Abstract
The human cathelicidin antimicrobial protein-18 and its C terminal peptide, LL-37, displays broad antimicrobial activity that is mediated through direct contact with the microbial cell membrane. In addition, recent studies reveal that LL-37 is involved in diverse biological processes such as immunomodulation, apoptosis, angiogenesis and wound healing. An intriguing role for LL-37 in carcinogenesis is also beginning to emerge and the aim of this paper was to explore if and how LL-37 contributes to the signaling involved in tumor development. To this end, we investigated the putative interaction between LL-37 and growth factor receptors known to be involved in tumor growth and progression. Among several receptors tested, LL-37 bound with the highest affinity to insulin-like growth factor 1 receptor (IGF-1R), a receptor that is strongly linked to malignant cellular transformation. Furthermore, this interaction resulted in a dose-dependent phosphorylation and ubiquitination of IGF-1R, with downstream signaling confined to the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK)-pathway but not affecting phosphatidylinositol 3 kinase/Akt signaling. We found that signaling induced by LL-37 was dependent on the recruitment of β-arrestin to the fully functional IGF-1R and by using mutant receptors we demonstrated that LL-37 signaling is dependent on β-arrestin-1 binding to the C-terminus of IGF-1R. When analyzing the biological consequences of increased ERK activation induced by LL-37, we found that it resulted in enhanced migration and invasion of malignant cells in an IGF-1R/β-arrestin manner, but did not affect cell proliferation. These results indicate that LL-37 may act as a partial agonist for IGF-1R, with subsequent intra-cellular signaling activation driven by the binding of β-arrestin-1 to the IGF-1R. Functional experiments show that LL-37-dependent activation of the IGF-1R signaling resulted in increased migratory and invasive potential of malignant cells.
Supplementary information
The online version of this article (doi:10.1038/onc.2011.239) contains supplementary material, which is available to authorized users.
Keywords: LL-37, breast cancer, IGF-1R, invasion, metastasis
Introduction
Antimicrobial peptides, AMPs, are important components of the innate immune system in most living organisms because they exhibit broad antimicrobial function against bacteria, fungi, yeast and viruses (Radek and Gallo, 2007). A common feature of AMPs is their cationic and amphiphatic structure, allowing interaction with and disruption of microbial membranes, which typically contain a high degree of negative charge (Gennaro and Zanetti, 2000). In mammals, two important subgroups of AMPs have been described: defensins and cathelicidins. Cathelicidins are stored in cells in an unprocessed form, each comprising a highly conserved signal sequence, a sequence homologous to the cathepsin L inhibitor cathelin and a more variable bioactive C-terminus. In humans, only one cathelicidin has been identified; the unprocessed form is termed human cationic antimicrobial protein 18 (hCAP-18) and the cleaved, mature C-terminal peptide consisting of 37 amino acids is accordingly named LL-37 (Zanetti, 2004). hCAP-18 is expressed in leukocytes and epithelial cells and LL-37 is cleaved and released from the precursor through the action of serine proteinases. In physiological conditions, LL-37 assumes an alpha helical structure and displays broad antimicrobial activity, neutralizes lipopolysaccharide bioactivity (Turner et al., 1998) and acts as a chemoattractant for inflammatory and immune cells (De et al., 2000).
The functional repertoire of LL-37 is rapidly expanding and the peptide is currently implicated in multiple processes such as angiogenesis, wound healing and apoptosis (Heilborn et al., 2003; Barlow et al., 2006; Shaykhiev et al., 2008; Chamorro et al., 2009). Given their cytotoxic activity at high concentrations, LL-37 and other antimicrobial proteins have been proposed as therapeutic agents for the treatment of cancer (Mader and Hoskin, 2006). In contrast, several recent publications propose a role for LL-37 in tumor development (Heilborn et al., 2005; Coffelt and Scandurro, 2008; von Haussen et al., 2008). Cumulative data from these reports show overexpression of hCAP-18/LL-37 in breast, ovarian and lung cancer cells and that treatment with LL-37 peptide stimulates the proliferation, migration and invasion of cancer cells as well as promoting tumor growth and metastasis in animal models (Heilborn et al., 2005; Coffelt et al., 2008; von Haussen et al., 2008; Weber et al., 2009). Thus, LL-37 is suggested to act as a survival molecule released from cancer cells or from stromal cells surrounding cancer, although the mechanisms responsible for these effects are unknown. One hypothesis is that LL-37 exerts its oncogenic effects through activation of specific signaling pathways. So far, several receptor pathways have been implicated in the non-antimicrobial effects induced by LL-37. The G-protein coupled receptor, formyl peptide-receptor-like-1, is involved in stimulation of chemotaxis and angiogenesis (Koczulla et al., 2003) and the purinergic P2X7 ion channel participates in apoptosis, neutrophil recruitment and cytokine processing stimulated by LL-37 (Elssner et al., 2004). Furthermore, LL-37 was proposed to transactivate the epidermal growth factor receptor, EGFR, inducing cytokine release and cell migration (Tjabringa et al., 2003; Tokumaru et al., 2005). However, the tumorigenic effects associated with LL-37 could not be fully explained through the interaction with any of these receptors (Weber et al., 2009). Therefore, the main aim of this study was to explore additional putative receptors and pathways for LL-37.
Receptor tyrosine kinases constitute an important family of plasma membrane receptors, composed of 59 related members with similar structural and functional characteristics (Hubbard and Miller, 2007). Among them, insulin-like growth factor 1 receptor (IGF-1R) is one of the crucial players in cancer development (Adams et al., 2000; Baserga, 2000; Larsson et al., 2005). Most tumor cell types including breast, prostate and lung cancer express high amounts of IGF-1R and conditions in the tumor microenvironment, such as hypoxia, can lead to enhanced responsiveness to IGF-1 (All-Ericsson et al., 2002; LeRoith and Roberts, 2003; Ulfarsson et al., 2005; Pollak, 2008). In addition, IGF-1R confers protection against apoptosis, maintains the malignant phenotype and protects against antitumor therapy (Girnita et al., 2000a; Baserga et al., 2003; Pollak, 2008). The multiple functions of IGF-1R in cancer development coupled with its redundancy in physiological cell growth make this receptor an attractive target for cancer treatment (Girnita et al., 2000b, 2004; Baserga, 2005; Menu et al., 2006; Clemmons, 2007; Tornkvist et al., 2008; Yin et al., 2010). Owing to its ubiquitous expression pattern and its role in promoting cell growth, strategies to inhibit IGF-1R actions are being pursued for treatment of diverse conditions such as short stature, atherosclerosis and diabetes (LeRoith and Roberts, 2003; Razuvaev et al., 2007; Pollak, 2008). To date IGF-1R has only one known ligand, IGF-1 a polypeptide hormone with a high degree of structural similarity to human proinsulin.
Results
LL-37 associates with IGF-1R in vitro
First, we investigated the potential binding of LL-37 to several growth factor receptors using a sandwich enzyme-linked immunosorbent assay. Antibodies against IGF-1R, insulin receptor (IR), fibroblast growth factor receptor, platelet-derived growth factor receptor (PDGFR), Her2neu, vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) were employed to capture the corresponding receptors. Antibodies for LL-37 were used as positive and mouse and rabbit isotype immunoglobulin G as negative controls. Total protein lysate from MCF-7 cells, which are known to express these receptors, was added to the capture antibody. After the addition of LL-37, binding to respective receptors was detected using antibody against LL-37. Intriguingly, IGF-1R showed the highest affinity for LL-37 (Figure 1) and therefore we decided to investigate in detail the putative functional implications of this interaction.
Figure 1.
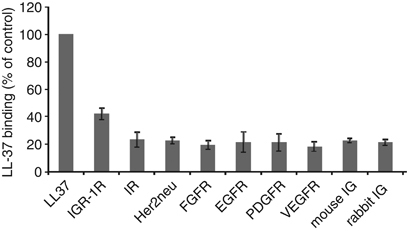
LL-37 associates with IGF-1R in vitro. A sandwich enzyme-linked immunosorbent assay, demonstrating the binding of LL-37 to different membrane receptors. Antibodies against IGF-1R, insulin receptor (IR), Her2neu, fibroblast growth factor receptor (FGFR), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor receptor (VEGFR) were employed to capture the corresponding receptors. Antibodies for LL-37 were used as positive and mouse and rabbit isotype immunoglobulin G as negative controls. Total protein lysate from MCF-7 cells, known to express these receptors, was added over the capture antibody. After the addition of LL-37, binding to receptors was detected using an antibody against LL-37, horseradish peroxidase conjugated. The reactions were quantified by measuring the absorbance and the relative LL-37 binding as percentage of LL-37 binding to the anti-LL-37 antibodies is shown. Mean values of three experiments are shown; bars, ±s.e.
