Abstract
Pulmonary surfactant lipoproteins lower the surface tension at the alveolar:airway interface of the lung and participate in host defense. Previous studies reported that surfactant protein A (SP-A) inhibits lymphocyte proliferation. We hypothesized that SP-A mediated modulation of T cell activation depends upon the strength, duration and type of lymphocyte activating signals. Modulation of T cell signal strength imparted by different activating agents ex and in vivo in different mouse models, and in vitro with human T cells show a strong correlation between strength of signal (SoS) and functional effects of SP-A interactions. T cell proliferation is enhanced in the presence of SP-A at low SoS imparted by exogenous mitogens, specific antibodies, APCs or in homeostatic proliferation. Proliferation is inhibited at higher SoS imparted by different doses of the same T cell mitogens, or indirect stimuli such as LPS. Importantly, reconstitution with exogenous SP-A into the lungs of SP-A-/- mice stimulated with a strong signal also resulted in suppression of T cell proliferation, while elevating baseline proliferation in unstimulated T cells. These signal strength and SP-A dependent effects are mediated by changes in intracellular Ca2+ levels over time, involving extrinsic Ca2+ activated channels late during activation. These effects are intrinsic to the global T cell population, and are manifested in vivo in naïve as well as memory phenotype T cells. Thus, SP-A appears to integrate signal thresholds to control T cell proliferation.
Introduction
The pulmonary alveolar epithelium is one of the most environmentally exposed tissues in the body. Although it is almost continually bombarded with both innocuous and pathogenic inhaled particles, it normally defends against pathogenic organisms while remaining free of a runaway immune response and inflammation. Many factors that contribute to pulmonary host defense have been identified, one of which is surfactant protein –A (SP-A) (1).
Pulmonary surfactant proteins –A, -B, -C, and -D are produced by the Type II alveolar epithelial cells and to some extent, Clara cells, and then secreted into airspaces in the lung. One function of surfactant is to reduce surface tension at the alveolar air:liquid interface, thereby increasing lung compliance and reducing the work of breathing. The immunomodulatory functions of surfactant are primarily mediated by SP-A and SP-D [reviewed in (2)]. SP-A and SP-D share both sequence and structural homology, and belong to the mammalian collectin family of proteins that includes mannose-binding lectin and conglutinin (3, 4). Surfactant collectins have an amino-terminal collagen-like stalk, a lipid-binding neck and a carboxy-terminal C-type lectin domain. SP-A and SP-D function as soluble scavenger receptors and opsonins, by utilizing their lectin-domains to bind carbohydrate-containing molecules including glycolipids and glycoproteins on the cell walls or membranes of infectious agents (5). This interaction triggers the innate immune response, leading to increased phagocytosis and clearance of inhaled pathogens (6, 7). SP-A, which is approximately 10 fold more abundant than SP-D, can also modulate levels of reactive oxygen and nitrogen intermediates and secretion of inflammatory cytokines (8). Indeed, SP-A deficient mice generally have increased susceptibility to intratracheal administration of bacteria and viruses, as well as enhanced LPS-induced lung inflammation (9). On the other hand, SP-A mediates resolution of inflammation and a runaway innate response through enhanced clearance of apoptotic neutrophils (10, 11), suppression of cytokine production induced by Gram-negative organisms (12), and inhibition of NADPH oxidase (13). SP-A also regulates T cell mediated adaptive immunity (14). However, unlike its beneficial effects on APC and neutrophil function, to date, SP-A has only been shown to suppress allergen- and mitogen-induced T cell proliferation (14-16) and IL-2 secretion (17). Previous work in our lab has demonstrated both an IL-2 dependent and – independent effect of functional SP-A interactions on T cells in vitro (18).
T cell activation is a complex, multistep process driven by both a primary signal through the TCR as well as a costimulatory signal. This initial interaction regulates multiple cellular processes, and is modulated by several factors, e.g. the affinity and avidity of the corresponding MHC:peptide complexes, and the frequency and duration of interaction. Although SP-A has been shown to bind CD93, CD91, SIRP-1α, TLR2 and TLR4 (19-21), none of these receptors are identified on naïve T cells, or enhanced on memory cells and the SP-A receptor involved in regulation of T-cells remains undefined. Polymorphisms in human SP-A have been associated with a range of conditions ranging from predisposition to allergic rhinitis and otitis media to associations with meningococcal disease and respiratory synctial virus (22-26). Naïve and memory T cells continually migrate through the lung, and are present in large numbers in the alveolar region as well as the lamina propia of the bronchi (27, 28). The SP-A mediated suppression of T cell proliferation seems surprising in light of the importance of these cells in modulating both adaptive and innate immune responses. Hence, we hypothesized that SP-A may play a differential role in modulating T cell activation depending upon the strength of the activating signal. In the present study, we utilized a variety of different stimuli, including APC-MHC:peptide interactions, mitogens and pharmacological agents to demonstrate dose-dependent opposing responses of SP-A interactions in both mouse and human T cells ex vivo, and to establish a possible mechanism for the observed effects. Additionally, we demonstrate similar phenotypes in vivo, utilizing baseline maintenance proliferation (usually driven by weakly agonist self-peptides) and exogenously added stimuli to mimic weak and strong signals respectively.
Materials and Methods
Mice and human samples
SP-A-/- mice were generated as previously described (29) and back-crossed to C57BL/6N background for 12 generations. WT mice were obtained from littermates in heterogeneous breedings or from Charles River Laboratories (CRL, Wilmington MA). Mice aged 8-12 week were used for all experiments, which were performed independently with both male and female mice. All mice were housed in a barrier facility, and all procedures were performed according to local and National Institutes of Health guidelines and were approved by the Duke University Institutional Animal Care and Use Committee. Human blood samples from healthy volunteers were collected in BD Vacutainer tubes per IRB guidelines and used for isolation of T cells as described below.
SP-A preparation and analyses
SP-A was purified from the lung lavage fluid of patients with alveolar proteinosis as described previously (30). Briefly, the lavage fluid was initially treated with butanol to extract the SP-A. The resulting pellet was then sequentially solubilized in the detergent octylglucoside and 5 mM Tris, pH 7.4. Extracted SP-A was then passed over a polymyxin B-agarose column to reduce endotoxin contamination. SP-A preparations had final endotoxin concentrations of <0.01pg/mg SP-A as determined by the Limulus amoebocyte lysate assay according to manufacturers’ instructions (QCL-1000, BioWhittaker (Lonza), MD). In some experiments, SP-A was labeled according to the standard Molecular Probes protocol, and density of labeling was calculated using an extinction coefficient of 239000 for AF647 and 72000 for SP-A. While no active TGFβ was detected in the SP-A preparations utilized for this study, we did find varying amounts of inactive (‘total’) TGFβ ranging from ~1.6-2.8 pg/μg of purified SP-A using both a bioassay (CCL64 cells) as well as an ELISA (R&D Systems). A CCL64 mink lung epithelial cell line with a PAI1 luciferase reporter system was utilized to determine biological activity of TGFβ (diluted to contain <0.4% FBS during assay) using either purified human platelet derived or recombinant TGFβ (Peprotech or R&D Systems) as a reference standard.
Media and antibodies
RPMI 1640 with 5% heat inactivated FBS (Hyclone), 25 mM HEPES, 5 μM 2-mercaptoethanol, penicillin-streptomycin (100 U/ml), and 2 mM L-glutamine (all from Gibco Invitrogen, NY) was utilized as the primary culture medium (complete RPMI). All antibodies used for activation or flow cytometry were obtained from BioLegend, eBioScience or BD Pharmingen. Low-endotoxin azide free anti-CD3 (clone UCHT1 human, 145-2C11 mouse) and anti-CD28 (clone CD28.2 human, 38.51 mouse) were used for ex vivo activation assays.
T cell and BMDC isolation
Splenocytes or lymphocytes were gently teased out from the tissue onto uncoated polystyrene plates. Lungs were diced and digested with collagenase A (Roche) and DNAse I (Worthington) for 30 minutes, and the reaction was stopped with excess EDTA and FBS. Cells from either source were filtered through a 40-μm nylon strainer to obtain a single cell suspension. The cell suspension was then subject to density gradient centrifugation using Histopaque 1083 (Sigma). The large left lobe from each mouse was used for flow cytometric analysis. Human T cells were enriched from blood samples using Histopaque 1077 or OptiPrep density gradients. RBC lysing solution (BioLegend) was utilized to remove any residual RBCs. Purified T cells were then obtained by negative selection using an appropriate MACS antibody cocktail and paramagnetic microbeads (Miltenyi Biotec or Dynal Invitrogen) to deplete contaminating cells. For some experiments, CD4+ T cells were subsequently negatively selected using paramagnetic microbeads (Miltenyi), or CD8+ cells purified using the Lympholyte Pure system (Cedarlane Labs). T cell purities averaged 96-98% for pan-T cells, and 99% for highly purified subpopulations. BMDCs were generated using marrow from the tibia and femur bones of mice, which was harvested, washed and cultured in complete RPMI 1640 supplemented 5% GM-CSF conditioned medium for 6 days (30). Loosely attached cells were harvested and negatively selected with biotinylated-Gr-1 Abs (BD Pharmingen) and streptavidin paramagnetic microbeads (Miltenyi Biotec).
