN from naïve BALB/c mice and cultured them in vitro with IL-33 for 2 or 4 days. IL-33 induced robust production of IL-5 and IL-13 by lung cells in a time-dependent manner, but not by the cells from the other organs examined (Figure 2A). IFN-γ production was observed in splenocytes and mesenteric LN cells cultured with IL-33, but not in lung cells (data not shown). By intracellular IL-5 staining and flow cytometric analysis (Figure 2B), the lung cells that stained positive for IL-5 did not express authentic markers for T cells (CD3, CD4, CD8), B cells (B220), macrophages (F4/80), mast cells/basophils (FcεRIα), or granulocytes (Gr-1), but they clearly expressed Thy1.2, CD25 and CD44. Thus, novel population(s) of immune cells that are present in the lungs of naïve mice produce Th2-type cytokines in response to IL-33; CD25 and CD44 are likely to be useful to identify this cell type(s) among the lung cells.
Figure 2.
Lung cells cultured with IL-33 produce IL-5 and IL-13 in vitro.
(A) Single-cell suspensions were obtained from lungs, spleens, thymi and mediastinal and mesenteric LNs of naive BALB/c mice and cultured for 2 or 4 days with IL-33 (10 ng/ml). The concentrations of IL-5 and IL-13 in the cell-free supernatants were measured. (B) Single cell suspensions of lung cells from naive BALB/c mice were cultured with IL-33 (10 ng/ml) for 48 h and stained for intracellular IL-5 and cell surface markers. Data are representative of 4 different experiments showing similar results.
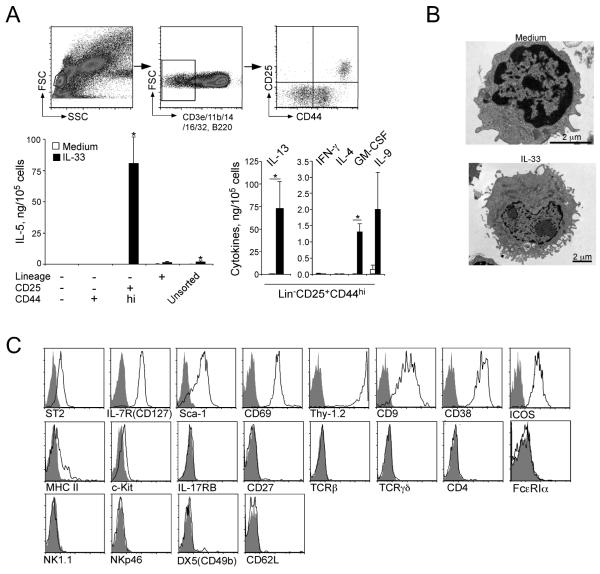
To address this hypothesis directly, lung cells were FACS-sorted from naïve BALB/c mice based on lineage markers (CD3ε, CD11b, CD14, CD16/CD32 and B220), as well as CD25 and CD44 (Figure 3A, upper panels). Three populations of the sorted lung Lin− cells, as well as unsorted lung cells and Lin+ cells, were cultured with medium alone or IL-33 for 96 h. Notably, when cultured with IL-33, the Lin−CD25+CD44hi population produced approximately 50x more IL-5 than unsorted cells. The amount of IL-5 produced by this Lin−CD25+CD44hi population was extremely high, reaching 750 ng IL-5 per 1×106 cells. In contrast, the Lin−CD25−CD44−, Lin−CD25−CD44+ or Lin+ populations did not produce detectable IL-5. Furthermore, the Lin−CD25+CD44hi population produced a large quantity of IL-13 as well as GM-CSF and IL-9 in response to IL-33; these cells did not produce detectable IL-4, IFN-γ or IL-17 (Figure 3A, and data not shown).
Figure 3.
Lin−CD25+CD44hi lymphoid cells in the lungs produce a large quantity of IL-5 and IL-13 in response to IL-33 in vitro.
(A) Four populations of lung cells, including Lin+ cells, Lin−CD25−CD44− cells, Lin− CD25−CD44+ cells and Lin−CD25+CD44hi cells were isolated from naïve BALB/c mice by FACS sorting; the upper panels show the gating strategy. Sorted and unsorted lung cells were cultured with medium alone or with 10 ng/ml IL-33 for 96 h, and the levels of cytokines in the supernatants were measured by ELISA. Data shown are means ± SEMs of 4-9 independent experiments. *, p<0.05 compared to cells cultured with medium alone. (B) FACS-sorted Lin−CD25+CD44hi lung cells were cultured with medium alone or 10 ng/ml IL-33 for 96 h and examined under electron microscopy. Original magnifications; 25,000x (medium alone, top) and 12,000x (IL-33, bottom). (C) Expression of various cell surface markers on the Lin−CD25+CD44hi lung cell population was examined by flow cytometry. Solid line depicts staining with antibody to the indicated marker; filled grey areas are isotype control staining. Data are representative of three experiments showing similar results.
