Abstract
T cell subset specific migration to inflammatory sites is tightly regulated and involves interaction of the T cells with the endothelium. T helper 17 (Th17) cells often appear at different inflammatory sites than T helper 1 (Th1) cells, or both subsets appear at the same sites but at different times. Differences in T cell subset adhesion to endothelium may contribute to subset-specific migratory behavior, but this possibility has not been well studied. We examined the adhesion of mouse Th17 cells to endothelial adhesion molecules and endothelium under flow in vitro and microvessels in vivo, and characterized their migratory phenotype by flow cytometry and qRT-PCR. More Th17 than Th1 cells interacted with E-selectin. Fewer Th17 than Th1 cells bound to TNF-α activated E-selectin deficient endothelial cells, and intravital microscopy studies demonstrated that Th17 cells engage in more rolling interactions with TNF-α treated microvessels than Th1 cells in wild type mice but not in E-selectin deficient mice. Th17 adhesion to ICAM-1 was dependent on integrin activation by CCL20, the ligand for CCR6, which is highly expressed by Th17 cells. In an air pouch model of inflammation, CCL20 triggered recruitment of Th17 but not Th1 cells. These data provide evidence that E-selectin and ICAM-1 dependent adhesion of Th17 and Th1 cells with endothelium are quantitatively different.
Introduction
Different subsets of CD4+ helper T cells (Th) play distinct roles in protective immunity and immune-mediated diseases (1). Th1 cells secrete IFN-γ, protect against intracellular infections, and are implicated in the pathogenesis of granulomatous responses to infections and in various organ-specific autoimmune diseases. T helper type 2 (Th2) cells secrete IL-4, -5, and -13, protect against helminthes, and Th2 responses to environmental antigens underlie the pathogenesis of allergic diseases. In addition to the distinct cytokines produced by Th1 and Th2 cells, these two subsets have different migratory properties. Th17 cells, which were discovered more recently (2,3) secrete IL-17A and IL-17-F, IL-22 and IL-21 (2,4,5). Th17 cells participate in several human immune/inflammatory diseases, such as multiple sclerosis, inflammatory bowel disease (IBD), rheumatoid arthritis and psoriasis, as well as in different animal models of autoimmune/inflammatory diseases (3,5–8). The critical roles of Th17 cells during the immune response against extracellular bacteria and fungi have also been demonstrated (9). The selective recruitment of a particular Th subset is tightly regulated and depends on the type of inflammatory response and the expression of adhesion molecules on both the vascular endothelium and the T cells (10,11). Adhesive interactions between specific ligands expressed on the T cell surface with their respective adhesion molecule expressed on the vascular endothelium, in combination with appropriate chemokines, are believed to underlie selective Th subset recruitment. Interestingly, Th1 and Th2 cells have different migratory phenotypes. In general, Th1 cells express more functional E- and P-selectin ligands (12–14) and have higher levels of the chemokine receptors CXCR3 and CCR5, compared to Th2 cells. In contrast, Th2 cells may express more CCR3, CCR4 and CCR8 (15). These differences are believed to support different patterns of migration of Th1 and Th2 cells. In contrast, the mechanisms underlying recruitment of Th17 cells remain less well characterized.
Emerging data on the dominance of Th17 versus Th1 cells in certain organ-specific autoimmune diseases raise the question of whether selective migration of Th17 cells occurs. Flow cytometry analyses have shown that human Th17 cells are CCR6+CCR4+ or CCR2+CCR5−, in contrast with Th1 cells, which are CCR6+ CXCR3+ or CCR2+CCR5+ (15,16). In mice, E-selectin ligand positive CD4+ T cells found in inflamed skin included Th1 and Th17 cells, both of which were CCR4+ (17), suggesting that migration of Th1 and Th17 into some tissues may share the same mechanisms. Also in the mouse, CCR6 is reported to be a marker of Th17 cells and necessary for their migration into the gut (18) and the CNS in the setting of EAE (19). In contrast to studies of chemokine receptor expression, to date, there has been no assessment of adhesion molecule expression and function on murine Th17 cells as assessed by physiologically relevant flow conditions.
In the present study, we have used an in vitro flow chamber that simulates flow conditions in postcapillary venules with live time videomicroscopy, and confocal intravital microscopy to examine the interactions of mouse Th17 cells with immobilized recombinant endothelial adhesion molecules, with mouse heart endothelial cells (MHEC) in culture, and with microvessels in inflamed cremaster muscle in vivo. We report significant differences between Th1 and Th17 cells with respect to E-selectin rolling and ICAM-1 chemokine dependent arrest on activated endothelium both in vitro and in vivo, and examine recruitment of in vivo generated Th17 vs Th1 cells using an air pouch model of inflammation.
Materials and Methods
Reagents
Recombinant mouse IL-23, E-selectin, P-selectin, VCAM-1, ICAM-1 and MAdCAM-1 Fc-chimeras, and anti-CCR6-PE were from R&D Systems (Minneapolis, MN). Recombinant mouse IL-12, IL2, IL-6, TNF-α, recombinant human TGF-β, and the following antibodies to mouse cytokines, chemokine receptors or adhesion molecules: IL-4 (clone 11B11), IFNγ (clone XMG 1.2), IL-2 (clone JES6-1A12), CD4 (clone GK 1.5), CD3 (clone145-2C11), CD28 (clone 37.51), IL-17A (clone 2C11-18H10.1),CD43 activation-associated glycoform (clone 1B11) and Thy 1.1-Alexa 488 (CD90.1) are all from Biolegend (San Diego, CA). Antibodies to CXCR4, CD11a, PSGL-1, ICAM-2 and PECAM-1 were purchased from BD-Pharmingen (San Jose, CA), and SDF-1-α, TNF-α and carrier free CCL20 from Preprotech (Rocky Hill, NJ). PMA and ionomycin were from SIGMA (St. Louis, MO). Vibrant CFDA-SE, CMRA and Alexa-680 cell tracker stains are from Invitrogen (Carlsbad, CA). Collagenase was from Worthington (Lakewood, NJ).
Mice
All mice used were bred in the pathogen free facility at Harvard Medical School New Research Building, in accordance with the guidelines of the committee of Animal research at the Harvard Medical School and the NIH Animal research guidelines. C57Bl/6 (WT) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Strain matched E-selectin deficient mice (E-sel−/−) (20) were purchased from Jackson Laboratories (Bar Harbor, ME) or obtained from Dr. Richard Hynes (MIT, Cambridge, MA). These animals have been backcrossed onto the C57Bl/6 background more than 10 generations. IL-17-RFP (21) and Ifng/Thy1.1 BAC-In (22) transgenic cytokine reporter mice were obtained from Dr. Cheng Dong (MD Anderson, Houston, TX) l and Dr. Casey Weaver (University of Alabama, Birmingham, AL) respectively. Mice were sacrificed at 7–12 weeks of age for harvest of naïve T cells, and 8–12 days of age for harvest of heart endothelial cells.
Preparation of naïve and effector T cells
CD4+ cells were isolated from spleen and lymph node cell suspensions of WT, IL-17-RFP or Ifng/Thy1.1 BAC-In mice using negative selection by immunomagnetic beads (Invitrogen, Carlsbad, CA). Th1 cells were derived from the naïve T cells by anti-CD3 and anti-CD28 stimulation in the presence of IL-12 and IFN-γ, as previously described (23). To achieve Th17 differentiation, naïve T cell were stimulated with anti-CD3 in the presence of human TGF-β (3ng/ml), mouse IL-6 (30ng/ml), mouse IL-23 (20ng/ml), plus anti-IFN-γ (10ug/ml), anti-IL-4 (10µg ml), and anti-IL-2 (10µg/ml) mAb. On day 3, Th1 and Th17 cultures were diluted 1:1 with fresh medium containing IL-2 (25U/ml) and IL-23 (20ng/ml), respectively. Cells were harvested on day 5 and immediately used in experiments. Th1 cells derived from Ifng/Thy1.1 BAC-In mice and Th17 cells from I-17-RFP mice were sorted by flow cytometry to prepare 100% reporter expressing cells before being used in the adhesion experiments.