Ex vivo activation assays and ELISA
Purified, freshly isolated T cells (~100,000) were incubated under different experimental conditions in 96-well round-bottom plates in complete RPMI. Each well received 0.8 μCi of [3H]thymidine (6.7 Ci/mmol; MP Bio) and cells were incubated for another 15 h. Incorporated radioactivity (as an indicator of proliferation) was measured by liquid scintillation using CytoScint ES (MP Bio) on a TriCarb 2100TR (Packard Instruments) or MiniBeta counter. To track cell divisions, freshly isolated T cells were labeled with 0.4 μM CFSE (Molecular Probes #C34554). Excess label was quenched and activation assays were performed. At the indicated time points, cells were harvested, stained as described and data were acquired on a BD LSRII flow cytometer. All ELISAs were performed using eBioScience Ready-Set-Go kits according to manufacturer instructions. The amount of IL-2 in culture supernatants was determined using a 4-parameter fit equation derived from values obtained known amounts of standard cytokine after background subtraction (BMG Labtech / Optima software).
In vivo studies
Two methods were utilized to track cell division in vivo. In some experiments, BrdU was instilled in mice to track proliferation. Mice were initially injected ip with 200 μl of 4 mg/ml BrdU (Sigma). Barrier cages were fitted with deprivation caps, and mice were allowed access to 0.8 mg/ml BrdU in 4 or 5% sucrose supplemented drinking water ad libitum (32). For some experiments, purified SP-A (unlabelled or AF647 labeled, 15 μg/50 μl/dose) and/or PMA+I (250 ng, 250 nM) was administrated intratracheally in order to determine whether exogenous SP-A could modify the lung T cell phenotype. For CFSE tracking studies, a combination of SP-A, PMA+I, LPS (Sigma, E. coli O55:B5) or CFSE (Molecular Probes) administration was performed by an oropharyngeal aspiration method. Briefly, mice were anesthetized by isoflurane, and suspended by their upper incisors with wire on a ~70° inclined frame. The tongue was gently extended and SP-A/PMA+I/LPS/CFSE solution or USP saline (Sigma) was pipetted into the mouth. Brief occlusion of the nose forced the animal to inhale through the mouth, thereby aspirating the solution into the respiratory tract in one or two breaths. Animals were subsequently removed from the support and observed closely until fully recovered from anesthesia.
Flow cytometry and fluorimetry
Flow cytometry was performed using a BD LSRII (BD Biosciences, CA) at the Duke University Human Vaccine Institute Flow Cytometry Core, which is supported by the National Institutes of Health Award AI-51445. Multiple panels of multi-color surface staining on T cells were set up using anti-CD3, CD4, CD8, CD25, CD44, CD62L, and FR4 to distinguish specific cell populations. For intracellular staining using Foxp3, IL-4 or anti-BrdU antibodies, cells were washed well after surface staining, fixed in 10% neutral buffered formalin, and permeabilized with 0.3% saponin in HBSS+0.5% FBS for BrdU or Foxp3 staining, or 0.1% Triton X100 in HBSS+0.5% FBS for cytokine staining. Brefeldin A for intracellular cytokine staining was obtained from eBioScience, and treatment was performed for ~5-6 h prior to staining. Since the anti-BrdU antibody was conjugated to biotin, an additional intracellular staining with either streptavidin-AF633 (Molecular Probes) or streptavidin-AF488 (BioLegend) was performed. All FACS data were analyzed with FlowJo software (TreeStar Inc., OR) using default or CFSE-specific plugins. Free Ca2+ levels in the cytoplasm were measured by loading the cells with 1 μM Fluo-4 acetoxymethyl ester (Molecular Probes) at 37°C for 45 minutes, or with Fluo-4-Direct+probenicid at 37°C for 60 minutes. The esterified dye is cell permeable, and after hydrolytic cleavage within the cell, the fluorophore is caged intracellularly. The increase in fluorescence intensity with greater levels of [Ca2+]i was measured on a LSRII or BMG Labtech Optima fluorimeter. Pluronic F127 (Calbiochem) was used as a dispersant in the regular formulation. The Fluo-4-Direct buffer + water soluble probenicid helps reduce fluorescence caused by dye leakage out of the cells.
Statistics
Data are expressed as mean ± SEM unless otherwise indicated. Statistical significance was tested with an unpaired Student's t test or non-parametric ANOVA using Prism 4b (GraphPad Software Inc, CA). Statistically significant differences were determined by p<0.05.
Results
SP-A-/- mice display inverse T cell proliferation profiles compared to WT mice in response to low and high dose LPS in vivo
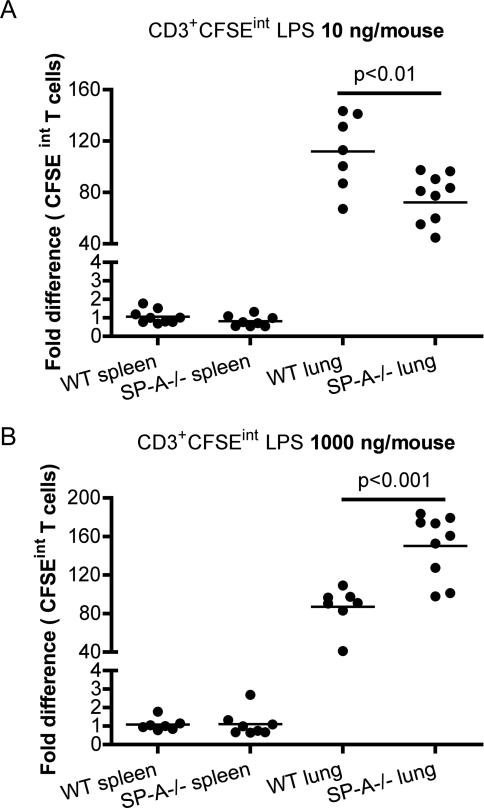
In order to determine the functional role of SP-A in regulating T cell responses to low and high signal strengths, we utilized the broad spectrum response invoked by LPS to activate T cells in the lung. The total number of cell divisions in vivo was tracked using CFSE. CFSE is a vital dye that binds covalently to lysine residues of intracellular proteins. Excess, unbound dye is typically cleared in 12-15 h. Since previous studies have determined that it is non-toxic (33), a high dose CFSE treatment (125 μM instead of the 0.5 μM dose used for pre-labeling cells in vitro) was performed to label cells in vivo. As the cell divides, CFSE fluorescence is reduced by half with every division, giving a range of intermediate fluorescence intensities in actively dividing cells. Thus, the presence of intermediate levels of fluorescence of CFSE (CFSE-int) indicates the occurrence of cell division. Wild type and SP-A-/- mice were oropharyngeally instilled with different concentrations of LPS, in conjunction with CFSE, in (Figs. 1A-B). CFSE-int gates were defined based on CFSE treated lung lymphocytes (undivided, CFSE-hi) and splenocytes (unlabeled, CFSE-lo) isolated from WT saline treated mice. Less than 0.5% of T cells from spleens of saline treated mice were present in the CFSE-int gates, since labeling was performed in the lung. Total CD3+ T cells in the lung remain nearly identical at baseline, with no significant differences. At very low concentrations of LPS (10 ng per mouse), SP-A-/- mice had significantly lower proportions of CFSE-int T cells, indicating reduced proliferation compared to that seen at higher concentrations of LPS (with up to six division cycles tracked). At concentrations of LPS which correspond to strong activation (1,000 or 10,000 ng per mouse), the profile is reversed in SP-A-/- mice, indicating that the SP-A inhibits T cell proliferation in vivo. This aspect is reflected in the T cell numbers isolated: WT saline range: 0.794-1.323×106; SP-A-/- saline: 0.713-1.198 ×106, WT LPS-lo: 1.795-2.258 ×106, SPA-/- LPS-lo: 1.509-2.081 ×106, WT LPS-hi 2.307-2.415 ×106, SPA-/- LPS-hi 2.794-3.138 ×106. Thus, SP-A enhances T cell proliferation with low-grade stimulation, and inhibits proliferation with stronger activating signals.
Figure 1. SP-A can either enhance or suppresses exogenous stimulation induced T cell activation in the lung in vivo.