By electron microscopy, lung Lin−CD25+CD44hi cells cultured with medium alone for 4 days displayed a lymphoid morphology and did not contain apparent intracellular granule structures (Figure 3B). When cultured with IL-33, they increased in size (note the difference in the scale bars) and developed pronounced endoplasmic reticulum and Golgi apparatus. By FACS, unstimulated Lin−CD25+CD44hi cells highly expressed ST2 (IL-1RL1), CD127 (IL7Rα), Sca-1, CD69, Thy1.2, CD9, CD38, and ICOS (Figure 3C). The histograms of all of these cell surface markers showed a unimodal distribution, suggesting that the FACS-sorted Lin−CD25+CD44hi subset likely consists of a single cell population. A modest expression of MHC class II and minimal expression of c-Kit were also detected, but there was no detectable expression of IL-17RB (IL-25R), CD27, TCRβ, TCRγδ or CD4. The conventional markers for basophils/mast cells (FcεRIα) and NK cells (NK1.1, NKp46, and DX5) were also negative. Collectively, these findings suggest a Lin−CD25+CD44hi lymphoid cell population, which responds vigorously to IL-33 and produces abundant Th2-type cytokines, is present in the lungs of naïve mice.
IL-7 receptor is essential for the development of lung Lin−CD25+CD44hi cells
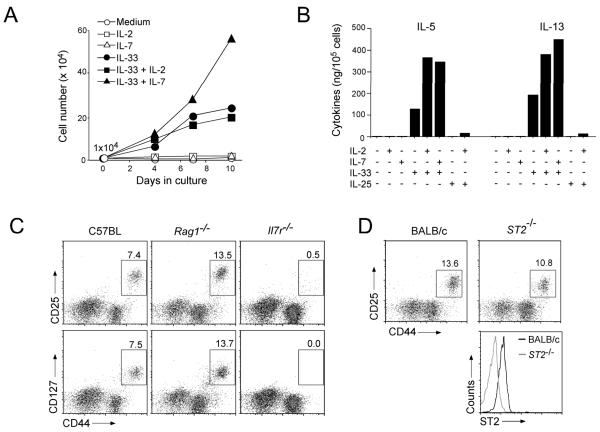
Flow cytometry analysis indicated abundant cell surface expression of the components of IL-7R (CD127, IL-7 α-chain) and IL-2R (CD25, IL-2 α-chain) in lung Lin−CD25+CD44hi cells. Because IL-2R and IL-7R share the common γ chain and both IL-2 and IL-7 are considered T cell growth factors (30), we examined whether these cytokines play any roles in the development or expansion of Lin−CD25+CD44hi cells. Lin−CD25+CD44hi cells were FACS-sorted from the lungs of naïve BALB/c mice and cultured with cytokines for up to 10 days in vitro. Culture with IL-33 alone increased the number of Lin−CD25+CD44hi cells robustly, e.g. 20-fold by day 10 (Figure 4A). IL-7 alone did not exert any effect on the cell number but, when added together with IL-33 to cell culture, it synergistically increased the number of Lin−CD25+CD44hi cells. IL-2 did not show any apparent effects with or without IL-33. When we measured the amounts of IL-5 and IL-13 in the cell-free supernatants of these cells, neither IL-2 alone nor IL-7 alone was capable of inducing these cytokines (Figure 4B). On the other hand, when added together with IL-33 to the cell culture, both IL-7 and IL-2 enhanced the IL-33-induced production of IL-5 and IL-13. Furthermore, unlike recently identified novel Lin− lymphoid cell population(s) in mesenteric LN and the peritoneal cavity that respond to IL-25 (23-26), lung Lin−CD25+CD44hi cells produced little IL-5 or IL-13 after in vitro culture with IL-25 with or without IL-2. Thus, while IL-2 may enhance IL-33-driven Th2 cytokine production by Lin−CD25+CD44hi cells, IL-7 may be involved in Lin− CD25+CD44hi cell proliferation, activation or both.
Figure 4.
The roles of IL-2 and IL-7 in proliferation of and cytokine production by Lin− CD25+CD44hi lung cells.