Isolation of endogenous Th17 cells and expansion ex-vivo
WT mice were immunized with MOG peptide as described (24). Briefly, mice were immunized subcutaneously, sacrificed at day 8 post-immunization, and the inflamed lymph nodes were harvested. Cells were further cultured ex-vivo with MOG peptide (1µg/ml) and IL-23 (25ng/ml) for 72h, and IL-23 was supplemented for two additional days before the cells are used.
Preparation of Mouse Heart Endothelial Cells
Mouse heart endothelial cells (MHEC) were prepared as described (25) except that hearts from newborn animals (7–9 days old) were used. This modification yielded cells that more consistently form uniform monolayers than cells from adult mice.
Measurement of interactions of T cells with adhesion molecules and MHEC under defined flow conditions in vitro
T cell interactions with immobilized adhesion molecules or MHEC were observed by videomicroscopy (20× objective) under defined laminar flow conditions in a parallel plate apparatus (25). Accumulation of T cells on immobilized molecules was determined in 8 different fields after the initial minute of each flow rate. T cell interactions with confluent TNF-α (100ng/mL, 5h) activated MHEC grown on glass coverslips were monitored by live cell fluorescence microscopy as follows: Th1 and Th17 cells were labeled with CFSE or CMRA following the manufacturer’s instructions, and either mixed together or kept separate and drawn in the flow chamber at an estimated shear stress of 0.76 dynes/cm2 for 1 minute, followed by flow buffer alone for 10 minutes (26). Accumulation of Th1 and Th17 cells on WT cells was measured by counting accumulated cells in 5 different fields of view using a 20X objective. Accumulation of Th1 and Th17 cells on E-selectin deficient endothelial cells was compared to accumulation on wild type endothelial cells, using the following formulas: “[# accumulated Th1 cells on E-selectin−/− MHEC/ # accumulated Th1 cells on WT] × 100”, and “[# accumulated Th17 cells on E-selectin−/− MHEC/ # accumulated Th17 cells on WT] × 100”.
Flow cytometry
Flow cytometry was performed to corroborate the differentiation of Th17 cells as described (18). The data were acquired on a FACScaliber flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo (Ashland, OR).
Quantitative RT-PCR (qRT-PCR) analysis
Total RNA was extracted from cultured T cells by RNeasy kit (QIAGEN Inc., Valencia, California, USA), and reverse-transcribed using the ThermoScript RT-PCR system and random hexamer primers according to the manufacturer’s instructions (Invitrogen Carlsbad, CA), and amplified by real-time PCR with SYBR Green PCR mix (Applied Biosystems) and Step-One Detection System (Applied Biosystem) according the manufacturer’s instructions. The sequences of the primers are available on request.
Competitive rolling assay for T-cell adhesion in vivo in the cremaster muscle
Intravital microscopy studies of the mouse cremaster muscle microcirculation were performed as described (27,28). Mouse recombinant TNF-α (0.5 µg in 200µL saline) was injected intrascrotally 1.5h prior to cremaster exteriorization. Mice were anesthetized and a microcatheter was introduced into the right femoral artery to enable retrograde injection of fluorescently-labeled Th1 and Th17 cells (28). Transmitted light and fluorescent cremaster imaging was performed with an Olympus FV1000 confocal intravital microscope using a 20X water immersion objective. Fluorescence imaging was done sequentially at 473nM and 635nM to reduce the potential for channel crosstalk. The centerline red blood cell velocity (Vcl) in each venule was measured in real time with an optical doppler velocimeter (Texas A&M, College Station, TX) and Vcl was used to determine the wall shear rate and critical velocity (Vcrit) (28). CFSE-labeled Th1 and Alexa 680-labeled Th17 cells were suspended at 33×107 cells/ml and small boluses (3×106 of each cell type) of a mixture of both cells were injected retrograde into the femoral artery catheter to visualize their adhesion in the post capillary venules. Microvessel images were analyzed off-line using Imaris software (South Windsor, CT).
Air pouch model of inflammation
Air pouches were created in the dorsal side of the back of wild type and E-Selectin deficient mice as previously described (29). PBS, TNF-α (500ng) or CCL20(400ng) were injected in the air pouch and 20h later cell infiltrates were harvested by pouch lavage by repeated washes with PBS. Single cell suspensions were permeabilized and stained for intracellular IL-17 or IFN-γ and analyzed by flow cytometry.
Statistical analysis
Data are expressed as the mean ± SD unless otherwise stated. Statistical analyses by student’s T test or by ANOVA followed by the Newman Keuls post test were performed with GraphPad Prism software and were considered statistically significant at p<0.05.
Results
Functional interactions of Th17 cells with endothelial cell adhesion molecules under flow conditions
Th17 and Th1 cells were generated in vitro from naïve mouse CD4+ cells as described in methods. Importantly, there were no Th1 cells contaminating the Th17 cultures or vice-versa, Th17 and Th1 cells expressed ROR-γT and T-bet respectively, and Th17 cells also expressed IL-22 (Fig 1A,B). In addition, we added anti-IL-2 neutralizing antibody to avoid the differentiation of Tregs (< 1% Foxp3+) under Th17 polarizing conditions (data not shown). Note that studies of T cell subsets in many laboratories use populations that do not uniformly produce the detectable defining cytokines by flow cytometric assays and usually the signature cytokine producing cells are less than 40% of the population (14,18,30,31). This does not mean that only a minority of the cells has undergone the appropriate differentiation steps, but more likely reflects the nature of the assays, which cannot induce synchronized cytokine expression in all the cells.
Figure 1.
Phenotype of in vitro differentiated T cell populations. Naïve CD4+ T cells isolated from the spleen and lymph nodes of C57/BL6 mice were cultured under Th17 or Th1 cell induction conditions during 5 days, as described in materials and methods. A. Intracellular IFNγ and IL17A were detected in PMA plus ionomycin stimulated Th cells by flow cytometry (see methods). The dot plot data shown are representative of 12 separate cell preparations that were also used in flow chamber studies and adoptive transfer studies presented in this article. B. Th17 and Th1 cells that were differentiated for 5 days were analyzed by qRT-PCR. Results were normalized to β-actin (Actb) gene expression and relative gene expression levels are indicated. Data show the mean ± SD of 4 different experiments, ** p<0.001.