Mice were oropharyngeally instilled with low (10 ng/mouse) or high (1000 ng/mouse) LPS in conjunction with CFSE. Single cell suspensions from spleen and lung digests were prepared after 68-72 h, stained with T cell markers and analyzed for CFSE-int (divided cells) by flow cytometry (1A-B). Data is normalized to WT spleen, and represented as the fold difference in CFSE-int T cells from either 8 or 9 mice per group, with three independent experiments. Proliferating BrdU+ T cells in lungs or spleen were identified by intracellular flow cytometric staining in conjunction with surface markers. * p<0.05 compared to WT mice.
SP-A-/- mice have reduced levels of memory T cell proliferation at baseline
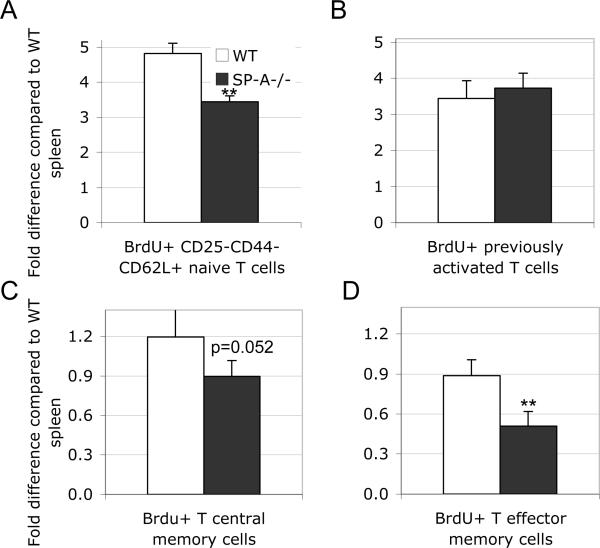
Since SP-A appears to have differential effects depending on the signal strength in vivo, we continued to investigate the significance of SP-A mediated change from a stimulatory to an inhibitory response. Basal T cell proliferation in vivo is driven by weakly agonist self-peptide:MHC interactions. To further elaborate the effects of SP-A in cells with weak signals and to confirm our LPS mediated low SoS observations, we examined this baseline T cell proliferation using BrdU labeling in vivo. BrdU incorporation was tracked over 4 days in both WT and SP-A-/- mice without any exogenous activation, and sub-populations of T cells from the lung were analyzed as described in Materials and Methods. BrdU incorporation by the analyzed cell types in the spleen from WT mice in each experiment was used to normalize the BrdU levels in lung cell populations. Naïve phenotype CD3+ T cells showed a reduction in homeostatic proliferation in the lung in the SP-A-/- mice (Fig. 2A), while no differences were observed in previously activated cells (Fig. 2B). Central and effector memory cells (Figs. 2C-D) tended to show reduced proliferation in the absence of SP-A, although statistically significant differences were only seen in effector memory cells. Thus, SP-A enhances T cell proliferation with weak or homeostatic maintenance signals. These results suggest that SP-A affects multiple T cell phenotypes even under normal homeostatic conditions, and not just in response to inflammatory or allergic stimuli.
Figure 2. SP-A enhances basal low level proliferation of T cell subsets in the lung in vivo.
Wild type or SP-A-/- mice were treated with i.p. BrdU and in drinking water over 4 d as described in Methods. Single cell preparations from the lungs were labeled with T cell markers to determine naïve, pre-activated, central memory or effector memory phenotypes (2A-D). All populations were concurrently analyzed for BrdU incorporation in the nucleus. Data is representative of 3 pooled samples from 3-4 mice in 3 independent experiments, and is depicted as mean fold difference normalized using the appropriate splenic T cell population as unity. *p<0.02, **p<0.01 compared to respective WT conditions.
SP-A specifically enhances or suppresses human and mouse T cell activation
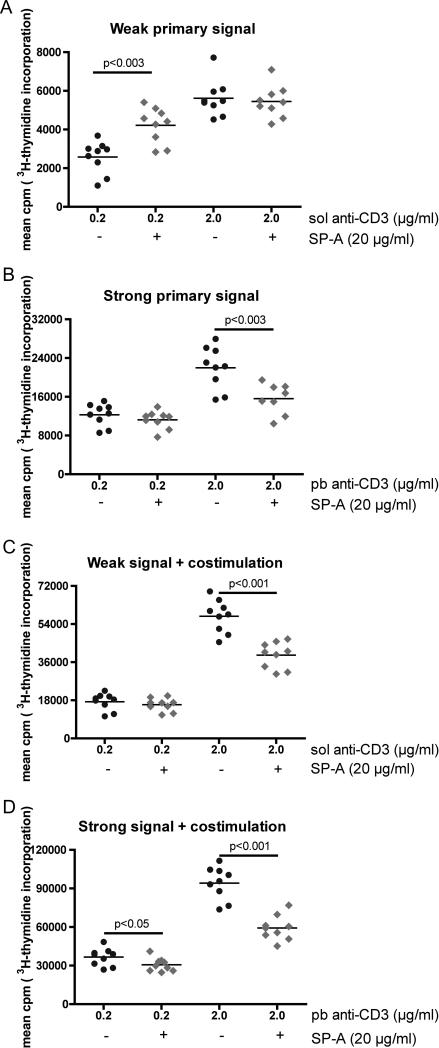
To determine whether human T cells also have a signal strength dependent response with SP-A, T cells from multiple normal subjects were purified from PBMCs and activated with anti-CD3 in a variety of different doses and presentations to mimic a range of different intensities of TCR stimulation (strength of signal, Fig. 3A). Plate-bound (pb) anti-CD3 immobilized onto tissue culture plates forms a solid support matrix that allows for maximal cross-linking and elicits a stronger strength of signal compared to soluble (sol) anti-CD3, which is flexible and generates a weaker signal (34, 35). The effect of signal strength on cell proliferation was determined by measuring incorporation of [3H]-thymidine by actively dividing cells. Increased incorporation corresponds to increased cpm as presentation is switched to a plate bound format, with comparable amounts of anti-CD3 (Fig. 3B). A further increase in thymidine incorporation was observed when exogenous costimulation imparted by anti-CD28 is present, in both sol and pb anti-CD3 stimulated conditions (Fig. 3C-D). As previously reported (17), the addition of purified human SP-A into cultures activated with a relatively high strength of signal resulted in a suppression of T cell proliferation. However, when SP-A was added to cells activated with the lowest strength of signal, we observed a consistent 30-50% enhancement in cell proliferation (weak signal, no costimulation, Fig. 3A). These results were further corroborated by cell cycle analyses (supplementary table 1), where we observed a SP-A-induced increase in proliferation with sol anti-CD3, and inhibition of proliferation with pb anti-CD3. The stronger signal imparted by anti-CD28 mediated costimulation invariably resulted in inhibition of proliferation in the presence of SP-A.
Figure 3. Human T cells respond to SP-A in an integrated strength of signal dependent manner.
Purified human T cells from PBMCs were stimulated for a total of 75 h with either soluble anti-CD3 (sol aCD3: A and C) or plate-bound anti-CD3 (pb aCD3: B and D) in the absence or presence of anti-CD28. Control Ig or purified human SP-A (20 μg/ml) was added as indicated. Mean cpm of [3H]-thymidine incorporation over 15 h by proliferating cells from replicate wells in 9 individual subjects is depicted. Scales for mean cpm on the Y-axis expand with increasing levels of stimulation. p values from the Mann-Whitney test are as indicated in the plots.