(A) Isolated Lin−CD25+CD44hi cells (1×104 cells/well) were cultured with medium alone or with IL-2 (50 ng/ml), IL-7 (10 ng/ml), or IL-33 (10 ng/ml) or their combinations for up to 10 days. Expansion of Lin−CD25+CD44hi cells was determined by counting the cell number at each time point. Data from one of three experiments showing similar results are presented. (B) Isolated Lin−CD25+CD44hi cells were cultured with medium alone or with IL-2 (50 ng/ml), IL-7, IL-25, IL-33 (10 ng/ml each) or their combinations for 96 h. The levels of IL-5 and IL-13 in the supernatants were determined by ELISA. Data are representative of three experiments showing similar findings. (C) Single-cells suspensions of lung cells from naive C57BL, Rag1−/− and Il7r−/− mice were stained for lineage markers, CD44, and CD25 or CD127 (IL-7Rα), and Lin− lung cells were analyzed as described in Figure 3A. (D) Single-cell suspensions of lung cells from BALB/c and ST2−/− mice were stained with lineage markers, CD44, CD25 and ST2. Top panels: Lin− lymphocytic cells were analyzed as described in Figure 3A. Bottom panel: expression of ST2 on Lin−CD25+CD44hi cells is depicted in BALB/c (black line) and ST2−/− (red line) mice.
To examine the roles of IL-7 in the dynamics of the lung Lin−CD25+CD44hi population in vivo, we examined IL7r−/− C57BL mice. In naïve wild type C57BL mice, the lung Lin−CD44hi population expressed both CD25 and CD127 (i.e. the IL-7R α-chain) (Figure 4C); >90% of Lin−CD44hi cells were positive for both CD25 and CD127 (data not shown). Lin−CD25+CD44hi cells consisted on average of 5.1% of Lin− cells, 0.67% of lymphocytic cells, or 0.25% of the total cells in the lungs; lungs of naïve C57BL mice contained approximately 3×104 Lin−CD25+CD44hi cells/mouse. The same Lin− CD25+CD44hi cell population was detected in the lungs of Rag1−/− C57BL mice, likely in increased levels compared to the wild type mice. In contrast, the Lin−CD25+CD44hi cell population was nearly absent in the lungs of Il7r−/− C57BL mice. The Lin−CD25+CD44hi population was also present in the lungs of wild type BALB/c mice as well as in the lungs of mice deficient in the IL-33 receptor (i.e. ST2−/− on BALB/c background) (Figure 4D).
Exposure to a ubiquitous aeroallergen, Alternaria, induces an innate Th2-type cytokine response and eosinophilic inflammation in the airways
In humans, the association between asthma and exposure to fungal allergens, especially Alternaria alternata, has been established clinically and epidemiologically (27, 28). Severe asthma and life-threatening acute exacerbations of asthma have also been associated with increased airborne exposure to Alternaria spores (31, 32). Therefore, to design a mouse model relevant to human asthma, we intranasally exposed non-sensitized naïve BALB/c mice to the culture extracts of Alternaria alternata, three times, three days apart. On day 7 (i.e. 24 h after the last exposure to Alternaria), wild type BALB/c mice exposed to Alternaria demonstrated marked peribronchial infiltration of inflammatory cells, epithelial hyperplasia (Figure 5A), and pronounced airway eosinophilia (Figure 5B). Rag1−/− BALB/c mice also developed airway eosinophilia when they were exposed to Alternaria (Figure 5B). The magnitude of airway eosinophilia in Rag1−/− BALB/c mice was roughly comparable to that in the wild type BALB/c mice for up to 5 days (Figure 5B); airway eosinophilia further increased at a later time point in the wild type but not in Rag1−/− mice. These findings suggest that airway Th2-type immune responses to Alternaria exposure consist of at least two arms; an initial innate immune response and a subsequent involvement of adaptive immunity.
Figure 5.
Airway exposure of mice to Alternaria extract induces rapid Th2-type cytokine responses through IL-33-dependent innate immune mechanism(s).
(A) Naïve BALB/c mice were exposed i.n. three times to Alternaria extract, as described in Materials and Methods. Lungs were collected 24 h after the third exposure, formalin fixed, paraffin embedded and stained with H&E. (B) Naïve BALB/c or Rag1−/− mice were exposed i.n. three times to Alternaria extract or PBS on days 0, 3, and 6. BALs were collected 24 h after the first exposure and 48 h after the second and third exposure, and the eosinophil numbers were determined. Data shown are means ± SEMs of 3-5 mice per group and representative of 3 individual experiments. (C) Naïve BALB/c mice were exposed i.n. once to Alternaria extract or PBS. BAL fluid was collected 1 or 12 h later, and the levels of IL-25, IL-33, and TSLP in the supernatants were determined. Data shown are means ± SEMs of 3-6 mice per group and representative of 3 individual experiments. *, p<0.05; **, p<0.01. (D) Naïve C57BL or Rag1−/− mice were exposed i.n. once to Alternaria extract or PBS. Lungs were collected 12 h later and the levels of IL-5 and IL-13 in lung homogenates were examined. Data shown are means ± SEMs of 4-8 mice per group and representative of 2 individual experiments. *, p<0.05; **, p<0.01. (E) Naïve BALB/c or ST2−/− mice were exposed i.n. once to Alternaria extract or PBS. Lungs were collected 12 h later, and the levels of cytokines in lung homogenates were determined. Data are shown as means ± SEMs of 8-16 mice per group, a pool of 2 individual experiments. **, p<0.01. (F) Naïve BALB/c or ST2−/− mice were exposed i.n. three times to Alternaria extract or PBS as described in B. BAL fluid was collected 48 h after the second exposure (i.e. Day 5) or 24 h after the third exposure (i.e. Day 7), and the numbers of eosinophils were determined. Data are shown as means ± SEMs of 8-16 mice per group from a pool of 2 individual experiments. **, p<0.01.