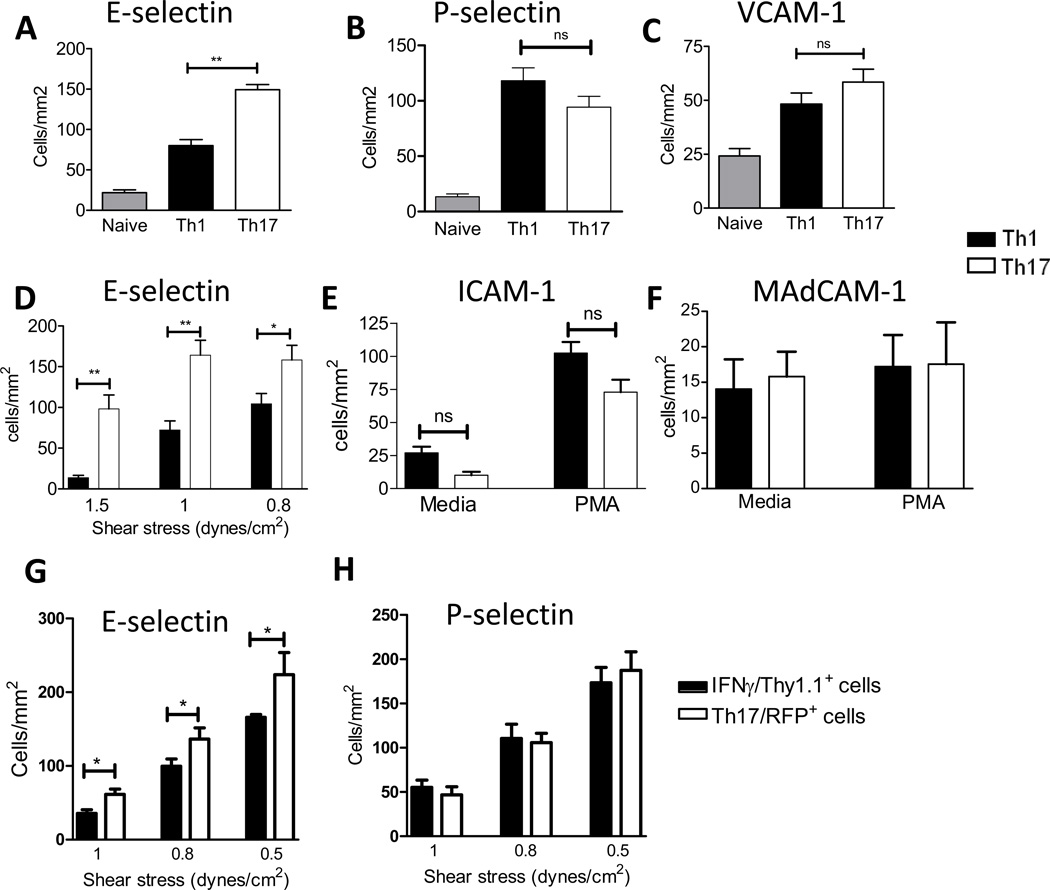
The capability of the in vitro differentiated Th17 cells to initially attach to E- and P- selectins, ICAM-1, VCAM-1 or MAdCAM-1 was assessed under defined flow conditions. Few naïve T cells accumulate on immobilized E- and P selectin due to the lack of functional selectin ligands, whereas significant numbers of Th1 cells can adhere to both E- and P- selectin, as well as to ICAM-1 and VCAM-1 (32,33). These properties make these two T-cell subsets the appropriate positive and negative controls for comparison with Th17 cells. We found that Th17 cells avidly interacted with E-selectin, P-selectin, ICAM-1, VCAM-1 and MAdCAM-1 under flow conditions (Fig 2). Interestingly, significantly more Th17 than Th1 cells accumulated on immobilized E-selectin. These differences were sustained across the range of shear stresses tested (Fig 2A, D). In contrast, there were no significant differences between Th17 and Th1 cell accumulation on P-selectin or VCAM-1 (Figs2B,C). Accumulation of T cells on ICAM-1 under flow conditions requires activation of the β2 integrins LFA-1(αLβ2) and Mac-1(αMβ2), and this can be induced by a variety of agents including chemokines and phorbol myristate acetate (PMA) (34,35). We did not observe any differences between binding of Th17 and Th1 cells to ICAM-1 upon PMA activation (Fig2E). Given the relevance of Th17 cells in intestinal barrier function, we examined the Th17 cell accumulation on immobilized mucosal addressin cell adhesion molecule-1 (MAdCAM-1), which is expressed in the high endothelial venules of mesenteric lymph nodes, Peyer’s patches and lamina propria (Fig2F). The total numbers of Th17 or Th1 cells that accumulated on MAdCAM-1 were low, even under low stringency conditions of 0.5dynes/cm2. Binding was not enhanced upon T cell activation with PMA, and we found that similar numbers of Th17 and Th1 cells bound to MAdCAM-1. We also generated Th17 and Th1 cells from IL-17 and IFNγ reporter mice, respectively, sorted them and the uniform reporter positive cell populations were used in flow chamber adhesion assays. Th17 cells obtained this way also showed enhanced adhesion to E-selectin, as compared to Th1 cells (Fig 2G), whereas no differences were observed on adhesion to P-Selectin (Fig2H).
Figure 2. Interactions of Th17 cells with endothelial cell adhesion molecules under flow conditions.
Naïve and in vitro differentiated Th1 and Th17 cells from WT mice were drawn across coverslips coated with the indicated adhesion molecules within a flow chamber at shear stress of 1 dyne/cm2 (A–C), 0.8 dynes/cm2 (E) or 0.5 dynes/cm2 (F), or at decreasing levels of shear stress (D). In vitro differentiated Th1 cells derived from splenic CD4+ T cells from Ifng/Thy1.1 BAC-In mice (22) and Th17 cells derived from IL17-RFP mice (21), were sorted to obtain 100% cytokine reporter positive cells, which were then drawn across coverslips coated with E-Selectin (G) or P-Selectin (H) at decreasing levels of shear stress. T cell interactions with the adhesion proteins were recorded with a phase contrast objective (20X) and videomicroscopy (Ed Marcus Laboratories, Boston, MA). Accumulation of T cells was determined after the initial minute of each flow rate by counting adherent cells in eight fields. Data show the mean ±SD values from 6 different experiments. **p<0.01, * p<0.05.
Selectin ligand, integrin, and chemokine receptor phenotypic analysis of in vitro differentiated Th17 cells
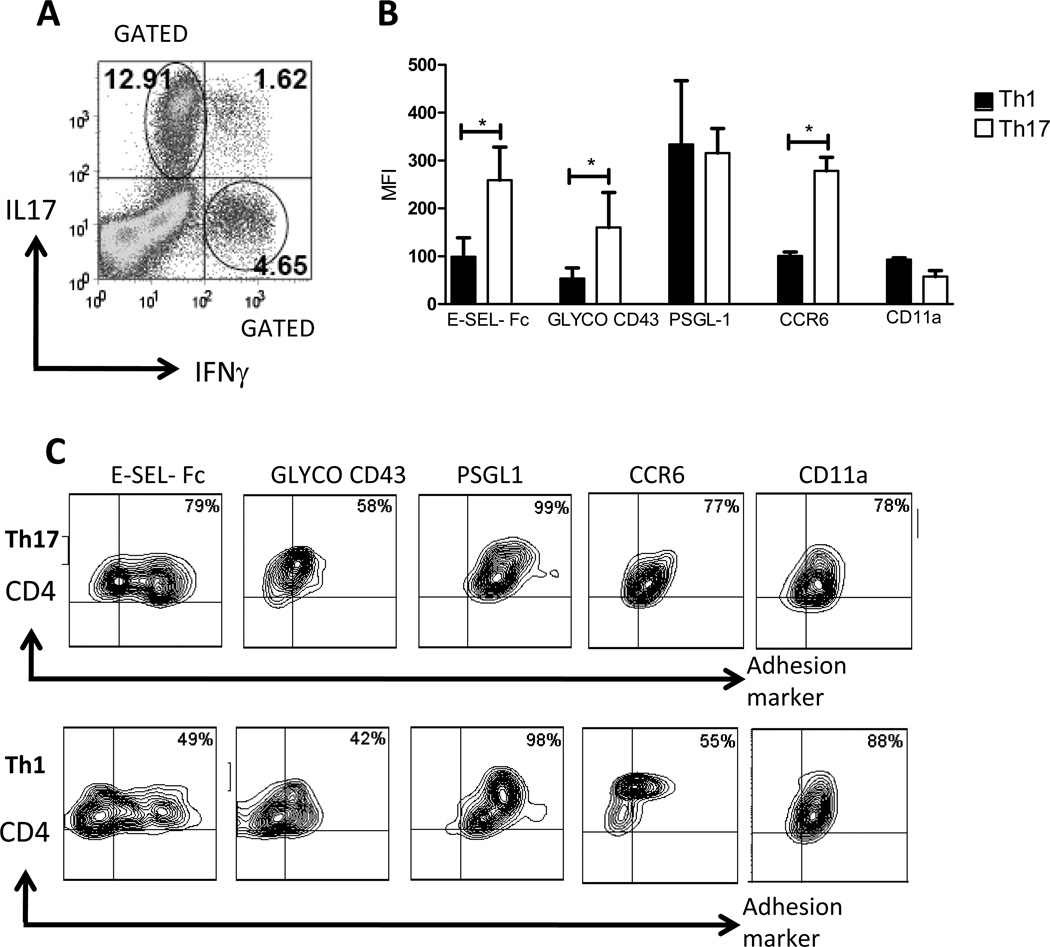
To corroborate our findings of Th17 cell binding to E-selectin under flow conditions, we examined Th17 vs. Th1 binding of E-selectin using a flow cytometric assay and soluble murine E-selectin-IgG chimera (14). We also analyzed the expression of glycoCD43, a known E-selectin ligand expressed by Th1 cells (23,36), and the expression of PSGL-1, a ligand for both P-selectin and E-selectin. We found that Th17 cells bound significantly more E-selectin-Fc protein than did Th1 cells, and Th17 cells expressed more glycoCD43 than Th1 cells. However, the levels of PSGL-1 surface protein were not different (Fig3A, B).