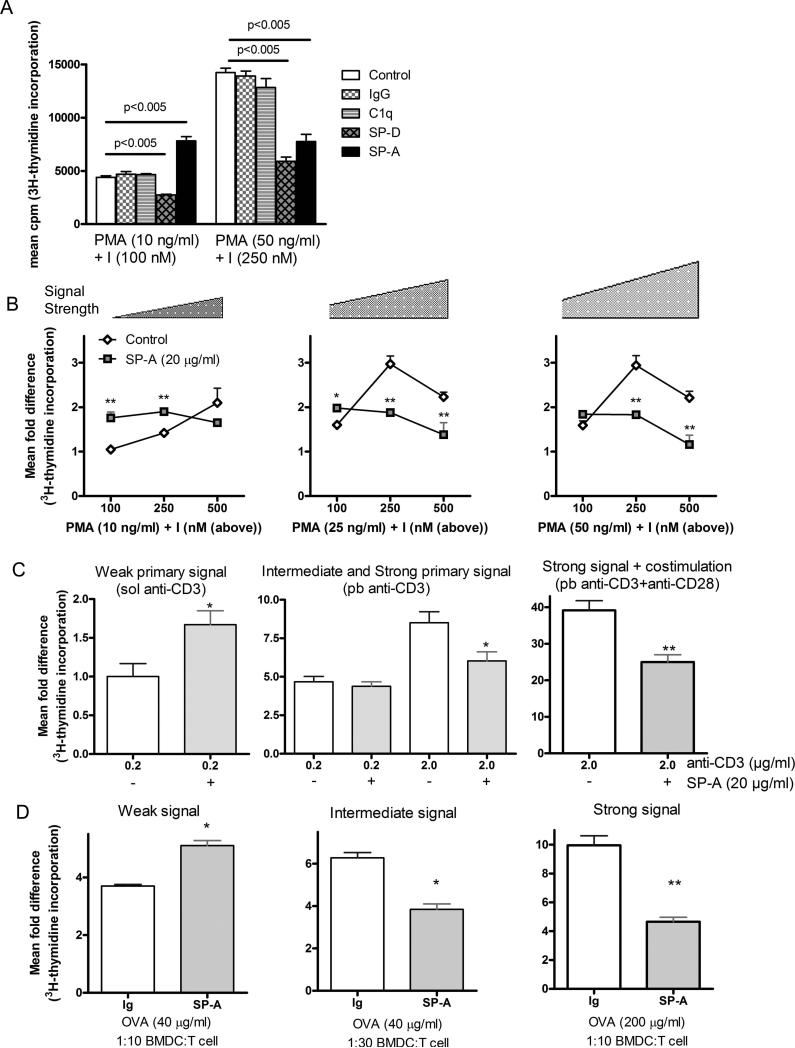
Neither the structurally similar protein C1q nor a mouse Ig control exhibited strength of signal dependent effects on mouse T cell proliferation, using low and high doses of the pharmacological mitogens, PMA and ionomycin (PMA+I) (Fig. 4A), suggesting that the effects observed with SP-A are not shared even by a structurally homologous protein. The other immune surfactant collectin, SP-D, also showed no SoS dependent effects on proliferation, inhibiting cell division at both low and high SoS, consistent with previous observations (36). These results suggest that SP-A acts as a putative costimulator to specifically enhance cell proliferation in both human and mouse T cells stimulated with weak activation signals.
Figure 4. SP-A mediated modulation of T cell proliferation is specific and depends upon the strength of activating signal.
Primary mouse T cells were purified and activated with the indicated proteins (20 μg/ml) and defined amounts of PMA+I or anti-CD3 ± anti-CD28 for 50 h with a [3H]-thymidine pulse during the last 15 h. Data is represented as mean cpm (4A) or as fold difference normalized to the lowest dose of PMA+I (4B) or soluble anti-CD3 alone (4C) and are representative of 3-6 independent experiments with pooled cells obtained from 5-9 mice per experiment. A total of nine different concentrations of PMA and I covering a range of defined signal strengths are depicted. p values from pairwise comparisons are as indicated in Fig. 2A. *p<0.05, **p<0.005 compared to respective no-SP-A treated condition. Purified T cells from OVA-primed mice were activated with different amounts of ovalbumin and varying ratios of OVA-primed BMDCs to establish different levels of signaling. Ratios depicted (4D) include those with 10,000 BMDCs for 100,000 purified T cells (1:10) or 3350 BMDCs for 100,000 T cells (1:30). Control Ig or exogenous SP-A (20 μg/ml) treatment was performed as indicated in the plots. The activation culture was performed for a total of 65 h, with a [3H]-thymidine pulse during the last 15 h. Data is represented as mean fold difference to corresponding ratios of non-primed BMDCs and T cells without OVA. An increased cpm in the control conditions corresponds to greater signal strength, as indicated by the scale bar on the plots. *p<0.05, **p<0.02 compared to respective control Ig treated conditions. Data is representative of three to five independent experiments with pooled cells obtained from 3-4 mice per experiment.
SP-A affects T cell responses with multiple activation models to modulate signal strength in vitro
To elucidate the role of signal strength and SP-A in T cell activation, we used the pharmacological agents PMA+I. These agonists provide a defined activation signal that stimulates two key signal transduction pathways in T cells (PI3K-NFκB and Ca2+-NFAT, respectively), and their concentrations can be titered to show a linear response. These experiments demonstrated a switch from a SP-A mediated stimulatory response at low PMA+I, to a suppressive response as the concentration of PMA+I was increased (Fig. 4B), consistent with SoS dependent effects of SP-A observed with direct stimulation of the TCR (Fig. 3). Signal strength in this system showed a greater dependence on ionomycin induced [Ca2+]i flux compared to PI3K activation by PMA, as evidenced by the gradation of response in the left panel with low PMA and increasing amounts of ionomycin. However, both PMA and ionomycin were required for a T cell response (also shown in (37)).
SP-A induced enhancement of proliferation was also observed with purified mouse T cells activated with low dose sol anti-CD3 (Fig. 4C). As previously observed with human T cells, stronger signals (manifested by increased incorporation of [3H]-thymidine compared to cells stimulated with sol anti-CD3 alone) imparted by pb-cross-linked anti-CD3 or the presence of CD28 resulted in the suppression of proliferation in the presence of exogenous SP-A. These SP-A mediated effects saturated at 12.5-15.0 μg/ml of SP-A from four low endotoxin SP-A preparations. Thus, as the strength of activating signal imparted to T cells increases, the SP-A mediated enhancement of proliferation is replaced by an inhibitory response.
To determine whether SP-A exhibited SoS dependent effects in the presence of APC mediated activation, we also stimulated lung T cells from i.p. OVA treated mice with different ratios of ovalbumin (OVA)-primed BMDCs, and different amounts of OVA. Proliferation was assayed by [3H]-thymidine incorporation and different ratios of APCs and OVA amounts were tested. In this system, signal strength is non-linearly defined by both ratio of BMDC: T cells (within limits, lower numbers of BMDCs counter-intuitively provided better T cell stimulation), as well as concentration of OVA. Hence, we used a series of different ratios and amounts of OVA, and three representative conditions with steadily increasing mean cpm (from left to right panels), are shown in Fig. 4D, and reported as fold difference compared to unstimulated control (T cells+unprimed BMDCs). In the weakly activating condition, at a 1:10 ratio of BMDCs to T cells with 40 μg/ml OVA, lung-derived T cells show ~30% enhanced proliferation in response to exogenously added SP-A. However, when the ratio is changed to 1:30 with the same amount of OVA (40 μg/ml), these T cells showed a 40% suppression of proliferation in the presence of SP-A. Even greater suppression was observed when OVA concentration increased to 200 μg/ml 1:10 BMDC:T cell ratio. Thus, proliferation over baseline proportionately increased as the total signal strength increased from left to right, with the greatest degree of SP-A mediated suppression at the highest signal strength.
SP-A enables early division cycles in lung T cells with low and high signal strength but suppresses later divisions with a strong signal
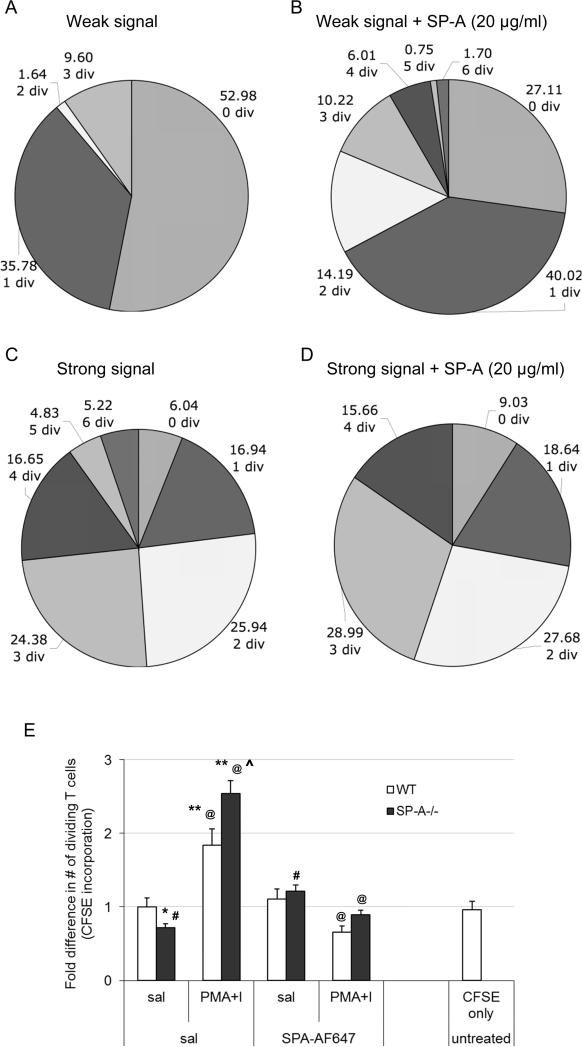
Next, we determined the kinetics of the SP-A mediated activation signal integration mechanism. CFSE-labeled purified T cells were activated with different SoS in the absence or presence of SP-A, and cell division analysis was performed. As expected, low SoS resulted in a lower magnitude of proliferative response, with a large proportion of undivided cells, and few cells with two or more complete division cycles (Fig. 5A). At low signal strength in the presence of SP-A, we observed ~50% decrease in undivided cells with a concomitant three-fold increase in the number of cells with two or more cell divisions, as well as significantly more cells with a greater number of cell divisions (Fig. 5B). This profile was reversed with high SoS (Figs. 5C and D), where fewer cell divisions were observed in the presence of SP-A. Interestingly, since the initial cell divisions are very comparable in both conditions, it appears that the presence of SP-A under strong activation signals does allow for initial rounds of cell division. As seen in figure 5 C and D, cells within the first 3 divisions account for 73.3% and 84.34% of total T cells in the absence or presence of SP-A respectively. However, in the presence of a strong signal in the absence of SP-A, 26.7% of cells are at 4 or more divisions, compared to 15.66% in the presence of SP-A. Thus, the suppressive effect becomes predominant by the 3rd and 4th rounds of cell division.