LL-37 associates with IGF-1R in a cell system
We next examined whether LL-37 binds IGF-1R in a more physiological context, in living cells. In the first set of experiments, MCF-7 cells were cultured overnight in the absence of serum and then treated for 10 min with 9 μg/ml of LL-37. The cells were harvested, IGF-1R was immunoprecipitated from total protein lysates with an anti-IGF-1R antibody and the immunoprecipitate analyzed by western blot with an anti-LL-37 antibody. As shown in Figure 2a, LL-37 co-immunoprecipitated with IGF-1R in MCF-7 cells both in the presence and absence of IGF-1. To verify the specificity of the association, we used small interfering RNA (siRNA) to downregulate IGF-1R expression in MCF-7 cells. In the absence of IGF-1R, following siRNA treatment, the IGF-1R antibody failed to co-immunoprecipitate LL-37, indicating a specific binding of LL-37 to IGF-1R (Figure 2b). As an alternative experimental model, we used mouse embryonic fibroblasts with a targeted disruption of the IGF-1R (R− cells) or overexpressing IGF-1R (R+). Consistent with the above results, the IGF-1R immunoprecipitation showed a preferential binding of LL-37 to R+ over R− cells (Figure 2c). Finally, we investigated the specificity of the LL-37/IGF-1R interaction by using a pull-down assay followed by in vitro ligand-receptor interaction. IGF-1R was isolated by immunoprecipitation from R+ or MCF-7 cells and the beads were incubated for 10 min with 9 μg/ml LL-37 or with the same concentration of the 3L–7L scrambled peptide—a synthetic peptide with identical amino-acids composition as LL-37 but with a slightly different order (Figure 2c).
Figure 2.
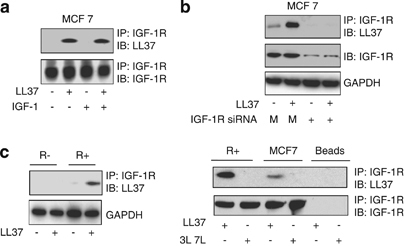
LL-37 associates with IGF-1R in a cell system. MCF-7 cells were cultured overnight in the absence of serum and then treated or not for 10 min with 9 μg/ml LL-37 in the presence or absence of the IGF-1. The cells were harvested, IGF-1R was immunoprecipitated from total protein lysates with an anti-IGF-1R antibody and the levels of LL-37 binding were assayed by western blot with an anti-LL-37 antibody. As loading control, the total levels of IGF-1R were determined using an antibody against IGF-1R (a). The IGF-1R/LL-37 association is specific: siRNA to IGF-1R impaired IGF-1R/LL-37 association. MCF-7 cells were treated with siRNA to downregulate IGF-1R expression or with non-silencing siRNA (M) for 48 h then treated or not for 10 min with 9 μg/ml LL-37. The cells were harvested, IGF-1R was immunoprecipitated from total protein lysates with an anti-IGF-1R antibody and the levels of LL-37 binding were assayed by western blot (b). The effect of IGF-1R silencing by siRNA was assayed by measuring the total IGF-1R expression in the total cell lysate; GAPDH was used as loading control (b). LL-37 binding to the IGF-1R was measured by western blot as described above in mouse embryonic fibroblast with a targeted disruption of the IGF-1R (R− cells) or overexpressing IGF-1R (R+) (c, left panel). The specificity of the LL-37/IGF-1R interaction was investigated after immunoprecipitation of the IGF-1R in MCF-7 or R+ cells followed by beads incubation for 10 min with 9 μg/ml LL-37 or with the same concentration of 3L–7L scrambled peptide. The binding of LL-37 or 3L–7L was measured by western blot as described above (c, right panel).
Taken together, these findings show that both endogenous and overexpressed IGF-1R can interact with LL-37 in living cells.
Effects of LL-37 on IGF-1R signaling
Although our data clearly demonstrates that IGF-1R and LL-37 are detected together in protein complexes, it does not address whether this interaction is functional and affects intracellular signaling. The next set of experiments was designed to test this hypothesis. One consequence of ligand (IGF-1) binding to IGF-1R is phosphorylation of a group of three tyrosine residues (Y1135, Y1131 and Y1136) within the activation loop of IGF-1R. Therefore, we used an antibody raised against IGF-1R phosphorylated at tyrosine 1131 to investigate potential activation of IGF-1R following stimulation with LL-37. MCF-7 breast cancer cells and cells overexpressing IGF-1R (R+) were incubated in the absence of serum with and without LL-37. Treatment with LL-37 showed a clear increase in IGF-1R phosphorylation in both MCF-7 and R+ cells (Figure 3a). In agreement with a functional role, LL-37-induced IGF-1R phosphorylation in a dose-dependent manner up to LL-37 concentrations of 20 μg/ml in both of these cell lines (Figure 3b). Furthermore, LL-37-dependent activation of the IGF-1R generated intracellular signaling, as demonstrated by detection of dose-dependent phosphorylation of extracellular signal-regulated kinase (ERK)1/2 in parallel to phosphorylation of IGF-1R (Figure 3b). We next investigated the time-course of ERK activation induced by LL-37 in both MCF-7 and R+ cells and compared it with ERK activation by IGF-1. Maximum activation of ERK signaling was achieved after 10-min stimulation with LL-37, as with IGF-1, with the latter being more potent in MCF-7 cells (Figure 3c). To better understand the role of LL-37 in IGF-1R signaling, we also explored the second major pathway known to be activated by IGF-1R: the phosphatidylinositol 3 kinase (PI3K)/Akt pathway. As expected, IGF-1 stimulation resulted in a time-dependent phosphorylation of Akt in MCF-7 cells whereas in contrast, LL-37 was ineffective at inducing Akt phosphorylation (Figure 3c). Finally, we investigated the specificity of the LL-37-induced ERK activation in MCF-7 cells, by using the 3L–7L scrambled peptide. As shown in Figure 3d, 10-min stimulation with IGF-1, LL-37 or serum similarly increases ERK phosphorylation whereas a slight modification of the AA sequence of the LL-37 fully abolished its ERK activation potential. Consistently, in a time-response experiment, 3L–7L could not activate ERK signaling although the cells were treated with the scrambled peptides up to 60 min (Figure 3d).
Figure 3.
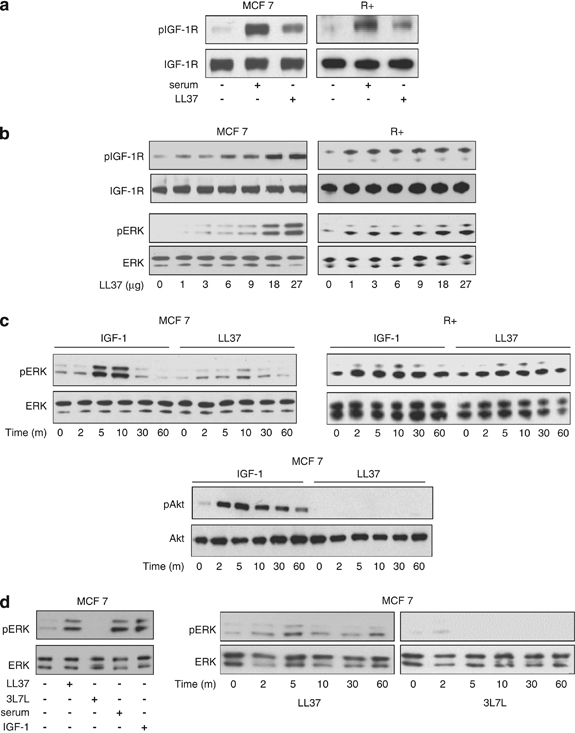
Effects of LL-37 on IGF-1R signaling. LL-37 induces IGF-1R phosphorylation. MCF-7 and R+ cells were serum-starved for 24 h and then stimulated with LL-37 or serum for 10 min. Total protein lysates were analyzed by western blot for phosphorylated IGF-1R using phospho-specific antibodies; expression of the total IGF-1R was used as loading controls (a). LL-37-induced IGF-1R phosphorylation in a dose-dependent manner. Cell lysates from MCF-7 and R+ cells stimulated with increased LL-37 concentrations were prepared as described in (a). Protein lysates were analyzed by western blot for IGF-1R phosphorylation, IGF-1R expression, ERK phosphorylation and ERK expression (b). Kinetics of LL-37-induced ERK activation. The cells were serum-depleted for 24 h and then stimulated with IGF-1 or LL-37 for 0, 2, 5, 10, 30 or 60 min. The lysates were analyzed for pERK1/2 versus ERK1/2 and pAkt versus Akt by western blotting (c). Specificity of the LL-37-induced ERK activation in MCF-7 cells. MCF-7 cells were stimulated for 10 min with IGF-1, LL-37, serum or 3 l–7 l or were stimulated for different times with the 3L–7L scrambled peptide whereupon the cell lysates were prepared as described in (a) and analyzed by western blot for ERK phosphorylation and ERK expression (d).