Figure 3. Expression of adhesion molecules and chemokine receptors on Th17 cells, and their chemokine-dependent interactions with ICAM-1.
A–D. In vitro differentiated Th1 and Th17 cells were stimulated with PMA and ionomycin in the presence of brefeldin during 4h and stained with E-selectin-human IgG fusion protein, or the indicated E-selectin ligands (A–B), integrins or chemokine receptors (C, D) and co-stained with antibodies specific for IL17A and IFNγ. The expression levels of the indicated markers were analyzed in gated IL17+ or IFNγ+ cells by flow cytometry. Dot plot data is shown representative of 12 separate experiments using cells that were also used in flow chamber studies and adoptive transfer experiments presented in this article. B, D. Data show the mean ± SD of 10–12 different cell preparations. In vitro differentiated Th17 (E) or Th1 (F) cells treated with media or PMA (10ng/ml) for 5 minutes at 37C, were drawn across ICAM-1 coated coverslips. Media treated cells were drawn across coverslips co-coated with either SDF-1α or CCL20. The shear stress within a flow chamber was 0.8 dynes/cm2. Data show the mean ±SD values from 5 different experiments. **p<0.01,* <0.05.
Recent reports have shown a different pattern for chemokine receptors expressed by Th17 and Th1 cells. In particular, CCR6 is more abundantly expressed by Th17 than Th1 cells (18,19). We performed flow cytometric analysis, gating on either CD4+IL17+ or CD4+IFN-γ+ cell populations to determine the levels of expression of CCR6, CXCR4 and LFA-1 (CD11a) (Fig3C,D). We found higher expression of CCR6 on Th17 cells, as previously described (18,19), and additionally, we found similar levels of expression of CD11a (Fig 3C), α4 and α4β7 integrins (data not shown). We obtained similar results when we analyzed whole Th17 vs. Th1 populations (i.e. not gated on IL-17 vs. producing cells) or when we analyzed the percent positive cells for the indicated adhesion molecules within the gated or ungated IL-17+ and IFN-γ+ cells (Supplemental Figure 1) as used in the in vitro and in vivo functional adhesion studies described below.
Interactions of Th17 cells with ICAM-1 under flow conditions are dependent on chemokine activation via CCR6
We also studied the interaction of Th17 cells with immobilized ICAM-1 under flow conditions. We found similar numbers of Th17 and Th1 cells adhered to ICAM-1 upon integrin activation by PMA. We next examined a more physiologically relevant stimulus, i.e. chemokines presented on the endothelium that trigger rapid integrin activation (34,35). For this purpose, we measured the accumulation of Th17 cells on coverslips coated with ICAM-1 and coimmobilized with CXCL12 (SDF-1α) or CCL20 (MIP3α), ligands for the chemokine receptors CXCR4 and CCR6, which are expressed at low and high levels, respectively, in Th17 cells (37). Interestingly, the number of Th17 cells that accumulated on ICAM-1 in the presence of CCL20 was greater than that found in the presence of SDF-1α. Similar numbers of Th17 cells bound to ICAM-1 following integrin activation by either CCL20/CCR6 or PMA (Fig3E). In contrast, binding of Th1 cells to ICAM-1 was enhanced by both PMA and SDF1-α but not by CCL20 (Fig3F).
Phenotypic analysis of adhesion molecules in Th17 cells induced in vivo
The Th17 and Th1 cells used in the studies described above were derived in vitro by activation of naïve T cells via TCR and co-stimulatory signals in the presence of a cocktail of cytokines. In order to corroborate that the migratory phenotype of these T cells faithfully represents the phenotype of Th17 and Th1 primed in vivo, we used an immunization protocol known to generate Th17 and Th1 cells in Experimental Autoimmune encephalomyelitis (EAE) (24) (Fig4A). In vivo primed Th17 cells expressed significantly higher levels of E-selectin ligands as compared to the in vivo primed Th1 cells, similar to in vitro differentiated populations (compare Fig 4B,C, to Fig 3A–D). The in vivo primed Th17 and Th1 populations showed comparable expression levels of PSGL-1 and CD11a. Lastly, CCR6 levels on in vivo primed Th17 cells were higher than on Th1 (Fig4C). Also a higher percentage of Th17 vs. Th1 cells were positive for CCR6 and E-Selectin ligands whereas a similar percentage of Th17 and Th1 cells were positive for integrins α4 and α4β7 and a higher percentage of Th1 cells expressed the chemokine receptor CXCR4 (Supplemental Fig 2). Although the in vitro and in vivo generated Th17 cells showed slight differences in the total levels of expression of the various molecules, the relative expression of each molecule tested between subsets appeared to be comparable for both the in vitro and in vivo generated cells, thus validating the comparative results obtained with in vitro generated cells.
Figure 4. Expression of adhesion molecules and chemokine receptors in Th17 cells generated in vivo.
C57BL/6 mice were immunized with MOG peptide, and 8 days after immunization the cells isolated from the lymph nodes were re-stimulated with MOG peptide for 5 additional days in culture and analyzed by flow cytometry as in Fig 3. A. A representative dot plot data is shown from 6 separate experiments with similar results, and indicates the presence of in vivo generated Th17 and Th1 cells. B. The expression levels of the different adhesion molecules and chemokine receptors were analyzed by flow cytometry in the IL17+CD4+ or IFNγ+CD4+ gated populations. Data show the mean ± SD of 2–3 different experiments. C. A representative dot plot data is shown of 4 separate experiments. *p<0.05.
The interactions of Th17 cells with mouse heart endothelial cells (MHEC) in vitro and with the cremaster microvasculature, in vivo, are E-selectin dependent
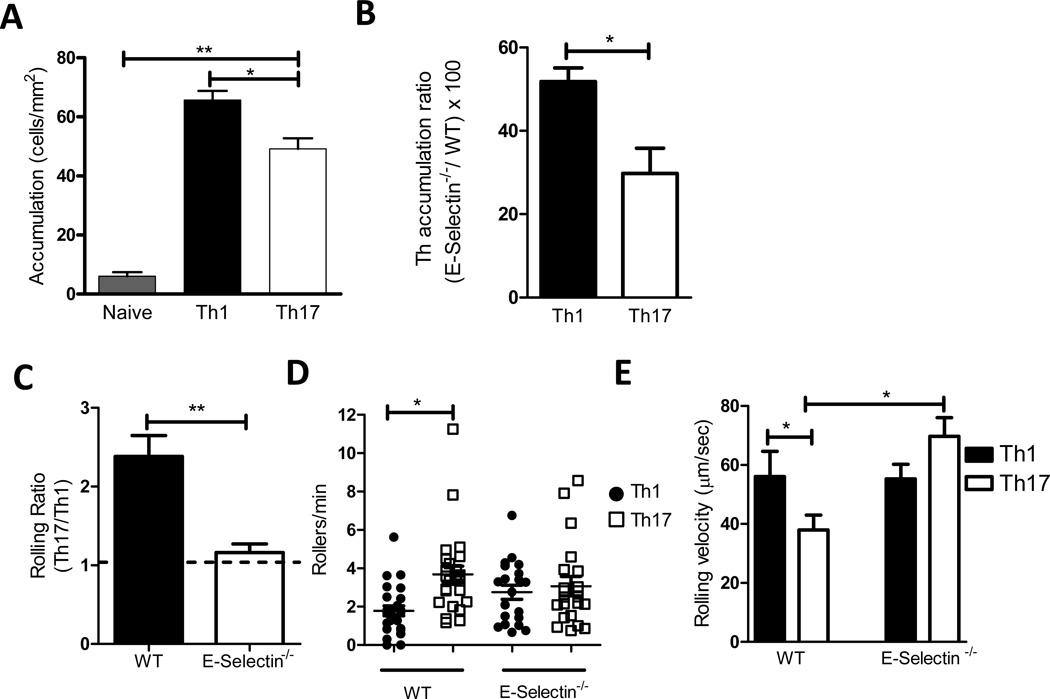
TNF-α activated MHEC express several adhesion molecules including E-selectin, P-selectin, ICAM-1 and VCAM-1 (25). We therefore sought to corroborate the above findings by quantifying naïve, Th17 and Th1 adhesion to TNF-α activated MHEC in an in vitro flow chamber. Th17 and Th1 cells were labeled with different color fluorescent dyes and both types of cells were combined together and drawn across the same MHEC monolayer to measure accumulation (see supplemental video 1). In contrast to the predicted outcome, the number of Th17 cells accumulating on activated MHEC was lower than the number of Th1 cells (Fig5A). As expected, few naïve cells adhered. Based on our data indicating both higher expression of E-selectin ligands and more accumulation on E-selectin by Th17 cells as compared to Th1 cells (Figs 2 and 3), we reasoned that in the absence of E-selectin, differences in Th17 vs. Th1 binding to MHEC would be diminished. Therefore we examined Th17 and Th1 cell binding to MHEC prepared from E-Selectin−/− mice. The lack of E-selectin resulted in decreased binding of both Th1 and Th17 cells, however, the decrease was significantly more pronounced with Th17 cells. The ratio ([Th17 accumulated on E-selectin−/− MHEC/Th17 accumulated on WT] × 100) was 30% indicating a 70% decrease in Th17 accumulation when E-selectin was absent, whereas the ratio for Th1 cells was 55%, indicating a 45% decrease in Th1 accumulation (Fig5B). This result confirms a higher dependence of Th17 cells on E-selectin in their interactions with MHEC compared to Th1 cells. Interactions of T cells with activated MHEC also depend on VCAM-1, P-selectin and ICAM-1. Thus, a 90% reduction of both Th1 and Th17 binding to E-selectin deficient activated endothelium was achieved by a combination of function blocking mAbs to ICAM-1 on the endothelial cells and to α4 integrin on the T cells (data not shown). Based on these in vitro findings, we next evaluated the interactions of Th17 and Th1 cells with activated endothelium in vivo using intravital microscopy.