To investigate the innate response further, we administered Alternaria extracts intranasally to naïve wild type or Rag1−/− BALB/c mice once and analyzed the immediate (i.e. 1 h) and early (i.e. 12 h) cytokine responses. In wild type BALB/c mice exposed to Alternaria, the IL-33 protein level in BAL fluid markedly increased within 1 h, and then it declined to baseline by 12 h (Figure 5C); little IL-25 and no TSLP protein was detectable in BAL fluid either at 1 or 12 h after exposure to Alternaria (note the difference in the y-axis scales). Increases in the lung levels of IL-5 and IL-13 proteins were also observed in the wild type as well as in Rag1−/− mice 12 h after Alternaria exposure (Figure 5D). Furthermore, these IL-5 and IL-13 responses were nearly abolished in ST2−/− mice (Figure 5E). When mice were exposed to Alternaria extracts up to three times, airway eosinophilia on day 5 or day 7 was significantly inhibited by >80% in the ST2−/− mice as compared to the wild type mice (p<0.01, Figure 5F). Together, these findings suggest that the airway immune responses to Alternaria exposure in naïve mice involve a rapid (< 1 h) increase in airway IL-33, which subsequently mediates Th2-type cytokine responses and airway eosinophilia by innate mechanism(s).
Lung Lin−CD25+CD44hi cells mediate Th2-type cytokine production and allergic airway inflammation in response to Alternaria exposure
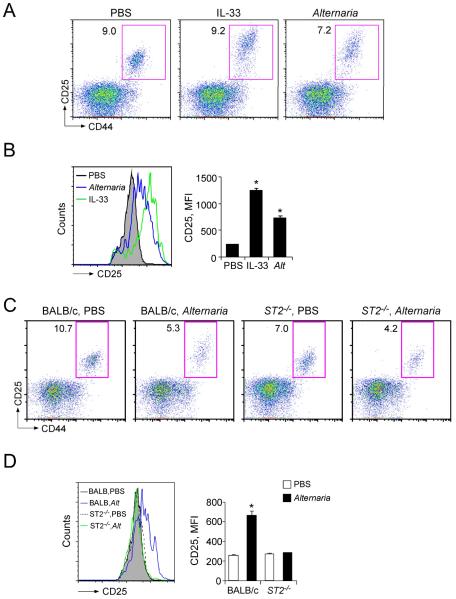
To investigate whether the lung Lin−CD25+CD44hi cells are involved in these innate type-2 responses to Alternaria, we first examined whether Lin−CD25+CD44hi cells are activated after airway exposure to Alternaria. Airway exposure to IL-33 significantly increased the expression of CD25, a marker of cell activation, in the Lin− CD25+CD44hi population as compared to the mice exposed to PBS (p<0.05, Figures 6A and 6B). Likewise, airway exposure to Alternaria significantly upregulated CD25 expression (p<0.05); this increase in CD25 expression was abolished in the ST2−/− mice (Figures 6C and 6D), suggesting that Lin−CD25+CD44hi cells are activated by endogenous IL-33 when mice are exposed to Alternaria extract.
Figure 6.
Lung Lin−CD25+CD44hi cells are activated when mice are exposed i.n. to IL-33 or Alternaria extract in vivo.