Taken together, these findings demonstrate that LL-37 activates mitogen-activated protein kinase (MAPK)/ERK signaling pathway through IGF-1R without affecting the PI3K/Akt pathway. This is in contrast to classical IGF-1 stimulation of the IGF-1R, which activates both pathways.
Mechanism of LL-37-induced ERK activation
So far, our data indicate that LL-37 is a partial agonist for IGF-1R as it binds and causes phosphorylation of the receptor, induces MAPK/ERK activation but does not affect the PI3K/Akt pathway. Recently, we showed that ubiquitination of the IGF-1R represents an important posttranslational modification of the IGF-1R, which together with receptor phosphorylation modulates the IGF-1R-dependent activation of intra-cellular signaling (Girnita et al., 2003; Sehat et al., 2008). In addition, we showed that β-arrestin-1 is a key protein involved in IGF-1R ubiquitination and MAPK/ERK activation and that β-arrestin-1 is able to preferentially direct IGF-1R signaling toward the MAPK/ERK pathway versus the PI3K/Akt pathway (Girnita et al., 2005, 2007). We also demonstrated that the C-terminal part of the IGF-1R is required for the preferential β-arrestin-1-dependent ERK signaling (Girnita et al., 2007; Vasilcanu et al., 2008). Therefore, as a next step we explored the mechanisms underlying LL-37-induced activation of ERK. Expression of IGF-1R is required for the activation of the MAPK/ERK pathway induced by LL-37 and IGF-1 since phosphorylated ERK was not detectable after 10-min stimulation with either agonist in cells lacking IGF-1R (R−) (Figure 4a). Treatment with serum served as a positive control demonstrating functional MAPK/ERK signaling in these cells. Notably, stable re-expression of increasing number of IGF-1R in R− cells (R12, 508 and R+ cells) restored ERK phosphorylation induced by LL-37 as well as IGF-1 (Figure 4a). In the next set of experiments, we used a range of IGF-1R mutants, with inactive functional key residues, to identify the IGF-1R domains involved in LL-37-induced MAPK/ERK activation. The following cells and conditions were used: R− cells stably transfected with mutant IGF-1Rs, (i) IGF-1R kinase defective cells (TM) where the activation domain tyrosine residues 1131, 1135 and 1136 are mutated to alanine to prevent phosphorylation of the activation loop; (ii) substrate binding defective mutants (SM) where tyrosine 950, the binding site for the two major IGF-1R substrates insulin receptor substrate (IRS) and Shc, is mutated to alanine; (iii) C-truncated mutants (CT) where the entire C-terminus of the IGF-1R is truncated after the 1245 residue and (iv) SM-CT cells that express IGF-1R with both truncated C-terminus and the Y950A substitution. In all of the tested cell lines, serum stimulation demonstrated functional MAPK/ERK signaling (Figure 4b). Interestingly, LL-37-dependent ERK activation required the presence of the IGF-1R C-terminal part of IGF-1R because Δ1245 truncated receptors failed to phosphorylate ERK in the presence of LL-37 (Figure 4b). However, the binding of LL-37 to IGF-1R was not affected by deletion of the C-terminus, as demonstrated by co-immunoprecipitation (Figure 4b, lower panel) indicating that the IGF-1R C-terminus is essential for the transmission of the LL-37 signals but not for its binding.
Figure 4.
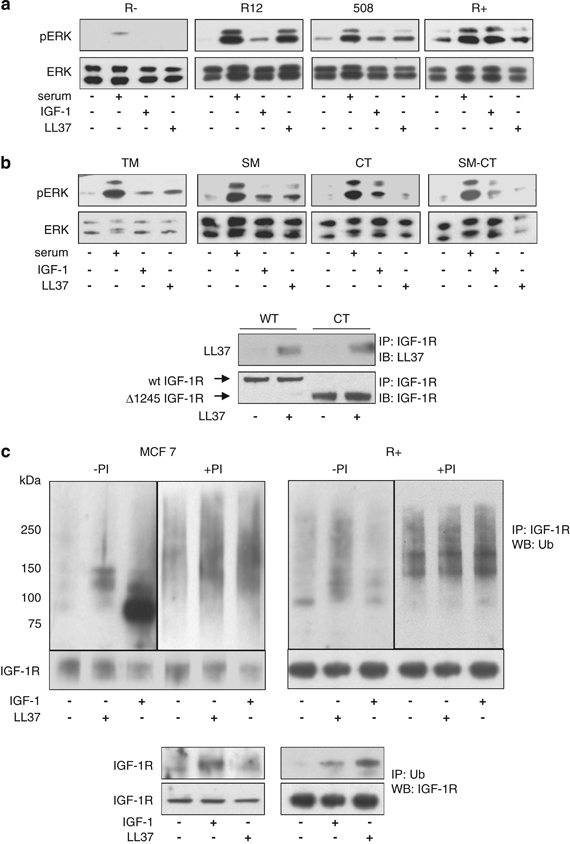
Mechanism of LL-37-induced ERK activation. Expression of IGF-1R is required for the activation of the MAPK/ERK pathway by LL-37. mouse embryonic fibroblast cells lacking IGF-1R (R−) or expressing increased numbers of human IGF-1R (R12, 508 and R+ cells) were serum-starved for 24 h and treated with serum, LL-37 or IGF-1 for 10 min and then assayed for ERK1/2 phosphorylation (a). LL-37-induced activation of ERK can occur independently of the tyrosine kinase signaling pathways TM (R− cells stably transfected with IGF-1R kinase defective triple mutant, where the activation domain tyrosine residues 1131, 1135 and 1136 are mutated to alanine), SM (R− stably transfected with IGF-1R with a substrate binding Y950 mutation to alanine), CT (R− stably transfected with IGF-1R and lacking the C-terminal), and SM-CT (R− stably transfected with IGF-1R with a Y950A mutation and lacking the C-terminal), cells were serum-depleted for 24 h and then stimulated as described in panel a (b). The LL-37 binding to the Δ1245 IGF-1R was verified by using the CT cells. Wild-type or CT cells were cultured overnight in the absence of serum and then treated or not for 10 min with 9 μg/ml LL-37. IGF-1R was immunoprecipitated from total protein lysates with an anti-IGF-1R antibody and the levels of LL-37 binding were assayed by western blot with an anti-LL-37 antibody. As a loading control, the total levels of IGF-1R were determined using an antibody against IGF-1R (b, lower panel). Effects of LL-37 stimulation on IGF-1R ubiquitination. MCF-7 and R+ cells were serum depleted for 24 h and then either untreated or treated with the proteasome inhibitor MG132 (PI) for 1 h before 10-min stimulation with IGF-1 or LL-37. IGF-1R was immunoprecipitated from the cell lysates and equal amounts of immunoprecipitates were fractionated and probed with an anti-ubiquitin antibody. Detection of the IGF-1R was used as a loading control (upper panel). IGF-1R ubiquitination in both MCF-7 and R+ cell lines was also verified by immunoprecipitating the cell lysates with an antibody to ubiquitin (P4D1) and immunoblotting with an antibody recognizing IGF-1R (lower panel) (c).