Figure 5. Th17 cells interactions with TNF-α activated mouse heart endothelial cells (MHEC) under flow conditions and with TNF-α activated cremaster muscle microvasculature in vivo.
(A, B) Naïve and in vitro differentiated Th1 and Th17 cells were drawn across TNF-α activated MHEC from C57/BL6 or E-selectin deficient mice within a flow chamber at a level of shear stress of 0.8 dynes/cm2. Accumulation (A) was calculated as described in the method section. Panel B is the ratio [(Th cells accumulated on E-Selectin−/− MHEC divided by Th accumulated on WT MHEC) × 100]. Data show the mean ±SD values from 3 different experiments. C. A competitive assay was performed to compare Th17 and Th1 cells rolling on the microvasculature. Equal numbers of in vitro differentiated Th17 and Th1 cells each labeled with a different fluorescent dye, were co-injected into C57/BL6 or E-selectin−/− recipient mice that had received TNF-α injection into the scrotal sac 2h before the cells were transferred. Recordings of each vessel were analyzed for 60 s, and rolling T cells were identified as the visible cells passing through a plane perpendicular to the vessel axis. Rolling ratio represents the ratio Th17/Th1 cells that roll on each vessel. The number of Th17 and Th1 rollers per minute in several independent vessels studied are also shown (D). E. The rolling velocity of high velocity cells (Vcell) was calculated to ensure that these cells qualified as rolling leukocytes, defined Vcell<Vcrit. Velocities of Th17 and Th1 cells from 4 independent preparations (33–44 Th17 or Th1 independent cells were analyzed rolling on WT or E-Selectin−/− vessels). Data represents mean ±SD values of 4 independent experiments using independent T cell preparations, and the numbers of mice and vessels are indicated in Table I. ** p<0.01, * p<0.05.
When mouse cremaster muscle is exposed to the mild trauma induced by surgical tissue preparation, leukocytes undergo rolling interactions on the vessel wall that is mediated by P-selectin and L-selectin (27). Tissue pre-exposure to TNF-α for 2h additionally induces local E-selectin expression and further elevates leukocyte adhesion (27,28). Hence, this model is ideal to study how Th17 interact with the vascular endothelium in vivo. Again, we chose to compare Th17 cells with Th1 cells, which have been previously described to interact with cremaster microvessels in vivo (30,31,38). Th17 and Th1 cells were labeled with different dyes and co-injected via the femoral artery after exteriorization of the TNF-α stimulated cremaster muscle, thus enabling comparison of Th17 and Th1 interactions in the same vessels and competitive rolling analysis. The microvessel parameters for wild type and E-selectin−/− mice are listed in Table I. Th17 and Th1 cells both exhibited rolling interactions in WT and E-selectin−/− mice. However, in WT mice the Th17 cells interacted with a significantly higher frequency compared to the Th1 cells (3.68±0.42 Th17 cells rolling/min vs. 1.78±0.25 Th1 cells rolling/min, p<0.0148, and a rolling ratio Th17/Th1 of 2.3). In contrast, in E-selectin−/− mice the Th17/Th1 rolling ratio remained close to 1, and Th17 and Th1 cells showed a similar rolling flux (3.01±0.54 Th17 rolling/min vs. 2.44±0.35 Th1 rolling/min, p=0.0426) (Fig5CD and supplemental video 2). The average rolling velocity of Th17 was slower than Th1 in WT mice, and similar rolling velocity was observed when Th17 and Th1 cells were co-injected in the E-Selectin−/− mice. Interestingly, Th17 cells rolled faster on E-Selectin−/− than on WT mice (Fig5E). It was also notable that there was heterogeneity in the rolling velocities of both Th17 and Th1 cells, with some cells rolling faster than others, but the percent of slow rollers was higher in Th17 than in Th1 cells, and there were no Th17 slow rollers (velocity ≤ 10µm/second) observed in the E-Selectin−/− mice (data not shown). These data show for the first time that Th17 cells roll on the microvasculature in vivo, and confirm the in vivo relevance of our finding that Th17 cells express more functional E-selectin ligands than Th1 cells.
Table I.
Microvessel parameters for WT and E-Selectin−/− mice.