(A) Naïve BALB/c mice were exposed i.n. once to PBS, IL-33 or Alternaria extract, and lungs were collected 12 h later. The cell surface expression of CD25 and CD44 on lung Lin− cells was analyzed, as described in Figure 2A. One of two experiments with similar results is shown. (B) Left panel: Representative histograms of CD25 intensity in Lin−CD25+CD44hi cells from mice exposed to PBS (grey filled histogram), IL-33 (green line) or Alternaria (blue line) are shown. Right panel: Mean fluorescence intensity (MFI) of CD25 staining in Lin−CD25+CD44hi cells was determined from flow cytometry, and data from 3 mice per group are summarized (means ± SEMs). *, p<0.05 compared to PBS administration. (C) Naïve BALB/c or ST2−/− mice were exposed i.n. once to PBS or Alternaria extract, and lungs were collected 12 h later. The cell surface expression of CD25 and CD44 on lung Lin− cells was analyzed, as described in Figure 2C. One of two experiments showing similar results is shown. (D) Left panel: Representative histograms of CD25 intensity in Lin−CD25+CD44hi cells from mice exposed to PBS (BALB/c, grey filled histogram; ST2−/−, dashed black line) or Alternaria (BALB/c, blue line; ST2−/−, green line) are shown. Right panel: MFI of CD25 staining in Lin− CD25+CD44hi cells was determined by flow cytometry, and data from 3 mice per group are summarized (means ± SEMs). *, p<0.05 compared to PBS administration.
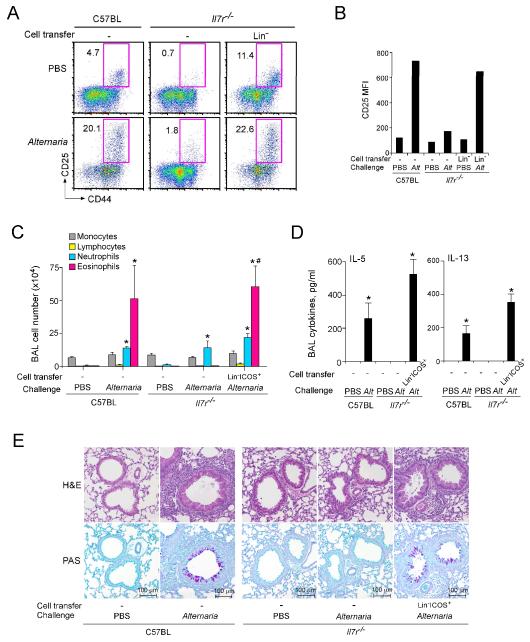
To directly examine the involvement of Lin−CD25+CD44hi cells in airway responses to Alternaria, a reconstitution approach was undertaken. Naïve Il7r−/− mice that are deficient in the lung Lin−CD25+CD44hi population (Figure 4) were adoptively transferred with 1×106 Lin− cells isolated from the lungs of naïve wild type mice; Lin− cells were intravenously injected into a retroorbital sinus. As expected, in Il7r−/− mice, the lung Lin−CD25+CD44hi population was nearly absent (Figure 7A). When Il7r−/− mice received lung Lin− cells and were subsequently exposed to PBS, the Lin−CD25+CD44hi population was clearly detected in the lungs of the recipients, suggesting a successful homing to the lungs without any microbial stimuli. Furthermore, increased expression of CD25 in these Lin−CD25+CD44hi cells was observed when wild type C57BL/6 mice or Il7r−/− mice that had received Lin− cells were exposed to Alternaria (Figures 7A and 7B).
Figure 7.
Adoptive transfer of Lin−CD25+CD44hi cells from wild type mice reconstitutes the Alternaria-induced immune responses in Il7r−/− mice.
(A) Lin− cells were isolated from the lungs of naïve C57BL mice as described in Materials and Methods, and they (1×106 cells/mouse) were adoptively transferred intravenously to naive Il7r−/− mice. Control naïve C57BL and Il7r−/− mice received PBS. Twenty-four h later, mice were exposed i.n. once to PBS or Alternaria extract. Lungs were collected 24 h later, and the expression of CD25 and CD44 on lung Lin− cells was analyzed, as described in Figure 2C. (B) MFI of CD25 staining in Lin−CD25+CD44hi cells was determined by flow cytometry. (C) Lin−ICOS+ cells were isolated from the lungs of naïve C57BL mice as described in Materials and Methods, and they (1×105 cells/mouse) were adoptively transferred intravenously to naive Il7r−/− mice. Control naïve C57BL and Il7r−/− mice received PBS. Beginning 24 h later, mice were exposed i.n. three times to PBS or Alternaria extract on days 0, 3, and 6. BAL fluid was collected 24 h after the last exposure, and the cell number and differentials were determined. Data shown are means ± SEMs from 2-4 mice per group. *, p<0.05 compared to the same strain of mice treated with PBS. #, p<0.05 compared to Il7r−/− mice without Lin−ICOS+ cell transfer but exposed to Alternaria extract. (D) The levels of IL-5 and IL-13 in the BAL fluid supernatants of the mice as described in C were determined. *, p<0.05 compared to the same strain of mice treated with PBS. (E) Representative histology (upper panels: H&E staining, lower panels: PAS staining) of the mice as described in C is presented.