As we previously demonstrated that the C-terminus is important for β-arrestin recruitment to the IGF-1R and for IGF-1R ubiquitination (Girnita et al., 2005), we next investigated the effects of LL-37 stimulation on IGF-1R ubiquitination. MCF-7 and R+ cells were incubated in the absence of serum with the proteasome inhibitor MG132, 1 h before stimulation and harvesting. IGF-1R was immunoprecipitated from the cell lysates and equal amounts of the immunoprecipitates (Figure 4c) were fractionated and probed with an anti-ubiquitin antibody. Control cells exhibited a low level of IGF-1R ubiquitination whereas stimulation with IGF-1 or LL-37 clearly increased IGF-1R ubiquitiation (Figure 4c). In the presence of the proteasome inhibitor MG132, the level of IGF-1R ubiquitination further increased suggesting that ubiquitinated IGF-1R is targeted for degradation. The high molecular weight (90–190 kDa) ubiquitin smear is typical for the multiple forms of mono or polyubiquitinated IGF-1R (Girnita et al., 2003). We verified the LL-37-induced IGF-1R ubiquitination in both MCF-7 and R+ cell lines by using a complementary approach: immunoprecipitation with an anti-ubiquitin antibody followed by detection with anti-IGF-1R antibody (Figure 4c, lower panel).
Involvement of β-arrestin-1 in LL-37-induced IGF-1R signaling
The above set of experiments were designed to test the hypothesis that preferential routing of the LL-37 signaling through ERK pathway is dependent on the C terminus of IGF-1R and is mediated by ubiquitination of IGF-1R. On the basis of our previous findings that IGF-1R stimulation by its cognate ligand (IGF-1) recruits β-arrestin-1 to the IGF-1R to activate ERK signaling, we wanted to test whether LL-37 also uses a β-arrestin-1-dependent mechanism. MCF-7 and R+ cells were cultured overnight in the absence of serum and stimulated with IGF-1 or LL-37. Confocal microscopy was used to explore whether LL-37 mediates recruitment of β-arrestin-1 to the plasma membrane. As shown in Figure 5a, under unstimulated conditions, in both MCF-7 and R+ cell lines, endogenous β-arrestins are diffusely scattered in the cytoplasm as determined by immunostaining with an antibody that detects both β-arrestin isoforms. LL-37 stimulation clearly induced formation of β-arrestin-1 signaling complexes similar to IGF-1 (Figure 5a). The presence of IGF-1R and β-arrestin-1 in these signaling complexes was demonstrated by co-immunoprecipitation followed by western blot (Figure 5b). Intriguingly, in MCF-7 cells, the IGF-1R that co-immunoprecipitate with β-arrestin-1 after LL-37 treatment migrate slightly slower than the one that recruits β-arrestin-1 after IGF-1 stimulation, perhaps because of different ubiquitination type induced by different ligands. To test the dependency of MAPK/ERK signaling on this association, we downregulated β-arrestin-1 expression by siRNA in MCF-7 cells (Figure 5c, upper panel). In agreement with our hypothesis, ERK phosphorylation induced by LL-37 was severely impaired by downregulation of β-arrestin-1 (Figure 5c, lower panel). Taken together, these experiments demonstrate that LL-37 binding to IGF-1R activates MAPK/ERK signaling and that this process is dependent on β-arrestin-1 recruitment and IGF-1R ubiquitination.
Figure 5.
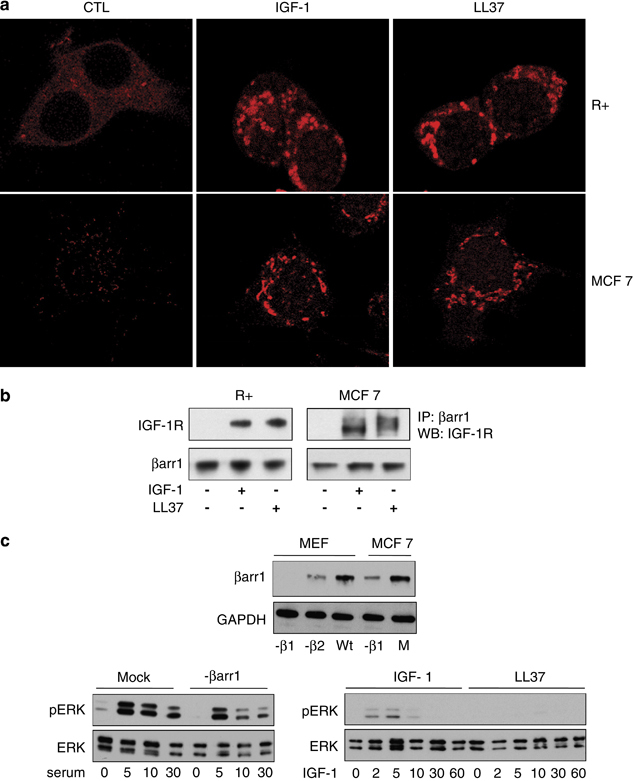
Involvement of β-arrestin-1 in LL-37-induced IGF-1R signaling. Subcellular distribution of β-arrestin-1 after LL-37 or IGF-1 stimulation. MCF-7 and R+ cells were cultured overnight in the absence of serum and then either remained unstimulated (left panels) or were stimulated with IGF-1 (50 ng/ml) for 30 min (middle panels) or LL-37 (right panels). The cells were subsequently fixed and permeabilized. Cellular distribution of β-arrestin was visualized by immunolabeling with anti-β-arrestin antibody, then an Alexa594 secondary antibody followed by confocal microscopy (a). The presence of IGF-1R and β-arrestin-1 in these signaling complexes was demonstrated by co-immunoprecipitation followed by western blot (b). Requirement of β-arrestin-1 for IGF-1-induced activation of ERK. MCF-7 cells were transfected with siRNA control (Mock) or β-arrestin-1 siRNA for 48 h. The expression of β-arrestin-1 was verified in the transfected stocks using total protein lysate of mouse embryonic fibroblast cells, negative for β-arrestin-1 or β-arrestin-2 as negative and positive controls, respectively. The cells were serum-depleted overnight and stimulated with 10% serum for 0, 5, 10 or 30 min (left panel), or IGF-1 (50 ng/ml) or LL-37 for 0, 2, 5, 10, 30 or 60 min (right panel). Cell lysates were analyzed for pERK1/2 versus ERK1/2 (c).
Biological effects of LL-37 activation of ERK signaling
So far we have demonstrated that LL-37-mediated activation of the IGF-1R results in receptor phosphorylation, β-arrestin-1 recruitment and subsequent activation of the ERK signaling pathway. In the next set of experiments, we investigated the biological effects of this activation. First, we evaluated the effects of LL-37 on cell proliferation. MCF-7 cells were serum-starved overnight and then stimulated with LL-37, IGF-1 or 10% serum for 48 h. Compared with serum-free controls, IGF-1 and serum clearly stimulated a 1.8 and 2.4-fold increase in cell number, respectively. LL-37 had a minor effect on cell proliferation (Supplementary Figure 1), suggesting that in the absence of an activated PI3K pathway, ERK activation is not sufficient to sustain cell proliferation. We next investigated the effects of LL-37 signaling activation on other cellular processes relevant to the cancer phenotype: cell migration and invasion. As a starting point, we used a classical in vitro wound-healing assay to evaluate the effects of LL-37 stimulation on cell migration. As shown in Figure 6a, in the absence of serum, the wound failed to close while serum stimulation-induced cell migration with almost complete healing of the wound after 24 h. IGF-1 and LL-37 were equipotent in inducing cell migration and gap closure of approximately 50% was observed (Figure 6a). We used the same experimental model to test whether the observed biological effect is dependent on IGF-1R, β-arrestin-1 or ERK activation. As described above, we used siRNA targeting IGF-1R or β-arrestin-1 to deplete the corresponding protein and U0126, a highly selective inhibitor of MEK 1 and MEK 2 to chemically inhibit the ERK pathway. The effects of siRNA targeting and ERK inhibition were verified by WB (Figure 6b). IGF-1R or β-arrestin-1 downregulation completely abrogated both IGF-1 and LL-37-induced migration, consistent with the observation that LL-37-activated ERK signaling is dependent on IGF-1R and β-arrestin-1 (Figure 6c and Supplementary Figure 2). Moreover, chemical inhibition of the ERK pathway had similar effects, indicating a major role of ERK signaling in cell migration (Supplementary Figure 2). In all of the above described experiments, we used serum stimulation as positive control, to verify the migratory capabilities of MCF-7 cells after various treatments. In the absence of IGF-1R, β-arrestin-1 or ERK activity, although it was impaired, MCF-7 cells still retain some migratory capabilities.
Figure 6.