| Genotype | Number of Mice | Number of Vessels |
Vessel diameter (µm) |
Wall shear rate (s−1) |
|---|---|---|---|---|
| Wild type (C57/BL6) | 8 | 28 | 32 ± 2.7 | 1,181 ± 162 |
| E-Selectin−/− | 5 | 22 | 34.8 ± 2.6 | 1,093 ± 112 |
TNF-α promotes recruitment of Th17 and Th1 cells into the air pouch of WT and E-Selectin−/− mice
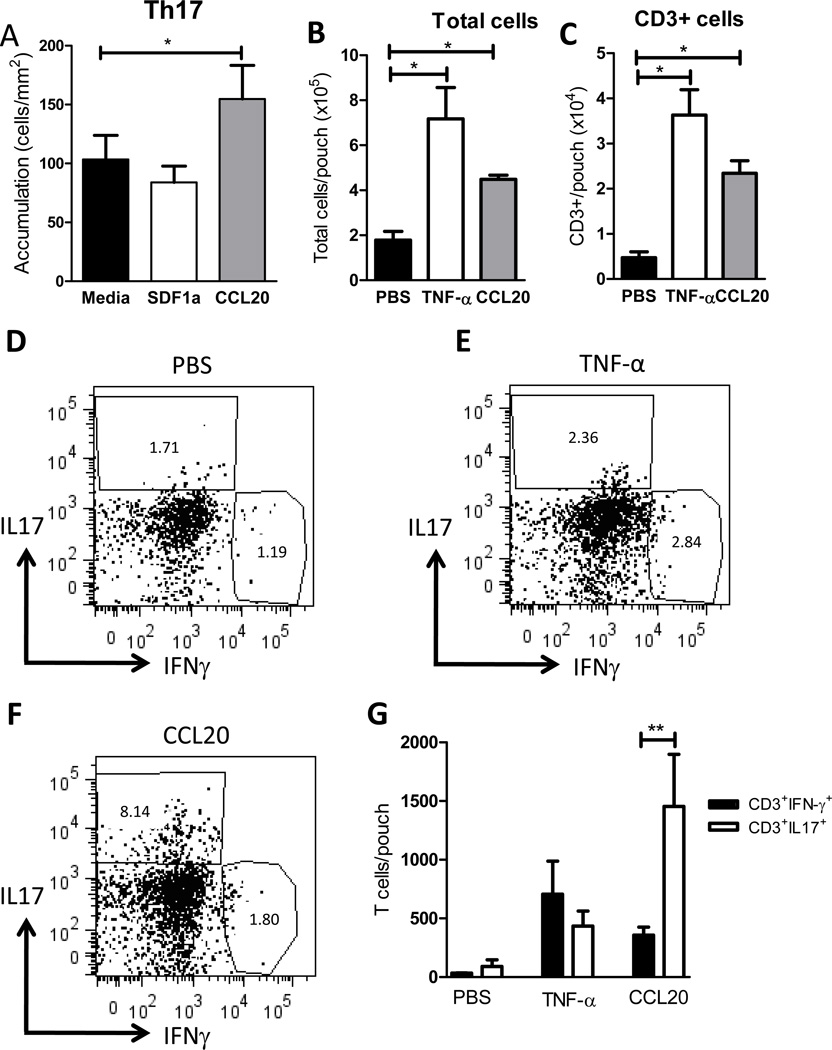
Leukocyte extravasation in a subcutaneous pouch is a well-characterized model used to examine leukocyte emigration in response to a specific stimulus (29,39). To further explore the differences in Th17 and Th1 migration during inflammation in vivo, we generated an air pouch in the dorsal side of WT and E-Selectin−/− mice and locally injected TNF-α as an inflammatory stimulus or PBS as control. 20h later we harvested the air pouches and analyzed the cellular infiltrates by flow cytometry. The total cells harvested per pouch were significantly increased in TNF-α treated mice compared to PBS injected mice and no quantitative differences were observed between WT and E-Selectin−/− mice (Fig6A). The number of cells recruited in PBS control mice was very low in both WT and E-Selectin−/− mice (Fig 6A and Supplemental Fig 3). The majority of the cell infiltrates were Gr1+ cells, as previously described in this model of inflammation (40) (data not shown). Interestingly, there was a component of cellular infiltrates that were CD3+ T cells, including Th17 cells and Th1 cells. However, no differences were observed in Th17 or Th1 cell recruitment between WT and E-Selectin−/− mice, indicating that E-Selectin deficiency did not result in decreased recruitment of endogenous Th17 or Th1 cells (Fig 6B–F). These data indicate that probably other selectins such as P-selectin overcome the lack of E-selectin and contribute to cell recruitment into the air pouch, similar with what has been described in E-Selectin deficient mice in other models of inflammation (20,41). More importantly, we can use this model to evaluate recruitment of Th subsets in response to other stimuli using TNF-α as a positive control of T cell recruitment.
Figure 6. Recruitment of Th17 and Th1 cells into the TNF-α injected air pouch.
WT or E-selectin−/− mice (5 mice per group) received 500ng/ml of TNF-α into a dorsal air pouch and cell infiltrates were harvested 20h post TNF-α injection and analyzed by cell counting (A) and flow cytometry (B–F). A representative dot plot of CD3+ gated cells is shown for the TNF- α injections in WT (C) and E-Selectin−/− (D) mice. Numbers in the gate represent % positive cells (C, D). Number of CD3+ cells/pouch was calculated based on the % positive CD3 cells relative to the total number of cells harvested per pouch (B,D). E. F. Data is calculated as in C, using the % positive cells within the CD3+ region.
The interactions of Th17 cells with mouse heart endothelial cells in vitro are CCL20/CCR6 dependent and CCL20 induces Th17 cell recruitment to the Air pouch in vivo
Based on the different expression pattern of CCR6 in Th17 vs Th1 cells, and our finding that CCL20 can specifically trigger Th17 cell arrest on immobilized ICAM-1 under flow conditions (Fig 3G,H), we next assessed the effect of CCL20 on Th17 cell accumulation on activated WT endothelium. Interestingly, we found that Th17 binding to MHEC was significantly enhanced in the presence of CCL20 while SDF-1α did not have any effect on Th17 accumulation (Fig 7A). This is in agreement with a recent publication that shows that human Th17 cells can arrest on CCL20 treated human umbilical vein endothelial cells and mouse fibroblasts expressing ICAM-1 (42). Taken together, these results indicate that CCL20 binding to CCR6 in Th17 cells mediates firm adhesion to activated endothelium via ICAM-1. In order to test this in vivo, we next asked the question if CCL20 would trigger efficient recruitment of Th17 cells in vivo using the air pouch model. Other chemokines have been previously shown to recruit inflammatory cells in the air pouch, however, to our knowledge, none of these studies have addressed T cell recruitment (43–45). Local injection of CCL20 into the air pouch resulted in statistically significant increase of cell recruitment as compared with PBS control (Fig 7B). The numbers of CD3+ recruited T cells into the CCL20 treated air pouch were significantly higher than the PBS control group and comparable to the TNF-α treated mice (Fig 7C). In response to TNF-α we observed that comparable numbers of Th17 and Th1 cells were recruited into the air pouch. Strikingly, CCL20 injection in the air pouch resulted in specific recruitment of Th17 cells but not Th1 cells (Fig 7D–G). Our data demonstrate that CCL20 cannot only induce Th17 adhesion to endothelium in vitro, but can also trigger recruitment of Th17 cells in vivo.
Figure 7. Th17 cell interactions with CCL20 treated MHEC and Th17 cell recruitment into CCL20 injected air pouch.
A. Naïve and in vitro differentiated Th1 and Th17 cells were drawn across TNF-α activated MHEC from C57/BL6 mice that had been treated with the indicated chemokines during 20 minutes before introducing the T cells within a flow chamber at a shear stress of 0.8 dynes/cm2. Data show the mean ±SD values from 3 different experiments. B–G. WT mice (5 mice per group) received PBS, 500ng/ml of TNF-α or 400ng of CCL20 into the air pouch and cell infiltrates were harvested 24h post injection and analyzed by cell counting (B) and flow cytometry (C–G). A representative dot plot representing cells gated within the CD3+ region is shown for the PBS (D), TNF-α (E) and CCL20 (F) injections. C. Number of CD3+ cells/pouch was calculated based on the % positive CD3 cells referred to the total number of cells harvested per pouch. G. Data is calculated as in C, using the % positive cells within the CD3+ gate. **p<0.01, *p<0.05.
Discussion
In this study we present a detailed description of the adhesion phenotype of murine Th17 cells and demonstrate that Th17 cells interact with activated endothelium differently than Th1 or naïve T cells in vitro and in vivo. We suggest these differences can contribute to temporal and spatial differences in recruitment of Th17 cells compared to other subsets during host defense and inflammation.
The evidence supporting our conclusions is as follows. First, we have dissected the different known adhesion pathways by testing interactions of Th17 cells with endothelial cell expressed adhesion molecules in isolation under flow conditions. Th17 cells have a high capacity to bind E-selectin, even higher than Th1 cells, which are well known to bind to E-selectin under flow conditions (14,23). We saw greater Th17 vs. Th1 biding to E-selectin under flow, using either unsorted Th1 and Th17 cell preparations which typically do not show uniform IFNγ or IL-17 expression, or using sorted 100% positive cytokine reporter expressing cells from IL-17-RFP or Ifng/Thy1.1 BAC-In mice. The expression of high levels of E-selectin ligands, including glyco-CD43, on Th17 cells, generated in vitro and in vivo, has also been demonstrated (Fig 3 and 4). Second, using flow cytometry and qPCR techniques, we have further characterized the level of expression of the integrin ligands for ICAM-1 and VCAM-1, and the expression of chemokine receptors expressed in Th17 cells. In agreement with previous reports (18,19), we have found that Th17 cells express high levels of CCR6. However, for the first time, we report that CCR6 interactions with its ligand CCL20 trigger robust Th17 arrest on immobilized ICAM-1. Third, these results were validated by analysis of Th17 cells generated in vivo by MOG immunization, and show that these cells also express high levels of E-selectin ligands and CCR6. Fourth, we have corroborated our findings by studying Th17 interactions with TNF-α activated endothelium using E-selectin−/− or WT MHEC pretreated with CCL20 chemokine. Lastly, the interactions of Th17 cells with activated endothelium are different from other T cell subsets in vivo. Thus, by intravital microscopy Th17 cells have a high capacity of rolling in cremaster microvessels in vivo and this rolling is highly dependent on E-selectin. Indeed, we have established differences between Th17 and Th1 cells in competitive rolling experiments using WT and E-Selectin−/− mice. In addition, using an air pouch model of inflammation, we have demonstrated that, Th17 cells are recruited into sites of acute inflammation differently than Th1 in response to CCL20. In summary, these findings demonstrate that Th17 cells interactions with activated endothelium are highly dependent on E-selectin, due, in part to the high levels of expression of E-selectin ligands and the ability to arrest on ICAM-1 in the presence of CCL20, and this is different from Th1 cells both in vitro and in vivo.