We FACS sorted Lin−CD25+CD44hi cells from the donor Lin− cells by staining with anti-CD25 and anti-CD44 antibodies, and attempted to reconstitute naïve Il7r−/− recipient mice with these cells. However, the experiments were unsuccessful, probably because the anti-CD25 or anti-CD44 antibodies adversely affected activation, homing, and/or survival of the isolated Lin−CD25+CD44hi cells in vivo. Therefore, to transfer the Lin−CD25+CD44hi cell population, we took an alternative approach and FACS sorted the cells by using Lin and ICOS as their identification markers. ICOS is highly expressed by Lin−CD25+CD44hi cells (Figure 3C), and the Lin−CD25+CD44hi cell is the only Lin− cell population that expresses ICOS (Supplemental Figure 2). Il7r−/− mice were reconstituted with sorted Lin−ICOS+ cells (1×105 cells per recipient) by retroorbital injection and exposed intranasally three times, three days apart, to Alternaria. On day 7 (i.e. 24 h after the last exposure), marked increases in the eosinophil number (Figure 7C) and the IL-5 and IL-13 proteins (Figure 7D) were observed in BAL fluid from the reconstituted Il7r−/− mice as well as the wild-type C57BL mice exposed to Alternaria extracts. Modest increases in the BAL neutrophil number were observed in both the wild type and Il7r−/− mice exposed to Alternaria extract irrespective of reconstitution. Pathologically, peribronchial infiltration of inflammatory cells, epithelial hyperplasia, and increased mucus production (Figure 7E) were observed in the Il7r−/− mice reconstituted with Lin−ICOS+ cells and exposed to Alternaria. In a separate experiment, no or minimal increases in the BAL levels of IL-5 and IL-13 or airway eosinophilia were observed in the reconstituted Il7r−/− mice exposed to PBS (Supplemental Figure 3). Together, these results demonstrate the potent capacity of lung Lin−CD25+CD44hi cells in mediating IL-5 and IL-13 production, type-2 airway inflammation and pathological features of asthma upon exposure to an airborne fungal allergen.
Discussion
Type-2 immune responses are induced by helminth parasites and are associated with allergic airway diseases. While CD4+ Th2 T cells are thought to be the main source of Th2-type cytokines, innate cellular sources for IL-4, such as basophils and eosinophils, have been proposed to initiate the type-2 immunity to intestinal helminth (33, 34). We now have identified a subset of lymphoid cells in the mouse lungs that are an important source of Th2-type cytokines during allergic airway inflammation. These lymphoid cells are resident in the lungs of naïve mice, respond quickly and vigorously to both exogenous and endogenous IL-33, and produce a large quantity of IL-5 and IL-13, but not IL-4. They are necessary and sufficient to mediate airway eosinophilia and asthma-like pathological changes in response to a common airborne allergen, even in the absence of T cells or B cells. Our findings provide a new mechanism by which type-2 immune responses are manifested in the airways, and reveal previously unrecognized immune cells that serve as an early source of IL-5 and IL-13 before the adaptive immune responses are established.
Intriguingly, the lung Lin−CD25+CD44hi cells are analogous to, but maybe distinct from, the innate type-2 cells found within the gastrointestinal organs (23-26). These intestinal innate type-2 cells commonly proliferate and produce IL-5 and IL-13, but may not produce IL-4, in response to IL-25 or IL-33 (23-26), and typically express c-Kit, Sca-1, IL-7Rα, Thy1.2 and CD44 (23, 24, 26). More recently, a comparable innate lymphoid cell population, which expresses c-Kit, Sca-1 and Thy1.2, has been identified in mouse lungs after influenza A virus infection (35). The lung Lin−CD25+CD44hi cells identified in this study proliferated and produced IL-5 and IL-13 in response to IL-33 both in vitro and in vivo and expressed Sca-1, IL-7Rα, Thy1.2 and CD44 (Figures 3 and 4). On the other hand, cell surface expression of c-Kit was minimal to undetectable in lung Lin−CD25+CD44hi cells, and these cells failed to express the IL-25 receptor (i.e. IL-17RB) and responded poorly to IL-25.