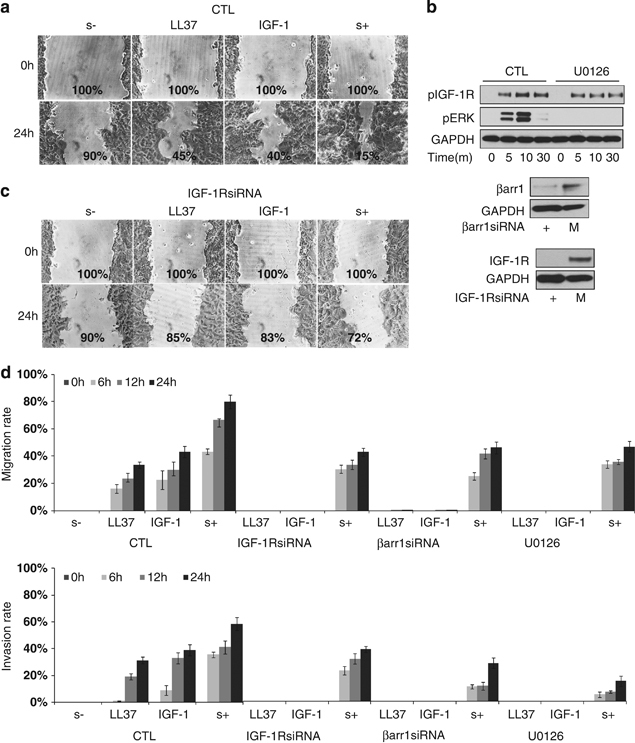
Effects of LL-37 on cell migration/invasion. The MCF-7 cells were grown to confluent monolayers and were serum-starved overnight. An artificial wound was made in the cell monolayer with a micropipette tip. After wounding, the cells were grown with IGF-1 (50 ng/ml), LL-37 (9 μg/ml) or 10% serum for 24 h. Wound closure was photographed and quantified by image analysis software and the efficiency of wound healing was calculated as percentage compared with starting point (a). MCF-7 cells were transfected with β-arrestin-1 siRNA or IGF-1R siRNA for 48 h. MEK inhibitor (U0126) was added to MCF-7 at a final concentration of 10 μM, 30 min before stimulation. The efficiency of siRNA targeting and MEK inhibitor was detected by WB (b) The effect of IGF-1R downregulation on serum, IGF-1 or LL-37-induced wound healing was investigated as describe above (c). Effect of LL-37 on cell migration and invasion. BD BioCoat Tumor Invasion System chambers were used to investigate MCF-7 cell migration (d, upper panel) and Matrigel invasion (d, lower panel) using LL-37 (9 μg/ml), IGF-1 (50 ng/ml) or serum 15%) as a chemoattractant for indicated times. MCF-7 cells were transfected or not with β-arrestin-1 siRNA or IGF-1R siRNA or treated with MEK inhibitor (U0126) as described in (b). The migration rate (d, upper panel) was calculated as the percentage of the migrating cells from the total cells added at the start of the experiment on the upper chamber. The invasion rate (d, lower panel) was calculated as % of cells that invaded through the Matrigel membrane divided by the migration rate. Each measurement was performed in triplicate. Columns, mean of three experiments; bars, s.d.
To further evaluate the effects of LL-37 on migration of MCF-7, we made use of a modified Boyden chamber assay (BD FluoroBlok cell culture inserts; BD Biosciences-Europe, Erembodegem, Belgium) in which the top and bottom compartments are separated by a light-tight polyethylene terephthalate (PET) membrane that efficiently blocks the transmission of light within the range of 490–700 nm, allowing detection of cells by fluorescent stain in a simplified and non-destructive manner. Once labeled, cells migrate through the membrane; they are easily detected by a bottom-reading fluorescence plate reader thus supporting kinetic migration and invasion assays. The MCF-7 cells were fluorescently labeled with BD Dilc12, before placing them on the top chamber, using either serum, LL-37 or IGF-1 as a chemoattractant in the lower chamber. Figure 6d upper panel, shows that after 24 h, almost 80% of serum-stimulated cells had migrated through the membrane, while in the negative control (serum free) no cells migrated. In the presence of IGF-1 or LL-37 about 40% of the cells migrated. These results imply that LL-37 efficiently stimulates cell migration along an LL-37 gradient in a time-dependent manner. By adding a reconstituted basement membrane (Matrigel) onto the pored membrane, we repeated the experiment to study the effect of LL-37 on basement membrane invasion. Of the serum-stimulated control cells that migrate, 55–60% passed through the basement membrane after 24-h incubation, while 30% of the LL-37 or 40% of IGF-1 stimulated, migrating cells, invaded through the Matrigel (Figure 6d, lower panel). Once more we tested the dependency of the observed migratory and invasive phenotype on IGF-1R, β-arrestin-1 and ERK activity as described above. As shown in Figure 6d, inhibition of IGF-1R, β-arrestin-1 or ERK completely abrogated the LL-37 and IGF-1-induced migration/invasion of MCF-7 cells.
Effects of LL-37 on various cell lines
To investigate if IGF-1R-mediated LL-37 signaling is cell-type dependent, we made use of a panel of malignant cell lines expressing IGF-1R including lung carcinoma (H1299), colon carcinoma (HCT116) and breast cancer (MCF-7 and ZR75-1). As shown in Figure 7, LL-37-induced ERK phosphorylation in all cell lines tested, regardless of their origin.
Figure 7.
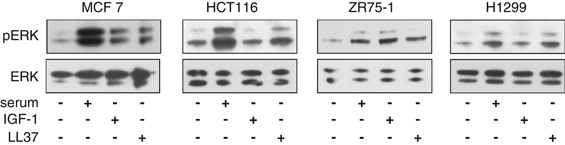
Effects of LL-37 on various cancer cell lines. A panel of malignant cell lines expressing IGF-1R including lung carcinoma (H1299), colon carcinoma (HCT116) and breast cancer (MCF-7 and ZR75-1) were serum-depleted for 24 h and then stimulated with 10% serum, IGF-1 (50 ng/ml) or LL-37 for 10 min. The lysates were analyzed for pERK1/2 versus ERK1/2 by western blotting.
Discussion
The purpose of this study was to investigate the potential binding and agonistic activity of the pro-inflammatory peptide LL-37 on different growth factors receptors. We found that LL-37 binds IGF-1R and behaves as a partial agonist, leading to receptor phosphorylation and ubiquitination and robust β-arrestin-1-dependent activation of the MAPK/ERK pathway. In contrast, stimulation of IGF-1R by LL-37 did not affect PI3K activity suggesting that LL-37 is a natural partial agonist for IGF-1R.
Epidemiological, clinical and experimental research indicates that the IGF-1R signaling pathway has a significant impact on the development and progression of cancer. Aberrant activation and/or overexpression of IGF-1R are associated with an aggressive phenotype, drug resistance and poor outcome in several tumors types including melanoma, breast, lung and prostate cancer (All-Ericsson et al., 2002; LeRoith and Roberts, 2003; Larsson et al., 2005). Multiple reports show a clear relationship between IGF-1 or IGF-binding proteins levels and cancer risk, tumor promotion, progression and outcome (LeRoith and Roberts, 2003; Baserga, 2005). IGF-1R has also been implicated in metastasis and was shown to interfere with expression of integrins, binding of extracellular matrix proteins and activity of matrix metalloproteinase 2 (Girnita et al., 2006; Samani et al., 2007), all of which are involved in invasion and metastasis.
Phosphorylation was previously thought to be the central process governing IGF-1R signaling because the activated IGF-1R tyrosine kinase in turn phosphorylates substrates including IRS-1/2 and Shc, leading to tumor transformation, overgrowth and metastasis (LeRoith and Roberts, 2003; Larsson et al., 2005). However, during the past decade, along with others, we have challenged this view by demonstrating that besides phosphorylation, ubiquitination is of critical importance for the downstream signaling of IGF-1R (Girnita et al., 2000a, 2003). Recently, we provided evidence that β-arrestins, otherwise known to be involved in the regulation of G protein-coupled receptors, serve as adaptors for the ubiquitination process, connecting the oncoprotein MDM2 to the IGF-1R (Girnita et al., 2005, 2007). In the case of G protein-coupled receptors, β-arrestins were initially discovered as negative regulators of G protein-mediated signaling by seven transmembrane receptors (7TMRs) (DeWire et al., 2007; Rajagopal et al., 2010), although they were later shown to be activators of cell signaling in their own rights (DeWire et al., 2007; Rajagopal et al., 2010). Similarly, in the case of IGF-1R, β-arrestins were demonstrated to have a similar dual role: receptor downregulation and activation of signaling (Girnita et al., 2005, 2007).