Figure 5. SP-A mediated regulation of proliferation is ongoing during the course of T cell activation.
CD4+ T cells were pulsed with CFSE immediately prior to activation with either a weak (PMA 10 ng/ml + I 100 nM) or strong signal (PMA 25 ng/ml + I 250 nM). Control Ig (5A and C) or SP-A (20 μg/ml, 5B and D) were added as indicated, and cells were analyzed by flow cytometry for total number of generations. Each section of the pie chart indicates a generational span, ranging from 0 (no cell divisions) to 7 (6 cell division events). The number of cell divisions, as well as the proportion of cells that have undergone those divisions is shown adjacent to each section. This data is representative of three independent experiments, each with either 3 or 4 mice per condition. In order to determine responses to a defined, strong exogenous stimulation (5E), WT or SP-A-/- mice were initially injected with CFSE i.v. All mice were then treated with saline or AF647-labeled SP-A i.t., following which either saline or PMA+I (250 ng/ml, 250 nM) was instilled i.t. as indicated. Lungs from individual mice were harvested 24-26 h later, stained with surface markers to identify T cells and analyzed by flow cytometry. Data is representative of samples from 3 independent experiments with 3 mice per condition, and is depicted as fold difference in % CFSE+ T cells that have undergone 1 or more cell divisions in vivo. *p<0.05, **p<0.01 compared to the WT saline treated condition. @ p<0.01 in a pair wise comparison between samples with exogenous activation in the absence or presence of instilled SP-A. ^p<0.05 compared to WT mice activated with PMA+I in vivo. # p<0.05 in a pair wise comparison with SP-A-/- mice treated with saline only, without any exogenous SP-A.
Reconstitution with exogenous SP-A results in elevated levels of proliferation at baseline and decreased proliferation with high dose PMA
To confirm our in vitro observations, we studied the proliferative response to defined signals in both WT and SP-A-/- mice in vivo. Mice were instilled with saline or PMA+I in the absence or presence of exogenous AF647-labeled SP-A, or left completely untreated. Single cell suspensions from the lungs showed ~5-12% labeling with CFSE (CFSE-hi), giving us a sampling efficiency of 1 of every 8 to 20 cells isolated. As previously observed with BrdU incorporation in Fig. 2A and 1C, a small but significant decrease in baseline proliferation was observed in saline treated SP-A-/- mice compared to WT mice (Fig. 5E, * p<0.05). This was completely reversed when exogenous SP-A was instilled into the lungs of SP-A-/- mice prior to stimulation with PMA+I (#p<0.05), suggesting that SP-A was playing a role in maintaining basal proliferation levels. Local instillation of PMA+I in vivo lead to increased levels of CFSE-int cells that had undergone one or more cell divisions, in both WT and SP-A-/- mice (** p<0.01). However, SP-A-/- mice showed a significant increase in the proportion of divided cells over their WT counterparts (^ p<0.05). The presence of exogenously added SP-A-AF647 resulted in a sharp decrease in percentage of divided T cells (p<0.05) in both WT and SP-A mice activated with PMA+I in vivo. No significant differences in the activation profiles were observed in parallel experiments performed to compare any functional differences between unlabeled and AF647-labeled SP-A (data not shown). Thus, these results indicate that SP-A enhances cell division with low dose activation and inhibits later stage division with strong activation.
SoS and SP-A dependent levels of [Ca2+]i over several hours provides a mechanism for differential T cell activation
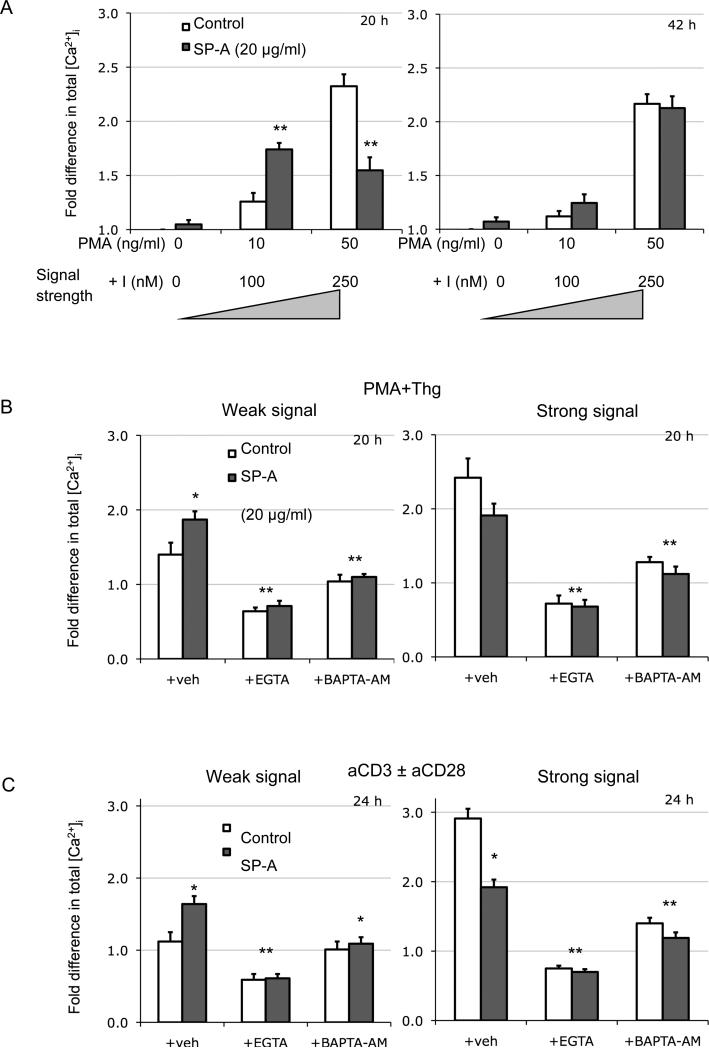
Based on our experiments with PMA+I where the dose of ionomycin appeared to play a dominant role in determining the signal thresholds, we also analyzed levels of intracellular Ca2+ in the presence and absence of SP-A several minutes to hours after activation with various SoS. The fact that initial cell divisions appeared to proceed normally even when SP-A eventually suppresses proliferation (Fig. 5), and lack of significant changes in the initial [Ca2+]i spike (data not shown) suggests that changes in initial proximal signaling are not playing a critical role in the observed effects of SP-A. Therefore, we also assayed for [Ca2+]i levels at varying intervals from 30 mins to 80 h post-activation with low or high doses of PMA+I in the absence or presence of SP-A. The concentrations of PMA+I used were derived from experiments in Fig. 4B to cover weak and strong signals (10+100 and 50 ng/ml+250 nM PMA+I respectively). The Ca2+ flux, triggered by the depletion of intracellular stores, has been previously reported to play a critical role in maintenance of B and T cell activation (38, 39). At intermediate periods of activation between 15-30 h (the 20 h time point is shown in Fig. 6A), [Ca2+]i levels mirrored the final T cell proliferative response. At low SoS, the presence of SP-A enhanced [Ca2+]i by ~140%, while at high SoS, [Ca2+]i levels were reduced ~60% compared to control. However, this difference in Ca2+ capacitance disappeared over time (e.g. at 42 h post activation), even as total [Ca2+]i levels gradually dropped to near baseline levels.
Figure 6. [Ca2+]i levels are enhanced in the presence of SP-A at lower signal strengths irrespective of mode of T cell activation.
[Ca2+]i measurements were performed from cells activated for various periods with low or high doses of PMA+I (7A), PMA+Thg (7B) or anti-CD3 ± anti-CD28 (7C) in the absence or presence of SP-A (20 μg/ml). During the last 60 mins of activation, cells were also pulsed with Fluo-4 and analyzed by flow cytometry. Two distinct time points representing the two [Ca2+]i profiles are depicted in Figure 7A. In 7B and C, cells were also suspended in buffer containing EGTA or BAPTA-AM during activation. All data is normalized to baseline [Ca2+]i levels in cells alone conditions at the indicated time points post-activation, and are representative of 3 or 4 independent experiments with pooled cells from the lungs of 5 mice. *p<0.05, **p<0.01 compared to respective control condition.