E-selectin is expressed at high density on the luminal plasma membrane of vascular endothelial cells at sites of inflammation (30,46) and mediates the initial contact of T cells with activated endothelium through two different functional E-selectin ligands expressed on murine activated T cells, PSGL-1 and glycoCD43. PSGL-1 is the best characterized selectin ligand on leukocytes that binds E-, P- and L-selectins (47), and the glycosylated form of CD43, glycoCD43, binds E-selectin but not P-selectin (23,36). Our prior studies with Th1 cells from PSGL-1:CD43 double deficient mice predict the existence of other E-selectin ligands in activated T cells (23). From our results and recent work of other groups it is clear that subsets of T helper cells differ in their expression levels of functional E- and P- selectin ligands, although no previous studies have examined Th17 (10,12–14,23). Hence, we provide new information that demonstrates Th17 cells bind avidly to both P-selectin and E-selectin and express high levels of both E-selectin ligands, PSGL-1 and glyco-CD43. It is notable that Th17 cells have a greater capacity to bind E-selectin than Th1 cells, given the high levels of both E- and P- selectin ligands on Th1 cells, and their ability to bind very avidly to both E- and P- selectins under flow conditions in vitro (10,12–14,23). The enhanced Th17 binding to E-selectin may be mediated in part by glycoCD43, but other E-selectin ligands may be involved, such as those detected by the E-selectin chimeric protein. The fact that E-selectin chimera staining is very high in Th17 cells is consistent with this, although the characterization of these new ligands would require additional biochemical analyses. Another E-selectin ligand candidate is CD44, which is expressed by both leukocytes and endothelium and contributes to T cell recruitment to sites of inflammation and immune reactions (30,48,49) Interestingly CD44 expressed by Th1 and Th2 cells has been shown to contribute to rolling interactions on TNF-α activated microvessels of the intestine (30,48). Intravital microscopy experiments have previously shown that E-selectin function in mediating leukocyte rolling in TNF-α stimulated vessels is largely redundant with that of P-selectin (20,50,51), and consequently, E-selectin deficient mice have only a subtle defect in leukocyte rolling, primarily in neutrophil low velocity rolling (20,50–52). We have found that this is also true for adoptively transferred cells Th17 and Th1 cells, which can also roll in the vasculature of E-selectin deficient mice (Fig 5). In contrast, the lack of E-selectin in mouse heart endothelial cells did result in significantly reduced binding of both T-cell subsets, and, more importantly, the defect in binding to MHEC was significantly more pronounced in Th17 cells. One likely explanation is that endothelium from heart tissue differs from cremaster tissue in cytokine-induced expression level of E- and P-selectin. Earlier studies support this explanation and investigators did find significant heterogeneity in the expression of E- and P-selectin in the vasculature of organs following LPS administration (46). Furthermore, we have shown for the first time that Th17 cells do roll in microvasculature in vivo, in a model that is highly dependent on E-selectin (27,28), and these studies support the high tropism of Th17 cells for E-selectin. Consistent with this idea, adoptively transferred Th17 cells roll more slowly than Th1 cells. Moreover, the competitive rolling assays show that the rolling ratio of Th17/Th1 in WT mice is significantly higher than in E-selectin−/− mice, indicating that the enhanced rolling of Th17 vs Th1 is abolished in the absence of E-Selectin. Taken together, these data support the conclusion that Th17 rolling in the cremaster vasculature is highly dependent on E-Selectin.
In contrast to our competitive rolling studies using adoptively transferred cells and studying rolling interactions within minutes, our air pouch data indicates that both Th17 and Th1 cells are efficiently recruited into the air pouch in response to TNF-α in WT and also in E-Selectin−/− mice. One explanation for this is that in the air pouch model T cells are recruited into the air pouch after several hours, and in that period of time, expression of P-selectin can overcome the absence of E-selectin, as it has been reported in other models of inflammation in which E-Selectin−/− mice are equally susceptible to inflammation (20,41). Th17 have also been found to be increased in the inflamed lung tissue in E-Selectin−/− mice in an experimental model of scleroderma; however this is a chronic inflammatory process with other vascular complications and the increased presence of Th17 cells in the lung cannot only be attributed to Th17-endothelial cell interactions (53).
The regulation of T cell subsets recruitment into tissues represents an important point in the control of the timing, type, and progression of an inflammatory response. Sequential leukocyte-endothelial cell interactions mediated by different adhesion molecules play a prominent role during the inflammatory response, and the fact that these are different between Th17 and Th1 cells can result in new ways of controlling sequential recruitment of effector T cells during the immune response. Chemotaxis studies performed in transwell assays have shown that Th17 cells migrate towards CCL20 whereas Th1 cells do not, and this is abolished in CCR6−/− mice (19), indicating that CCL20-CCR6 interactions are critical in Th17 recruitment during inflammation. Our data provides an additional mechanistic explanation of how this recruitment may work. We demonstrate that CCL20 enhanced binding of Th17 cells to ICAM-1 and to activated endothelial cells under flow, and this step is essential for leukocyte transendothelial migration from the bloodstream into the inflamed tissue. On the other hand, Th1 cells do not respond to CCL20, but do efficiently bind to ICAM-1 and activated endothelial cells in response to SDF-1α. A recent publication shows that human Th17 cells can arrest on CCL20 treated human umbilical vein endothelial cells and mouse fibroblasts expressing ICAM-1 (42). Our data using immobilized ICAM-1 and mouse Th17 cells could be extrapolated to mechanistically explain that arrest to endothelial cells is mediated by CCL20 activation of integrins to induce arrest. The presence of CCL20 in Peyer’s patches and intestinal lamina propria (18) may explain the high numbers of Th17 cells recruited during intestinal inflammation (54), and our findings would support this idea. An additional mechanism of how Th17 cells could be selectively recruited to the intestine could be by interacting with MAdCAM-1. However, our data shows that very few cells bound MAdCAM-1 under low stringency flow conditions, even in the presence of PMA activation or chemokines, and we did not observe differences between Th17 or Th1 cells (Supplemental Figure 4). To our knowledge, there is no published data defining whether Th cell subsets can accumulate on MadCAM-1 under flow conditions, or whether binding requires activation by chemokines. Abramson et al (55) showed that human CD4+CD45RA+ T cells producing IFN-γ bind to immobilized MAdCAM-1, although this was done under static conditions. Park et al (3) also showed binding of T cells activated with anti-CD3 and anti-CD28 mAb and retinoic acid to immobilized MAdCAM-1 under flow conditions, however these were not polarized towards any specific Th cell subset. Given these data sets, we suggest two explanations. First, our in vitro assays are not sensitive enough to detect Th subset adhesion to MAdCAM-1, or there is no difference in the ability of Th1 and Th17 to recognize MAdCAM-1, and therefore this pathway does not explain the Th17 dominance in parts of the intestine.