On the basis of the evidence provided in the present paper, we propose a novel concept of biased signaling of the IGF-1R. This concept is equivalent to the accepted model of β-arrestin biased signaling for G protein-coupled receptors: ligand binding to a G protein-coupled receptor activates in a balanced manner both G protein and β-arrestin-mediated pathways. Yet there are numerous reports on biased signaling, where signaling is mediated selectively through only one of these two pathways either by a biased agonist or by a biased receptor (Violin and Lefkowitz, 2007). Analogous to this model, we can consider two alternative signaling models for IGF-1R: (1) the classical pathway, originating on the substrate-binding site of the receptor (Y950), where IGF-I binding to IGF-1R results in receptor phosphorylation, increased kinase activity of the receptor and subsequent auto-phosphorylation of the tyrosine residues within IGF-1R; this pathway requires the substrate-binding site Y950. (2) The β-arrestin-1-mediated pathway requiring the C-terminus of IGF-1R being highly dependent on or resulting in IGF-1R ubiquitination. The natural IGF-1R agonist, IGF-1, is a balanced agonist, typically activating both Y950 and C-terminus (β-arrestin-1) signaling. In this study, by using biased receptors, in which the IRS/Shc and β-arrestin signaling pathways are dissociated by mutation of the Y950, involved in IRS and Shc binding or truncation of the C-terminus (β-arrestin-binding), we show that LL-37 activates MAPK/ERK pathways predominantly through the C-terminus signaling pathway.
Our current model, in which LL-37 is a biased agonist for IGF-1R capable of signaling mainly through β-arrestin, provides evidence for the concept of biased IGF-1R signaling and highlights the existence of natural biased agonists for tyrosine kinase receptors. To our knowledge, this is the first study reporting a natural biased agonist for a receptor tyrosine kinase.
It is generally accepted that cancer cells make use of physiologic signals for proliferation and/or antiapoptosis to gain a growth advantage over normal cells. Besides growth factors, such selective signals are generated by the interaction of the ‘initiated’ cells with the surrounding tissue, including the adjacent inflammatory process and are transmitted by plasma membrane receptors. The notion that inflammation is a critical component of tumor development and progression has long-been appreciated (Balkwill and Mantovani, 2001; Coussens and Werb, 2002). Based primarily on epidemiological evidence, the major role of inflammation in cancer development is now fully recognized. Although the underlying molecular mechanisms are not completely understood the present study reveals a new and unexpected putative mechanism: activation of the highly cancer relevant IGF-1R, by the proinflammatory mediator, LL-37. A potential role for LL-37, and its pro-form, hCAP-18, in cancer development and progression is being debated. hCAP-18/LL-37 is strongly expressed in several human malignancies including breast (Heilborn et al., 2005), lung (von Haussen et al., 2008) and ovarian cancer (Coffelt et al., 2008). Overexpression of the peptide in experimental cancers was associated with increased growth and metastasis in animal models and treatment with neutralizing anti-LL-37 antibodies suppressed the growth of tumors in ovarian cancer, suggesting a tumor-promoting effect from LL-37 (Coffelt et al., 2008). LL-37 displays several biological activities that could explain these effects: LL-37 is reported to stimulate angiogenesis (Koczulla et al., 2003) suppress apoptosis (Chamorro et al., 2009), upregulate matrix metalloproteinases (Coffelt et al., 2008) and was recently shown to recruit multipotent mesenchymal stromal cells to the tumor environment (Coffelt et al., 2009).
It is generally accepted that IGF-1R is a critical regulator of cancer cell migration and proliferation (Baserga et al., 2003; Samani et al., 2007). Yet, these two cellular responses are mutually exclusive so at a given time a cell can either migrate or divide. This apparent contradiction could be explained by the fact that IGF-1R induces different and even opposite cellular responses through differential activation of intracellular signaling pathways (Baserga, 2000). In this study, we found that LL-37 preferentially stimulates the MAPK/ERK signaling with no detectable PI3K/Akt activation. This restricted signaling could not sustain cell proliferation. This is not surprising because the essential role of PI3K/Akt signaling for the IGF-1R mitogenic activity has long been appreciated (Dufourny et al., 1997; Adams et al., 2000; Baserga, 2009). Nevertheless, our results strongly suggest that LL-37 is a potent mediator of migration and invasion in an IGF-1R-dependent manner, validating the role of IGF-1R in altering integrin signaling as well as affecting the activity of extracellular matrix proteases (Doerr and Jones, 1996; Dunn et al., 1998, 2001; Girnita et al., 2006; Samani et al., 2007).
In summary, we demonstrate that LL-37 (i) forms a complex together with the IGF-1R as shown by direct binding in vitro and co-immunoprecipitation experiments in cell system (ii) this complex requires the presence of both IGF-1R and LL-37 as shown by siRNA and R+/R− experiments and (iii) this binding results in IGF-1R activation as demonstrated by IGF-1R phosphorylation and ubiquitination. Moreover, our results strongly support the concept of β-arrestin-dependent activation of MAPK/ERK signaling by LL-37 through IGF-1R, as shown by β-arrestin recruitment to the IGF-1R following LL-37 stimulation, absence of signaling and biological effects in β-arrestin-deficient cells or in cells expressing mutant IGF-1R defective in β-arrestin binding. In this study, demonstrating for the first time a direct interaction between LL-37 and the IGF-1R pathway, we reveal a mechanism whereby LL-37 may promote essential processes of cancer metastasis like cell migration and invasion. Our present data add substantial evidence to the novel hypothesis that LL-37, in addition to its antimicrobial and immunological functions may be an attractive target for cancer therapy.
Materials and methods
Reagents
LL-37 (purity of 98%) was obtained from Polypeptide Laboratories, Hilleröd, Denmark. Monoclonal antibodies to phosphotyrosine (PY99), ubiquitin (P4D1) and polyclonal antibodies against IGF-1R (H-60), GAPDH (FL-335), β-arrestin-1 (K-16), PDGFR (C-20), VEGFR, mouse immunoglobulin G, rabbit immunoglobulin G and IR-α were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Polyclonal antibodies against pERK1/2, ERK1/2, pAKT (serine 473) and AKT were purchased from Cell Signaling Technology (Danvers, MA, USA), as well as IGF-1R and pIGF-1R (Tyr1131). EGFR, HER2/ErbB2 and fibroblast growth factor receptor antibodies were from Cell Signaling Technology. The protein G sepharose was purchased from GE healthcare (Uppsala, Sweden). Dynabeads protein G was from Invitrogen (Carlsbad, CA, USA). Rabbit polyclonal anti-β-arrestin-1 antibody (A1CT) was a kind gift from Robert Lefkowitz (Duke University, Durham, NC, USA) and was previously described (Attramadal et al., 1992). U0126 (1,4-diamino-2,3-dicyano-1,4-bis (2-aminophe-nylthio butadiene)) (Calbiochem, Nottingham, UK) was dissolved in dimethylsulphoxide and used at a final concentration of 10 μM. Other reagents unless stated otherwise were from Sigma (St Louis, MO, USA).
Cell cultures
MCF-7 and ZR75-1 human breast cancer cell lines, H1299 human lung adenocarcinoma and HCT116 human colon carcinoma cell lines were obtained from ATCC (via LGC Promochem, Boras, Sweden). The MCF-7 and ZR75-1 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. H1299 and HCT116 were cultured in Iscove's modification of Dulbecco's medium supplemented 10% fetal bovine serum and 1% penicillin–streptomycin.
The R−, R+, R12, R508, 46, 48, 56 and 96 mouse cell lines were a generous gift from Dr Renato Baserga (Thomas Jefferson University, Philadelphia, PA, USA). The R− cells are mouse embryonic fibroblast with targeted disruption of the IGF-1R gene (Sell et al., 1994). All other cell lines were created following stable transfection of the R− cells with wild-type human IGF-IR (R+, R12 and R508) or various mutants of the human IGF-1R as previously described (Dews et al., 2000). The following mutants were used: IGF-1R kinase defective cells 48 (TM) where the activation domain tyrosine residues 1131, 1135 and 1136 are mutated to alanine to prevent phosphorylation of the activation loop; substrate-binding defective mutants 46 (SM) where tyrosine 950, the binding site for the two major IGF-1R substrates IRS and Shc, is mutated to alanine; C-truncated mutants 56(Δ1245) where the entire C-terminus of the IGF-1R is truncated after the 1245 residue; SM-Δ1245 96 cells, which express IGF-1R with both truncated C-terminus and the Y950A substitution. R12 cells express about 7000 receptors per cell while R508 expresses 17 000 molecules of IGF-IR per cell. The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum.