Notably, innate type-2 cells in previous reports, except for FALC cells (23), were induced by i.p. injection of IL-25 or IL-33 or by infection with either Nippostrongylus brasiliensis or influenza virus (24-26, 35). In contrast, a Lin−CD25+CD44hi cell population was present in the lungs of non-treated naïve BALB/c and C57BL mice (Figures 3 and 4). Furthermore, when Lin−CD25+CD44hi cells were isolated from the lungs of donor mice and injected into a retroorbital sinus of naïve recipient mice, they homed back to the lungs of the recipients without any immunological stimuli or exposure to infectious agents (Figure 7A). Therefore, lung Lin−CD25+CD44hi cells are present and readily available within the lungs of naïve mice. This may explain the extremely rapid production of IL-5 and IL-13 that takes place within 6 h or 12 h in naïve mice after airway exposure to exogenous IL-33 (Figure 1) or Alternaria extract (Figure 5), respectively. Further studies will be necessary to examine whether lung Lin− CD25+CD44hi cells (i.e. Lin−c-Kit−Sca-1+) develop into Lin−c-Kit+Sca-1+ cells (35) when mice are infected with certain respiratory viruses. Alternatively, they may be comprised of two distinct cell populations with different functional capacities; lung Lin− CD25+CD44hi cells may be specialized in airway inflammation and Lin−c-Kit+Sca-1+ cells may be involved in lung tissue remodeling. Unlike lung Lin−CD25+CD44hi cells, Lin−c-Kit+Sca-1+ cells did not induce eosinophilic airway inflammation (35), suggesting the latter account is more probable.
The lung Lin−CD25+CD44hi cell is likely a member of novel family of innate lymphoid cells (ILCs) that function in innate immune responses to infectious agents and tissue remodeling after injury (36). The ILC family includes prototypic NK cells and lymphoid tissue-inducer (LTi) cells; additional ILC populations, such as natural cytotoxicity receptor 22 (NCR22) cells (37), NK22 cells (38), and an IL-17-producing ILC subset (ILC17) (39), have been recently identified in mice or humans. NCR22 cells, NK22 cells, and ILC17 are present mainly in the intestine, produce IL-17 and/or IL-22, and mediate early protective immunity to colitis-inducing pathogens as well as intestinal pathology. The unifying characteristics of these ILCs are their dependence on hematopoietic cytokines that use γc, such as IL-7 and IL-15 (36, 37). We found in this study that IL-33-induced expansion of isolated lung Lin−CD25+CD44hi cells was enhanced synergistically by IL-7, but not by IL-2 (Figure 4). Furthermore, lung Lin− CD25+CD44hi cells were present in the lungs of Rag1−/− and ST2−/− mice, but were absent in the lungs of Il7r−/− mice (Figure 4). Therefore, similarly to other ILCs, IL-7 likely plays pivotal roles in the development and perhaps homing of lung Lin−CD25+CD44hi cells.
Asthma is likely initiated at the mucosal surface, where environmental allergens come in contact with the airway epithelia (2). Current models suggest that the release of epithelial cytokines, particularly IL-25, IL-33, and TSLP, along with chemokines, regulates important proximal events in initiating Th2-type airway inflammation (7, 40, 41). IL-33 was initially described as “nuclear factor from high endothelial venules” because it resides in the nucleus of high endothelial cells and other stromal cells (42). IL-33 is now considered as an “endogenous danger signal” or “alarmin” (43, 44), which is released during necrotic cell death associated with tissue damage during trauma or infection. In addition, IL-33 can be actively released by airway epithelial cells via mechanisms involving autocrine ATP and purinergic receptors (45). In this study, a large quantity of IL-33 was indeed detected within 1 h after airway exposure to Alternaria (Figure 5), and returned to a baseline level by 12 h. Airway epithelial cells are prone to damage or to become stressed from respiratory pathogens such as viruses, airway pollution and the protease activities of certain allergens. Thus, airway epithelial cells may serve as a sentinel against the atmospheric environment and thus initiate early inflammatory and possibly homeostatic innate immune responses by engaging the resident Lin−CD25+CD44hi cells via IL-33. Further studies are needed to elucidate whether and how epithelium-derived IL-33 and Lin−CD25+CD44hi cells may contribute to shape the adaptive immune responses when the airway mucosa is repeatedly exposed to respiratory pathogens and airborne allergens.
In summary, our findings suggest that Th2-type allergic airway inflammation can develop independently of adaptive immunity. Lung Lin−CD25+CD44hi cells were sufficient to induce airway inflammation in the absence of T cells or B cells when they were transferred into naïve Il7r−/− mice and also when these mice were exposed to the clinically relevant allergen Alternaria (Figure 7). Thus, Lin−CD25+CD44hi cells may contribute to airway inflammation and pathology of asthma and other allergic airway diseases similarly to CD4+ Th2 T cells, but in a non-redundant manner. Hopefully, these findings mark the beginning of a research effort that will progressively determine the effector potential of this previously unknown lymphoid cell population in the lungs. Recently, genetic evidence has been accumulating in support of an association between IL-33/ST2 and development of asthma. Single nucleotide polymorphisms (SNPs) in the IL-33 gene have been associated with asthma, increased eosinophils and allergic rhinitis (46, 47), and SNPs in the ST2 gene have been associated with asthma and airway function (47-49). In addition, genome wide associations study of over 10,000 patients with asthma revealed an association with both ST2 and IL-33 (50). Along with these genetic studies, the identification of lung Lin−CD25+CD44hi cells opens up new pathways to understand better the mechanisms of asthma and other allergic airway diseases from the perspective of the innate arm of type-2 airway immunity.