Despite the well documented presence of Th17 cells in various inflammatory diseases that affect different organs (56,57),very little is known about how Th17 cells, once primed in lymphoid organs, are recruited to these various inflamed tissues. Our results contribute to a better understanding of this process, and shed some light in describing not only what pathways Th17 cells use to migrate, but also how these are different from Th1 cells. Our air pouch studies in WT mice show that a proinflammatory cytokine like TNF-α can induce recruitment of both Th17 and Th1 cells into the subcutaneous air pouch, and no differences are observed among the two subtypes indicating that recruitment in this case is not highly dependent on E-Selectin. However, we show evidence for the first time that a single injection of CCL20 can specifically recruit Th17 cells in vivo.
In summary, our results indicate that Th17 and Th1 cells use different adhesion mechanisms to migrate to tissues during inflammation and these will be activated depending on the chemoattractants and adhesion molecules presented by the vascular endothelium, which may differ depending on the disease, vascular bed, and presence of other inflammatory cells. Taken together, these factors can determine selective recruitment of Th17 cells and partly explain how and why Th17 and Th1 cells appear often at different sites of inflammation, or at the same sites but at different times, and open a window to develop T cell subset-specific therapeutic interventions based in their distinct migratory phenotypes.
Supplementary Material
Acknowledgements
The authors acknowledge Dr. Nir Grabie for the helpful scientific discussion of this manuscript, Gabriel Griffin for contributing to the analysis of the intravital microscopy studies and other members of the Center for Excellence in Vascular Biology, BWH.
These studies were supported by NIH grants HL36028 (AHL, FWL), K99-HL097406 (PA), K08-HL086672 (KC), HL53993 (FWL), a Michael Lerner young investigator award (KC) and a Harris Family Foundation award (KC).
Footnotes
The authors declare no competing financial interests.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Park EJ, Mora JR, Carman CV, Chen J, Sasaki Y, Cheng G, von Andrian UH, Shimaoka M. Aberrant activation of integrin alpha4beta7 suppresses lymphocyte migration to the gut. J. Clin. Invest. 2007;117:2526–2538. doi: 10.1172/JCI31570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 5.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 9.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 10.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman AH, Ding H, Henault L, Vachino G, Camphausen R, Cumming D, Luscinskas FW. CD45RA−RO+ (memory) but not CD45RA+RO− (naive) T cells roll efficiently on E- and P-selectin and vascular cell adhesion molecule-1 under flow. J. Immunol. 1997;158:3640–3650. [PubMed] [Google Scholar]
- 12.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 13.Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J. Exp. Med. 1997;185:573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim YC, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J. Immunol. 1999;162:3193–3201. [PubMed] [Google Scholar]
- 15.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 16.Sato W, Aranami T, Yamamura T. Cutting edge: Human Th17 cells are identified as bearing CCR2+CCR5− phenotype. J. Immunol. 2007;178:7525–7529. doi: 10.4049/jimmunol.178.12.7525. [DOI] [PubMed] [Google Scholar]
- 17.Campbell JJ, O'Connell DJ, Wurbel MA. Cutting Edge: Chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J. Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal. Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J. Exp. Med. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 23.Alcaide P, King SL, Dimitroff CJ, Lim YC, Fuhlbrigge RC, Luscinskas FW. The 130-kDa glycoform of CD43 functions as an E-selectin ligand for activated Th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J. Invest Dermatol. 2007;127:1964–1972. doi: 10.1038/sj.jid.5700805. [DOI] [PubMed] [Google Scholar]
- 24.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr, Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am. J. Pathol. 2003;162:1591–1601. doi: 10.1016/S0002-9440(10)64293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood. 2008;112:1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, Flaumenhaft R, Furie BC, Furie B. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J. Exp. Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards JC, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J. Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- 30.Bonder CS, Clark SR, Norman MU, Johnson P, Kubes P. Use of CD44 by CD4+ Th1 and Th2 lymphocytes to roll and adhere. Blood. 2006;107:4798–4806. doi: 10.1182/blood-2005-09-3581. [DOI] [PubMed] [Google Scholar]
- 31.Mangan PR, O'Quinn D, Harrington L, Bonder CS, Kubes P, Kucik DF, Bullard DC, Weaver CT. Both Th1 and Th2 cells require P-selectin glycoprotein ligand-1 for optimal rolling on inflamed endothelium. Am. J. Pathol. 2005;167:1661–1675. doi: 10.1016/S0002-9440(10)61249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atarashi K, Hirata T, Matsumoto M, Kanemitsu N, Miyasaka M. Rolling of Th1 cells via P-selectin glycoprotein ligand-1 stimulates LFA-1-mediated cell binding to ICAM-1. J. Immunol. 2005;174:1424–1432. doi: 10.4049/jimmunol.174.3.1424. [DOI] [PubMed] [Google Scholar]
- 33.Lim YC, Xie H, Come CE, Alexander SI, Grusby MJ, Lichtman AH, Luscinskas FW. IL-12, STAT4-dependent up-regulation of CD4(+) T cell core 2 beta-1,6-n-acetylglucosaminyltransferase, an enzyme essential for biosynthesis of P-selectin ligands. J. Immunol. 2001;167:4476–4484. doi: 10.4049/jimmunol.167.8.4476. [DOI] [PubMed] [Google Scholar]
- 34.Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, Hirata T. CD43 functions as a ligand for E-Selectin on activated T cells. J. Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 37.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J. Immunol. 1999;162:186–194. [PubMed] [Google Scholar]
- 38.Norman MU, Zbytnuik L, Kubes P. Interferon-gamma limits Th1 lymphocyte adhesion to inflamed endothelium: a nitric oxide regulatory feedback mechanism. Eur. J. Immunol. 2008;38:1368–1380. doi: 10.1002/eji.200737847. [DOI] [PubMed] [Google Scholar]
- 39.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J. Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 40.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 41.Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 42.Ghannam S, Dejou C, Pedretti N, Giot JP, Dorgham K, Boukhaddaoui H, Deleuze V, Bernard FX, Jorgensen C, Yssel H, Pene J. CCL20 and beta-defensin-2 induce arrest of human Th17 cells on inflamed endothelium in vitro under flow conditions. J. Immunol. 2011;186:1411–1420. doi: 10.4049/jimmunol.1000597. [DOI] [PubMed] [Google Scholar]
- 43.Ali S, Robertson H, Wain JH, Isaacs JD, Malik G, Kirby JA. A non-glycosaminoglycan-binding variant of CC chemokine ligand 7 (monocyte chemoattractant protein-3) antagonizes chemokine-mediated inflammation. J. Immunol. 2005;175:1257–1266. doi: 10.4049/jimmunol.175.2.1257. [DOI] [PubMed] [Google Scholar]
- 44.Clish CB, O'Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gladue RP, Cole SH, Roach ML, Tylaska LA, Nelson RT, Shepard RM, McNeish JD, Ogborne KT, Neote KS. The human specific CCR1 antagonist CP-481,715 inhibits cell infiltration and inflammatory responses in human CCR1 transgenic mice. J. Immunol. 2006;176:3141–3148. doi: 10.4049/jimmunol.176.5.3141. [DOI] [PubMed] [Google Scholar]
- 46.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ. Res. 1996;79:560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 47.McEver RP. P-selectin and PSGL-1: exploiting connections between inflammation and venous thrombosis. Thromb. Haemost. 2002;87:364–365. [PubMed] [Google Scholar]
- 48.Buscher K, Riese SB, Shakibaei M, Reich C, Dernedde J, Tauber R, Ley K. The transmembrane domains of L-selectin and CD44 regulate receptor cell surface positioning and leukocyte adhesion under flow. J. Biol. Chem. 2010;285:13490–13497. doi: 10.1074/jbc.M110.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J. Exp. Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ. Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 51.Ley K, Allietta M, Bullard DC, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ. Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- 52.Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, Coggeshall KM, McEver RP. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood. 2010;116:485–494. doi: 10.1182/blood-2009-12-259556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa A, Akiyama Y, Muroi E, Hara T, Ogawa F, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, Tedder TF, Sato S. Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J. Immunol. 2010;185:2502–2515. doi: 10.4049/jimmunol.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 Drives Intestinal Inflammation through Direct Activity on T Cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abramson O, Qiu S, Erle DJ. Preferential production of interferon-gamma by CD4+ T cells expressing the homing receptor integrin alpha4/beta7. Immunology. 2001;103:155–163. doi: 10.1046/j.0019-2805.2001.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 57.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.