All cells were maintained in a humidified 5% CO2 atmosphere at 37 °C.
Small interfering RNAs
Chemically synthesized, double-strand siRNAs were purchased from Dharmacon (Pierce, Rockford, IL, USA). The siRNA sequence that was used to deplete endogenous β-arrestin-1 levels in MCF-7 cells was 5′-AAAGCCUUCUGUGCUGAGAAC-3′ (Ahn et al., 2003). A non-silencing RNA duplex (5′-AAUUCUCCGAACGUGUCACGU-3′), as the manufacturer indicated, was used as a control. The siRNA targeting human IGF-1R sequence 5′-GCAGACACCUACAACAUCAUU-3′ (Natalishvili et al., 2009) was used to deplete endogenous IGF-1R levels in MCF-7 cells. The cells were transfected at 40–50% confluency, in a 25-cm2 flask, using the DharmaFECT transfection reagent (Pierce) according to the manufacturer's instructions. After transfection, the cells were incubated for 24 h at 37 °C and then the media was replaced with serum containing growth media. After additional incubation for 24 h, cells were trypsinized and used for further experiments.
Immunoprecipitation
Cells were cultured to subconfluency in six-well plates. After indicated treatments, cells were lysed in phosphate-buffered saline (PBS) with 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (PBS-TDS) as described previously (Girnita et al., 2003). The protein concentration was determined by the bicinchoninic acid assay (BCA protein assay kit, Pierce). Dynabeads protein G (15 μl) (Invitrogen) or protein A/G—sepharose (15 μl) (GE) and 1 μg of antibody was added to 500 μg protein material. After overnight incubation at 4 °C on a rotator platform, the immunoprecipitate was collected, the supernatant discarded, the pellet was washed and then dissolved in a sample buffer for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE).
SDS–PAGE and western blotting
Protein samples were dissolved in lithium dodecyl sulfate (LDS) sample buffer (Invitrogen). Samples corresponding to 5–50 μg of cell protein were analyzed by SDS–PAGE with a 4–12% gradient separation gel. Molecular weight markers were run simultaneously. Following SDS–PAGE, the proteins were transferred for one hour to nitrocellulose membrane (Amersham Biosciences, Uppsala, Sweden) and then incubated for 1 h at room temperature in 5% (w/v) skimmed milk powder in 0.02% (w/v) Tween 20, PBS, pH 7.5. Incubation with appropriate primary antibody was performed for 1 h at room temperature. This was followed by washes with PBS and incubation with either a horseradish peroxidase-conjugated or biotinylated secondary antibody (Amersham Biosciences) for 1 h at room temperature. Following incubation with biotinylated secondary antibody, incubation with streptavidin-conjugated horseradish peroxidase was performed. Detection was made with ECL (Pierce). The films were scanned and quantified by Fluor-S imager (Bio-Rad, Hercules, CA, USA).
Receptor-binding assay
Binding of LL-37 to different membrane receptors was assayed using a sandwich enzyme-linked immunosorbent assay method as previously described (Girnita et al., 2004). Briefly, the capture antibody directed against growth factor receptor to be investigated, was bound to a solid phase in a polyvinylchloride microtiter plate. After washing and blocking unspecific binding sites with 2% bovine serum albumin in PBS, the antigen (total protein lysate of MCF-7 cells) was added and allowed to complex with the bound antibody. Unbound proteins were then removed by three washes, after which LL-37 was added to the plate followed by additional washes. The detection antibody, a peroxidase labeled antibody against LL-37 was allowed to bind the antigen, thus completing the sandwich. The assay was then quantified by assessing the amount of labeled secondary antibody bound to the matrix, through the use of a colorimetric substrate reaction and spectrophotometry (enzyme-linked immunosorbent assay reader). The results were calculated as percentage of positive control (LL-37 bound to the anti-LL-37 capturing antibodies).
Pull down assay and in vitro binding
IGF-1R was extracted from P6 or MCF-7 cells by immunoprecipitation using Dynabeads protein G. After washing with PBS, the pellet was incubated with 9 μg/ml LL-37 or the same amount of peptide containing the scrambled amino acids of LL-37 (3L–7L) in protein-binding buffer, overnight at 4 °C on a rotator platform. The beads and attached proteins were separated by magnetic field and washed three times with 0.1% Tween 20 in protein-binding buffer and the pellet was dissolved in lithium dodecyl sulfate sample buffer for analysis by SDS–PAGE.
Immunofluorescence MCF-7 and P6 cells were plated on collagen-coated 35-mm glass bottom dishes (Wilco Wells, Amsterdam, The Netherland) and serum starved for 8 h before stimulating with IGF-1 (50 ng/ml) or LL-37 (9 μg/ml) for 10 min. After washing three times with PBS, the cells were fixed with 4% paraformaldehyde diluted in PBS containing calcium and magnesium before confocal analysis. For immunostaining endogenously expressed β-arrestins, polyclonal β-arrestin-1/2 antibody (A1CT), and anti-rabbit ALEXA 594 (Invitrogen) were used as primary and secondary antibodies, respectively.
Cell proliferation assay
Cell proliferation was assessed using the Cell Proliferation kit II (XTT) (Roche, Mannheim, Germany) according to the manufacturer's instructions.
Wound-healing assay
The MCF-7 cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum at 37 °C and 5% CO2 to form confluent monolayers in 24-well plates and were serum-starved overnight. An artificial wound was made in the cell monolayer with a sterile plastic 10 μl micropipette tip. After wounding, the culture medium was removed and the cells washed twice with serum-free medium to remove detached cells. The cells were grown with IGF-1 (50 ng/ml), LL-37 (9 μg/ml) or 10% serum for 24 h. Wound closure was photographed at the same spot, using an inverted microscope equipped with a digital camera. The quantification of the wound closure was made using VisiCam 5.0 image analysis software (VWR, Stockholm, Sweden).
In vitro migration and invasion
The migratory/invasive potential of the cells was tested using BD BioCoat Tumor Invasion System (BD Biosciences-Europe). It consists of a BD Falcon FluoroBlok 24-Multiwell Insert Plate with an 8-μm pore size PET membrane coated with Matrigel—for invasion or uncoated—for migration. Cells were stained with Dilc12 for 2 h and seeded at 5 × 104 cells in 500 μl of serum-free Dulbecco's modified Eagle's medium onto the apical chamber. In all, 750 μl medium with IGF-1 (50 ng/ml), LL37 (9 μg/ml) or 15% serum was added to the bottom chamber for migration or both top and bottom chambers for invasion. Equal seeding was verified by measuring the top-fluorescence. The plates were incubated at 37 °C in a humidified 5% CO2 atmosphere for 24 h. During incubation, the fluorescence of the invaded cells on the bottom side of the membrane was measured using a Tecan Infinite M1000 plate reader (Tecan Group Ltd., Männedorf, Switzerland) at different time points. For migration, the data were calculated as the percentage of the migrating (bottom) cells of the total cells added at the start of the experiment. The invasion rate was calculated as number of cells that invaded through the Matrigel membrane divided by the number of cells that migrated through an uncoated membrane. Each measurement was performed in triplicate.
Experimental reproducibility
All experiments were repeated at least three times with consistent results.
Supplementary information
Acknowledgements
This work was supported by grants funded by the Swedish Cancer Society, Swedish Medical Council, Children Cancer Society, Welander Finsen Foundation, King Gustaf V Jubilee Foundation, Vinnova, Stockholm Cancer Society, the Stockholm County, and the Karolinska Institute. Dr Renato Baserga is greatly acknowledged for providing us with IGF-1R positive and negative cell lines as well as IGF-1R mutants' cells.