To attribute these effects to I-mediated increase in [Ca2+]i, we utilized another compound, i.e. Thapsigargin (Thg) in combination with PMA (10 ng/ml). Thg specifically increases [Ca2+]i by blocking [Ca2+]i transport to the sarcoplasmic and endoplasmic reticulum, and opens IP3 gated channels in the ER. While we still observed a small but significant increase with SP-A treated cells in the presence of a weak signal, the magnitude of this effect was much reduced. In addition, EGTA, which acts as an extracellular Ca2+ chelator, completely eliminated the SP-A mediated effect while simultaneously reducing total fluorescence (Fig. 6B). Since Thg preferentially acts on intracellular reserves of Ca2+, these results suggest that SP-A helps modulate the influx of extracellular Ca2+ into the cell. Since previous experiments utilized the pharmacological agents PMA, I and Thg which activate T cells bypassing TCR-CD3 signaling, we used anti-CD3, which triggers activation via the surface TCR-CD3 complex to further corroborate these observations (Fig. 6C). T cells were activated with low dose (0.2 μg/ml) sol anti-CD3 alone, which delivers a weak signal or high dose anti-CD3+anti-CD28. Weak anti-CD3 mediated activation led to a marginal (<10%) increase in [Ca2+]i compared to unactivated cells. However, [Ca2+]i was enhanced nearly 60% over baseline in the presence of exogenous SP-A. The opposite effect was observed with high dose anti-CD3+anti-CD28, where SP-A reduced [Ca2+]i by ~34%. The addition of either BAPTA-AM, an intracellular calcium chelator, or extracellular EGTA dramatically reduced [Ca2+]i as well as T cell proliferation. The dependence of T cell activation on extracellular Ca2+ fluxes has been very well established. EGTA has profound effects on T cell activation irrespective of the presence or absence of SP-A, and acts as an experimental control to block activation in order to determine the relative importance of internal stores (with Thg mediated activation) versus total calcium (with anti-CD3 mediated activation) with weak and strong activation signals. Thus, the Ca2+ channel induced Ca2+ flux might play a key role in predicating the final outcome of SP-A mediated modulation of T cell activation.
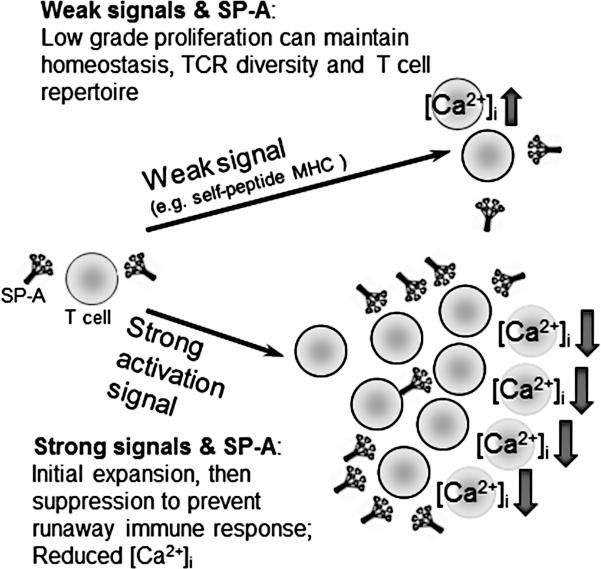
Taken together, as summarized in the model in Fig. 7, these data suggest that SP-A seems to affect T cell proliferation by functioning like a signal strength dependent rheostat, enhancing [Ca2+]i levels and influx of extracellular Ca2+ throughout the course of activation with low signals, while reducing [Ca2+]i levels with strong activation signals.
Figure 7. A schematic model summarizing the differential role of SP-A in modulating T cell activation and proliferation depending upon the strength of activating signal.
SP-A allows for low grade, basal levels of proliferation in part by maintaining [Ca2+]i levels, and thus may help maintain the immune repertoire in T cells. Initial expansion in response to strong signals does occur in the presence of SP-A, but it acts as a brake to prevent runaway inflammation at later stages of strong T cell responses. Since extensive proliferation and differentiation to terminal effector cells that ultimately undergo activation induced cell death is avoided, the generation of multifunctional T cells that eventually form memory cells might be enhanced in the presence of SP-A.
Discussion
This study demonstrates that SP-A differentially modulates responses of T cells from both humans and mice depending on the strength of activating signal. At high levels of signal strength, interaction with SP-A suppresses T cell activation, irrespective of the type of applied signal: pharmacological agents, anti-CD3 (soluble or plate bound), or MHCII:peptide presentation by APC. This suppression by SP-A is consistent with previous reports (15, 16, 40). Although we did not observe enhanced neutrophil or TNFα levels in SP-A-/- mice at 3 days post-LPS exposure, it is possible that some of the enhanced proliferation observed with high dose LPS in the SP-A-/- may be due to greater levels of initial inflammation at earlier time points. Reconstitution with exogenous SP-A into SP-A-/- mice stimulated with a strong signal also resulted in suppression of T cell proliferation (Fig. 5E). However, at low integrated SoS, SP-A interactions with T cells serve to enhance T cell activation and proliferation in the bulk T cell population, independent of the presence of accessory cells (Figs. 3-4). Indeed, this effect was observed in both naïve and memory phenotype CD4+ T cells in the lung. SP-A mediated enhanced proliferation at low SoS was observed on T cells irrespective of the source (human PBMCs, mouse spleen, lung, as well as mediastinal, mesenteric and inguinal lymph nodes). Finally, using CD4+CD25+ (Treg or preactivated) and CD4+CD44+ (memory phenotype) T cell depleted populations in proliferation assays we determined that the observed SoS dependent responses to SP-A were a property of the bulk T cell population, and were global T cell effects that were not significantly altered by native regulatory, pre-activated or memory T cells (Fig. S1). Additionally, no significant differences in overall numbers of T cells sourced from spleen, lung, inguinal, mediastinal or mesenteric LNs were observed between WT and SP-A-/- mice.
SP-A enabled extended numbers of cell divisions in T cells activated with low SoS, as evidenced by the greater number of cell division events, as well as greater proportions of cells in the higher generational spans of CFSE analysis (Fig. 5). While fold-differences are not large, the effects on proliferation are additive – in both weak and strong activating signals. Interestingly, IL-2 ELISAs from supernatants revealed that there is some disconnect between cytokine production and inhibition of proliferation. T cell proliferation in vitro usually requires the presence of IL-2, which is both produced as well as utilized in an autocrine and paracrine manner during the course of activation. We observed enhanced levels of IL-2 with increasing signal strengths, even when proliferation was suppressed in the presence of SP-A (Fig. S2), suggesting IL-2 independent effects on proliferation. A similar effect was also observed with sol and pb anti-CD3 mediated activation (Fig. S2; both human and mouse T cells), or using various concentrations of OVA peptide in DO11.10 TCR transgenic T cells (data not shown). We also assayed for expression of the high-affinity IL-2 receptor, CD25, to determine if a lack of expression of the receptor was responsible for the accumulation of IL-2. However, as seen in Fig. S2, no significant differences in CD25 expression were observed. At the same time, as previously observed with human cells, no enhanced apoptosis was observed by cell cycle analysis (supplementary table 1).
The maintenance of cell division in the presence of SP-A with otherwise weak stimuli might be a direct result of increased levels and Ca2+ signaling over the first ~30 h of activation. On the other hand, when T cells are stimulated with high SoS, the profile is reversed in the presence of SP-A, with lower Ca2+ levels in the first ~30 h of activation. Overall levels of [Ca2+]i drop steadily over the course of activation, and during later stages of activation, no significant differences were observed. In addition, SP-A did not affect the intensity maxima of the calcium spike. These results differ from those previously observed by Borron et al (16), where a reduction in the intensity of the initial Ca2+ spike was observed using purified normal bovine SP-A rather than the human SP-A used in the current studies. Since sustained [Ca2+]i capacitance rather than the initial spike is thought to be responsible for maintaining NFAT signaling responsible for optimal T cell proliferation (41-43), this kinetic effect may explain the delay in later cell divisions, and reduced total numbers of >4-generation cells in the presence of SP-A and a strong activating signal. NFAT and NFkB are two key signaling pathways common to activation induced by PMA+I, anti-CD3 or APC-MHC:peptide. While these pathways in tandem have an integral role in productive activation, Ca2+/calcineurin signaling that solely goes through the NFAT pathway induces a limited set of anergy associated genes (41). This phenomenon is worth noting because we have not observed enhanced cell death associated with SP-A mediated suppression (Supplementary table 1). Together, these results demonstrate that T cells, in conjunction with SP-A, respond uniquely to different activation conditions, and strength of activating signal.