Supplementary Material
Acknowledgements
We thank LuRaye S. Eischens for secretarial assistance.
Footnotes
This works was supported by R01 grants (AI34486 and AI49235) from the National Institutes of Health, Bethesda, MD, and the Mayo Foundation, Rochester, MN.
Abbreviations used in this paper: ACK, ammonium chloride/potassium; APC, allophycocyanin; FALC, fat-associated lymphoid clusters; ILC, innate lymphoid cells; i.n., intranasal; Lin, lineage; LN, lymph node; LTi, lymphoid; MBP, major basic protein; NCR, natural cytotoxicity receptor; PAS, periodic acid-Schiff; SNP, single nucleotide polymorphism; TSLP, thymic stromal lymphopoietin
References
- 1.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin. Exp. Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 2009;386:181–185. doi: 10.1016/j.bbrc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, Sudo K, Okumura K, Saito H, Nakae S. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, Bourne P, McClanahan TK, Pflanz S, de Waal Malefyt R. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int. Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 14.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J. Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 16.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J. Immunol. 2008;181:5981–5989. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 18.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J. Allergy Clin. Immunol. 2009;123:1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18660–18665. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 21.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, McKenzie AN, Teixeira MM, Liew FY, Xu D. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J. Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 23.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 24.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr., Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL-25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bush RK, Prochnau JJ. Alternaria-induced asthma. J. Allergy Clin. Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur. Respir. J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 29.Kephart GM, Alexander JA, Arora AS, Romero Y, Smyrk TC, Talley NJ, Kita H. Marked deposition of eosinophil-derived neurotoxin in adult patients with eosinophilic esophagitis. Am. J. Gastroenterol. 2010;105:298–307. doi: 10.1038/ajg.2009.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Nakamura M, Takeshita T. The common gamma-chain for multiple cytokine receptors. Adv. Immunol. 1995;59:225–277. doi: 10.1016/s0065-2776(08)60632-x. [DOI] [PubMed] [Google Scholar]
- 31.Delfino RJ, Zeiger RS, Seltzer JM, Street DH, Matteucci RM, Anderson PR, Koutrakis P. The effect of outdoor fungal spore concentrations on daily asthma severity. Environ. Health Perspect. 1997;105:622–635. doi: 10.1289/ehp.97105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O’Connell EJ, Ballard DJ, Sachs MI. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N. Engl. J. Med. 1991;324:359–363. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 33.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 34.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang Y-J, Kim HY, Albacker LA, Baumgarth N, McKenzie ANJ, Smith DE, DeKruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011 doi: 10.1038/ni.2045. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 37.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J. Exp. Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buonocore S, Ahern PP, Uhlig HH, Ivanov, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Lamkanfi M, Dixit VM. IL-33 raises alarm. Immunity. 2009;31:5–7. doi: 10.1016/j.immuni.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakashita M, Yoshimoto T, Hirota T, Harada M, Okubo K, Osawa Y, Fujieda S, Nakamura Y, Yasuda K, Nakanishi K, Tamari M. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin. Exp. Allergy. 2008;38:1875–1881. doi: 10.1111/j.1365-2222.2008.03114.x. [DOI] [PubMed] [Google Scholar]
- 47.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jonsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, Malerba G, Pignatti PF, Boner A, Pescollderungg L, Girelli D, Olivieri O, Martinelli N, Ludviksson BR, Ludviksdottir D, Eyjolfsson GI, Arnar D, Thorgeirsson G, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Gulcher J, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat. Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 48.Ali M, Zhang G, Thomas WR, McLean CJ, Bizzintino JA, Laing IA, Martin AC, Goldblatt J, Le Souef PN, Hayden CM. Investigations into the role of ST2 in acute asthma in children. Tissue Antigens. 2009;73:206–212. doi: 10.1111/j.1399-0039.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 49.Reijmerink NE, Postma DS, Bruinenberg M, Nolte IM, Meyers DA, Bleecker ER, Koppelman GH. Association of IL1RL1, IL18R1, and IL18RAP gene cluster polymorphisms with asthma and atopy. J. Allergy Clin. Immunol. 2008;122:651–654. doi: 10.1016/j.jaci.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 50.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WO. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.