Competing interests
Alvar Gronberg is a scientist and also an employee of Lipopeptide AB. Lipopeptide AB gave no financial support to this work and has denied rights regarding the work presented in this paper. Mona Ståhle is inventor of a patent regarding the use of LL-37 for wound healing. Mona Ståhle has received compensation as a speaker at sponsored symposia and as member of the scientific advisory boards of Pfizer and Abbott and Janssen Cilag. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41388-022-02567-5"
Change history
12/9/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41388-022-02567-5
References
- Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57:1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- All-Ericsson C, Girnita L, Seregard S, Bartolazzi A, Jager MJ, Larsson O. Insulin-like growth factor-1 receptor in uveal melanoma: a predictor for metastatic disease and a potential therapeutic target. Invest Ophthalmol Vis Sci. 2002;43:1–8. [PubMed] [Google Scholar]
- Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267:17882–17890. doi: 10.1016/S0021-9258(19)37125-X. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Barlow PG, Li Y, Wilkinson TS, Bowdish DM, Lau YE, Cosseau C. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- Baserga R. The insulin-like growth factor-I receptor as a target for cancer therapy. Expert Opin Ther Targets. 2005;9:753–768. doi: 10.1517/14728222.9.4.753. [DOI] [PubMed] [Google Scholar]
- Baserga R. The insulin receptor substrate-1: a biomarker for cancer? Exp Cell Res. 2009;315:727–732. doi: 10.1016/j.yexcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- Chamorro CI, Weber G, Gronberg A, Pivarcsi A, Stahle M. The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J Invest Dermatol. 2009;129:937–944. doi: 10.1038/jid.2008.321. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci USA. 2009;106:3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Scandurro AB. Tumors sound the alarmin(s) Cancer Res. 2008;68:6482–6485. doi: 10.1158/0008-5472.CAN-08-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Waterman RS, Florez L, Honer zu Bentrup K, Zwezdaryk KJ, Tomchuck SL. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–1039. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Dews M, Prisco M, Peruzzi F, Romano G, Morrione A, Baserga R. Domains of the insulin-like growth factor I receptor required for the activation of extracellular signal-regulated kinases. Endocrinology. 2000;141:1289–1300. doi: 10.1210/endo.141.4.7414. [DOI] [PubMed] [Google Scholar]
- Doerr ME, Jones JI. The roles of integrins and extracellular matrix proteins in the insulin-like growth factor I-stimulated chemotaxis of human breast cancer cells. J Biol Chem. 1996;271:2443–2447. doi: 10.1074/jbc.271.5.2443. [DOI] [PubMed] [Google Scholar]
- Dufourny B, Alblas J, van Teeffelen HA, van Schaik FM, van der Burg B, Steenbergh PH. Mitogenic signaling of insulin-like growth factor I in MCF-7 human breast cancer cells requires phosphatidylinositol 3-kinase and is independent of mitogen-activated protein kinase. J Biol Chem. 1997;272:31163–31171. doi: 10.1074/jbc.272.49.31163. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Ehrlich M, Sharp NJ, Reiss K, Solomon G, Hawkins R. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 1998;58:3353–3361. [PubMed] [Google Scholar]
- Dunn SE, Torres JV, Oh JS, Cykert DM, Barrett JC. Up-regulation of urokinase-type plasminogen activator by insulin-like growth factor-I depends upon phosphatidylinositol-3 kinase and mitogen-activated protein kinase kinase. Cancer Res. 2001;61:1367–1374. [PubMed] [Google Scholar]
- Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- Gennaro R, Zanetti M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers. 2000;55:31–49. doi: 10.1002/1097-0282(2000)55:1<31::AID-BIP40>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Girnita A, All-Ericsson C, Economou MA, Astrom K, Axelson M, Seregard S. The insulin-like growth factor-I receptor inhibitor picropodophyllin causes tumor regression and attenuates mechanisms involved in invasion of uveal melanoma cells. Clin Cancer Res. 2006;12:1383–1391. doi: 10.1158/1078-0432.CCR-05-1106. [DOI] [PubMed] [Google Scholar]
- Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–242. doi: 10.1158/0008-5472.CAN-03-2522. [DOI] [PubMed] [Google Scholar]
- Girnita L, Girnita A, Brodin B, Xie Y, Nilsson G, Dricu A. Increased expression of insulin-like growth factor I receptor in malignant cells expressing aberrant p53: functional impact. Cancer Res. 2000;60:5278–5283. [PubMed] [Google Scholar]
- Girnita L, Girnita A, Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA. 2003;100:8247–8252. doi: 10.1073/pnas.1431613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Girnita A, Lefkowitz RJ. [beta]-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem. 2005;280:24412–24419. doi: 10.1074/jbc.M501129200. [DOI] [PubMed] [Google Scholar]
- Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Vasilcanu D, Girnita A. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem. 2007;282:11329–11338. doi: 10.1074/jbc.M611526200. [DOI] [PubMed] [Google Scholar]
- Girnita L, Wang M, Xie Y, Nilsson G, Dricu A, Wejde J. Inhibition of N-linked glycosylation down-regulates insulin-like growth factor-1 receptor at the cell surface and kills Ewing's sarcoma cells: therapeutic implications. Anticancer Drug Des. 2000;15:67–72. [PubMed] [Google Scholar]
- Heilborn JD, Nilsson MF, Jimenez CI, Sandstedt B, Borregaard N, Tham E. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–719. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Miller WT. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007;19:117–123. doi: 10.1016/j.ceb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92:2097–2101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/S0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Mader JS, Hoskin DW. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs. 2006;15:933–946. doi: 10.1517/13543784.15.8.933. [DOI] [PubMed] [Google Scholar]
- Menu E, Jernberg-Wiklund H, Stromberg T, De Raeve H, Girnita L, Larsson O. Inhibiting the IGF-1 receptor tyrosine kinase with the cyclolignan PPP: an in vitro and in vivo study in the 5T33MM mouse model. Blood. 2006;107:655–660. doi: 10.1182/blood-2005-01-0293. [DOI] [PubMed] [Google Scholar]
- Natalishvili N, Axelson M, Girnita L, Larsson O, Vasilcanu D. Aberrant intracellular IGF-1R beta-subunit makes receptor knockout cells (IGF1R-/-) susceptible to oncogenic transformation. Exp Cell Res. 2009;315:1458–1467. doi: 10.1016/j.yexcr.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol. 2007;29:27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razuvaev A, Henderson B, Girnita L, Larsson O, Axelson M, Hedin U. The cyclolignan picropodophyllin attenuates intimal hyperplasia after rat carotid balloon injury by blocking insulin-like growth factor-1 receptor signaling. J Vasc Surg. 2007;46:108–115. doi: 10.1016/j.jvs.2007.02.066. [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- Sehat B, Andersson S, Girnita L, Larsson O. Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis. Cancer Res. 2008;68:5669–5677. doi: 10.1158/0008-5472.CAN-07-6364. [DOI] [PubMed] [Google Scholar]
- Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykhiev R, Behr J, Bals R. Microbial patterns signaling via Toll-like receptors 2 and 5 contribute to epithelial repair, growth and survival. PLoS One. 2008;3:e1393. doi: 10.1371/journal.pone.0001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- Tornkvist M, Natalishvili N, Xie Y, Girnita A, D'Arcy P, Brodin B. Differential roles of SS18-SSX fusion gene and insulin-like growth factor-1 receptor in synovial sarcoma cell growth. Biochem Biophys Res Commun. 2008;368:793–800. doi: 10.1016/j.bbrc.2008.01.162. [DOI] [PubMed] [Google Scholar]
- Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/AAC.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfarsson E, Karstrom A, Yin S, Girnita A, Vasilcanu D, Thoren M. Expression and growth dependency of the insulin-like growth factor I receptor in craniopharyngioma cells: a novel therapeutic approach. Clin Cancer Res. 2005;11:4674–4680. doi: 10.1158/1078-0432.CCR-05-0129. [DOI] [PubMed] [Google Scholar]
- Vasilcanu R, Vasilcanu D, Sehat B, Yin S, Girnita A, Axelson M. Insulin-like growth factor type-I receptor-dependent phosphorylation of extracellular signal-regulated kinase 1/2 but not Akt (protein kinase B) can be induced by picropodophyllin. Mol Pharmacol. 2008;73:930–939. doi: 10.1124/mol.107.040014. [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- von Haussen J, Koczulla R, Shaykhiev R, Herr C, Pinkenburg O, Reimer D. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Weber G, Chamorro CI, Granath F, Liljegren A, Zreika S, Saidak Z. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. 2009;11:R6. doi: 10.1186/bcr2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin SC, Girnita A, Stromberg T, Khan Z, Andersson S, Zheng HY. Targeting the insulin-like growth factor-1 receptor by picropodophyllin as a treatment option for glioblastoma. Neuro-Oncology. 2010;12:19–27. doi: 10.1093/neuonc/nop008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