Previous studies in animals and humans, using in vitro and in vivo models, have demonstrated that SP-A exhibits potent regulatory effects on immunity and inflammatory reactions in the lung although the mechanism(s) by which SP-A's effects are mediated remains undefined. Investigations to identify specific receptors for SP-A have been confounded by the fact that SP-A is a ‘sticky’ protein and promiscuously interacts with high affinity with several ligands including myosin, immunoglobulins, SP-D, TLR4, CD93 and CD91/calreticulin, as well as lipids and antibodies (19, 20, 40, 44-46). Most ‘classical’ receptors that have been reported to bind SP-A (e.g. TLR4, CD93) are not present on naïve T cells, even though there are multiple studies that highlight a functional role of SP-A on T cells. Another candidate for a receptor is SP-R210 (18), now identified as unconventional myosin 18A (47), which is largely expressed on monocytes, as well as on a fraction of T cells. A recent report showed that expression of SP-R210 was markedly increased in T lymphocytes after stimulation by M. tuberculosis (48). Thus, it is possible that activated T cells that express SP-R210 may become more susceptible to the effects of SP-A, which could then inhibit cell-cycle progression of T cells and attenuate intracellular Ca2+ levels. Another possible binding partner is CTLA4 (CD152), which has long been implicated in differential suppression of T cell responses, and recently been shown to interact with SP-D (49). Considered together, our work and other data show that SP-A not only mediates T cell proliferation induced by direct T cell agonists such as anti-CD3, -CD28, PMA and ionomycin, but also those induced via other cells. This is evidenced by the differential SP-A mediated T cell responses to low and high dose LPS in Fig. 1in vivo, (where neutrophils and macrophages followed by T cells are primary responders).
This study has many immunological implications. The strength of signal has been reported to have multiple effects on functional responses by T cells (50-52, 34). For example, in T cells, low affinity peptides generate reduced Ca2+ levels that result in the transcription of IL-4, whereas a stronger signal that activates both the Ca2+-NFAT and MAPK pathways is required to induce IFNγ for Th1 responses. In the presence of a strong signal in the lung, such as oropharyngeally administered high dose PMA+I, or an invading pathogen, SP-A may attenuate the later phases of the response, thereby preventing an overzealous runaway inflammatory response that can be very damaging to the delicate lung tissue. After activation of T cells with a strong signal, the SP-A mediated suppression might also help prevent the generation of T cells that express exclusively high affinity TCRs. SP-A mediated interactions with T cells allow the initial immune response to occur irrespective of TCR avidity (which corresponds to signal strength). However, later preferential inhibition of T cells with a high affinity TCR would decrease their competitive advantage and conceivably prevent these clones from dominating an immune response in perpetuity. The lungs and its associated secondary lymphoid tissues such as BALT and mediastinal lymph nodes have among the largest environmental exposure of any organ system in the body. This makes the lungs an ideal locale to ensure the generation of an adequate memory response capability by maintaining the diversity of antigen specific cells. When stimulation results in lower activation of T cells, SP-A mediated interactions enhance T cell proliferation. This would be exemplified by low TCR-signal due to low affinity peptides or cross-reactive peptides (all of which have lower Ca2+ responses in T cells). Thus, the presence of SP-A might enable the pro-survival, basal (homeostatic) low grade ‘maintenance’ proliferation from TCR-MHC:self-peptide interactions (with no or minimal exogenous stimulation) to occur normally in the lung. The reduced BrdU incorporation observed in naïve and central memory T cells from SP-A-/- mice (Figs. 2A-D) gives credence to this mechanism. However, even though Corse et al (53) make convincing arguments in terms of mimicking these weak maintenance signals with pMHC or soluble antibodies, it is very difficult to draw direct comparisons between these effects in vivo, versus in vitro activation. Duration and extent of TCR occupancy, (exemplified by the immunological synapse and “serial triggering” of the TCR), function as non-exclusive mechanisms to enhance the naturally low affinity of the TCR-MHC complex. It is likely that, by enhancing the generation of low affinity TCR clones, SP-A interactions might broaden T cell responses to cross-reactive antigens that may play important roles as pathogens mutate to overcome the host immune response, and delay the onset of T cell immunosenescence (54, 55) (summarized in Fig. 7). Future studies will address additional paracrine effects of SP-A interactions on T cell tolerance, homeostasis and trafficking, as well as explore the role of accessory cells such as the lung alveolar type II cells that produce SP-A.
In summary, SP-A modulates the activation threshold and functional outcome of both mouse and human T cell activation, depending upon the strength of activating signal, at least in part by a “store operated” [Ca2+]i entry mediated mechanism. Multiple T cell surface receptors are known to contribute to T cell activation. Thus, cumulative signals from these receptors might be ‘integrated’ to derive the lower threshold for enhanced proliferation, and the upper threshold to halt excessive proliferation (Fig. 7). SP-A interactions, possibly by cross-linking these surface receptors, enable SP-A to act as a molecular rheostat that integrates activation signals of varying strengths to modulate T cell activation.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health R01HL-060872, P50HL084917, RO1HL-0309023, P01AI-081672. We thank the Duke HVI flow cytometry core and Patti McDermott for their help.
Abbreviations
- pb
plate-bound
- PMA+I
PMA+Ionomycin
- OVA
ovalbumin
- sol
soluble
- SoS
Strength of Signal
- SP-A
Surfactant Protein-A
- SP-D
Surfactant Protein-D
- Thg
Thapsigargin
References
- 1.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 2.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 3.Sastry K, Ezekowitz RA. Collectins: pattern recognition molecules involved in first line host defense. Curr Opin Immunol. 1993;5:59–66. doi: 10.1016/0952-7915(93)90082-4. [DOI] [PubMed] [Google Scholar]
- 4.Thiel S, Reid KB. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989;250:78–84. doi: 10.1016/0014-5793(89)80689-1. [DOI] [PubMed] [Google Scholar]
- 5.Saitoh M, Sano H, Chiba H, Murakami S, Iwaki D, Sohma H, Voelker DR, Akino T, Kuroki Y. Importance of the carboxy-terminal 25 amino acid residues of lung collectins in interactions with lipids and alveolar type II cells. Biochemistry. 2000;39:1059–1066. doi: 10.1021/bi9917939. [DOI] [PubMed] [Google Scholar]
- 6.Tenner AJ, Robinson SL, Borchelt J, Wright JR. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR-and CR1-mediated phagocytosis. J Biol Chem. 1989;264:13923–13928. [PubMed] [Google Scholar]
- 7.Tino MJ, Wright JR. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996;270:L677–688. doi: 10.1152/ajplung.1996.270.4.L677. [DOI] [PubMed] [Google Scholar]
- 8.Hohlfeld JM, Erpenbeck VJ, Krug N. Surfactant proteins SP-A and SP-D as modulators of the allergic inflammation in asthma. Pathobiology. 2002;70:287–292. doi: 10.1159/000070744. [DOI] [PubMed] [Google Scholar]
- 9.Ledford JG, Goto H, Potts EN, Degan S, Chu HW, Voelker DR, Sunday ME, Cianciolo GJ, Foster WM, Kraft M, et al. SP-A preserves airway homeostasis during Mycoplasma pneumoniae infection in mice. J Immunol. 2009;182:7818–7827. doi: 10.4049/jimmunol.0900452. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 10.LeVine AM, Kurak KE, Wright JR, Watford WT, Bruno MD, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein-A binds group B streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A-deficient mice. Am J Respir Cell Mol Biol. 1999;20:279–286. doi: 10.1165/ajrcmb.20.2.3303. [DOI] [PubMed] [Google Scholar]
- 11.Reidy MF, Wright JR. Surfactant protein A enhances apoptotic cell uptake and TGF-beta1 release by inflammatory alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2003;285:L854–861. doi: 10.1152/ajplung.00439.2002. [DOI] [PubMed] [Google Scholar]
- 12.McIntosh JC, Mervin-Blake S, Conner E, Wright JR. Surfactant protein A protects growing cells and reduces TNF-alpha activity from LPS-stimulated macrophages. Am J Physiol. 1996;271:L310–319. doi: 10.1152/ajplung.1996.271.2.L310. [DOI] [PubMed] [Google Scholar]
- 13.Crowther JE, Kutala VK, Kuppusamy P, Ferguson JS, Beharka AA, Zweier JL, McCormack FX, Schlesinger LS. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;172:6866–6874. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- 14.Pastva AM, Mukherjee S, Giamberardino C, Hsia B, Lo B, Sempowski GD, Wright JR. Lung effector memory and activated CD4+ T cells display enhanced proliferation in surfactant protein A-deficient mice during allergen-mediated inflammation. J. Immunol. 2011;186:2842–2849. doi: 10.4049/jimmunol.0904190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scanlon ST, Milovanova T, Kierstein S, Cao Y, Atochina EN, Tomer Y, Russo SJ, Beers MF, Haczku A. Surfactant protein-A inhibits Aspergillus fumigatus-induced allergic T-cell responses. Respir Res. 2005;6:97. doi: 10.1186/1465-9921-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borron PJ, Mostaghel EA, Doyle C, Walsh ES, McHeyzer-Williams MG, Wright JR. Pulmonary surfactant proteins A and D directly suppress CD3+/CD4+ cell function: evidence for two shared mechanisms. J Immunol. 2002;169:5844–5850. doi: 10.4049/jimmunol.169.10.5844. [DOI] [PubMed] [Google Scholar]
- 17.Borron P, Veldhuizen RA, Lewis JF, Possmayer F, Caveney A, Inchley K, McFadden RG, Fraher LJ. Surfactant associated protein-A inhibits human lymphocyte proliferation and IL-2 production. Am J Respir Cell Mol Biol. 1996;15:115–121. doi: 10.1165/ajrcmb.15.1.8679215. [DOI] [PubMed] [Google Scholar]
- 18.Borron P, McCormack FX, Elhalwagi BM, Chroneos ZC, Lewis JF, Zhu S, Wright JR, Shepherd VL, Possmayer F, Inchley K, et al. Surfactant protein A inhibits T cell proliferation via its collagen-like tail and a 210-kDa receptor. Am J Physiol. 1998;275:L679–686. doi: 10.1152/ajplung.1998.275.4.L679. [DOI] [PubMed] [Google Scholar]
- 19.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 20.Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- 21.Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol. 2002;168:5989–5992. doi: 10.4049/jimmunol.168.12.5989. [DOI] [PubMed] [Google Scholar]
- 22.Deng Y, Chen S, Chen J, Tao Z, Kong Y, Xu Y, Xiao B, He Q. Relationship between surfactant protein A polymorphisms and allergic rhinitis in a Chinese Han population. Mol Biol Rep. 2011;38:1475–1482. doi: 10.1007/s11033-010-0254-4. [DOI] [PubMed] [Google Scholar]
- 23.Pettigrew MM, Gent JF, Zhu Y, Triche EW, Belanger KD, Holford TR, Bracken MB, Leaderer BP. Association of surfactant protein A polymorphisms with otitis media in infants at risk for asthma. BMC Med Genet. 2006;7:68. doi: 10.1186/1471-2350-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack DL, Cole J, Naylor SC, Borrow R, Kaczmarski EB, Klein NJ, Read RC. Genetic polymorphism of the binding domain of surfactant protein-A2 increases susceptibility to meningococcal disease. Clin Infect Dis. 2006;43:1426–1433. doi: 10.1086/508775. [DOI] [PubMed] [Google Scholar]
- 25.Thomas NJ, DiAngelo S, Hess JC, Fan R, Ball MW, Geskey JM, Willson DF, Floros J. Transmission of surfactant protein variants and haplotypes in children hospitalized with respiratory syncytial virus. Pediatr Res. 2009;66:70–73. doi: 10.1203/PDR.0b013e3181a1d768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lofgren J, Ramet M, Renko M, Marttila R, Hallman M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. J Infect Dis. 2002;185:283–289. doi: 10.1086/338473. [DOI] [PubMed] [Google Scholar]
- 27.Cose S, Brammer C, Khanna KM, Masopust D, Lefrancois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur J Immunol. 2006;36:1423–1433. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- 28.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 29.Korfhagen TR, Bruno MD, Ross GF, Huelsman KM, Ikegami M, Jobe AH, Wert SE, Stripp BR, Morris RE, Glasser SW, et al. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci U S A. 1996;93:9594–9599. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntosh JC, Swyers AH, Fisher JH, Wright JR. Surfactant proteins A and D increase in response to intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 1996;15:509–519. doi: 10.1165/ajrcmb.15.4.8879185. [DOI] [PubMed] [Google Scholar]
- 31.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L232–241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 32.Tough DF, Sprent J, Stephens GL. Measurement of T and B cell turnover with bromodeoxyuridine. Curr Protoc Immunol Chapter. 2007;4 doi: 10.1002/0471142735.im0407s77. Unit 4 7. [DOI] [PubMed] [Google Scholar]
- 33.Graziano M, St-Pierre Y, Beauchemin C, Desrosiers M, Potworowski EF. The fate of thymocytes labeled in vivo with CFSE. Exp. Cell Res. 1998;240:75–85. doi: 10.1006/excr.1997.3900. [DOI] [PubMed] [Google Scholar]
- 34.Brogdon JL, Leitenberg D, Bottomly K. The potency of TCR signaling differentially regulates NFATc/p activity and early IL-4 transcription in naive CD4+ T cells. J Immunol. 2002;168:3825–3832. doi: 10.4049/jimmunol.168.8.3825. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee S, Ahmed A, Malu S, Nandi D. Modulation of cell cycle progression by CTLA4-CD80/CD86 interactions on CD4+ T cells depends on strength of the CD3 signal: critical role for IL-2. J Leukoc Biol. 2006;80:66–74. doi: 10.1189/jlb.0505260. [DOI] [PubMed] [Google Scholar]
- 36.Borron PJ, Crouch EC, Lewis JF, Wright JR, Possmayer F, Fraher LJ. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol. 1998;161:4599–4603. [PubMed] [Google Scholar]
- 37.Garcia-Rodriguez C, Rao A. Requirement for integration of phorbol 12-myristate 13-acetate and calcium pathways is preserved in the transactivation domain of NFAT1. Eur J Immunol. 2000;30:2432–2436. doi: 10.1002/1521-4141(2000)30:8<2432::AID-IMMU2432>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed A, Mukherjee S, Nandi D. Intracellular concentrations of Ca(2+) modulate the strength of signal and alter the outcomes of cytotoxic T-lymphocyte antigen-4 (CD152)-CD80/CD86 interactions in CD4(+) T lymphocytes. Immunology. 2009;126:363–377. doi: 10.1111/j.1365-2567.2008.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang S, Milla C, Panoskaltsis-Mortari A, Hawgood S, Blazar BR, Haddad IY. Surfactant protein A decreases lung injury and mortality after murine marrow transplantation. Am J Respir Cell Mol Biol. 2002;27:297–305. doi: 10.1165/rcmb.2002-0035OC. [DOI] [PubMed] [Google Scholar]
- 41.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18:1–9. [PubMed] [Google Scholar]
- 42.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 43.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 44.Gupta N, Manevich Y, Kazi AS, Tao JQ, Fisher AB, Bates SR. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L436–446. doi: 10.1152/ajplung.00415.2005. [DOI] [PubMed] [Google Scholar]
- 45.Oosting RS, Wright JR. Characterization of the surfactant protein A receptor: cell and ligand specificity. Am J Physiol. 1994;267:L165–172. doi: 10.1152/ajplung.1994.267.2.L165. [DOI] [PubMed] [Google Scholar]
- 46.Tino MJ, Wright JR. Interactions of surfactant protein A with epithelial cells and phagocytes. Biochim Biophys Acta. 1998;1408:241–263. doi: 10.1016/s0925-4439(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 47.Yang CH, Szeliga J, Jordan J, Faske S, Sever-Chroneos Z, Dorsett B, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, et al. Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A. J Biol Chem. 2005;280:34447–34457. doi: 10.1074/jbc.M505229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samten B, Townsend JC, Sever-Chroneos Z, Pasquinelli V, Barnes PF, Chroneos ZC. An antibody against the surfactant protein A (SP-A)-binding domain of the SP-A receptor inhibits T cell-mediated immune responses to Mycobacterium tuberculosis. J Leukoc Biol. 2008;84:115–123. doi: 10.1189/jlb.1207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin KW, Jen KY, Suarez CJ, Crouch EC, Perkins DL, Finn PW. Surfactant protein D-mediated decrease of allergen-induced inflammation is dependent upon CTLA4. J Immunol. 2010;184:6343–6349. doi: 10.4049/jimmunol.0901947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 51.Badou A, Savignac M, Moreau M, Leclerc C, Foucras G, Cassar G, Paulet P, Lagrange D, Druet P, Guery JC, et al. Weak TCR stimulation induces a calcium signal that triggers IL-4 synthesis, stronger TCR stimulation induces MAP kinases that control IFN-gamma production. Eur J Immunol. 2001;31:2487–2496. doi: 10.1002/1521-4141(200108)31:8<2487::aid-immu2487>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 52.Schrum AG, Turka LA, Palmer E. Surface T-cell antigen receptor expression and availability for long-term antigenic signaling. Immunol Rev. 2003;196:7–24. doi: 10.1046/j.1600-065x.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 53.Corse E, Gottschalk RA, Allison JP. Strength of TCR-Peptide/MHC Interactions and In Vivo T Cell Responses. Journal of immunology. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 54.Hesse MD, Karulin AY, Boehm BO, Lehmann PV, Tary-Lehmann M. A T cell clone's avidity is a function of its activation state. J Immunol. 2001;167:1353–1361. doi: 10.4049/jimmunol.167.3.1353. [DOI] [PubMed] [Google Scholar]
- 55.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.