Abstract
Nematode parasites infect humans and domestic animals; treatment and prophylaxis require anthelmintic drugs because vaccination and sanitation is limited. Emodepside is a more recently introduced cyclooctadepsipeptide drug that has actions against GI nematodes, lungworm, and microfilaria. It has a novel mode of action which breaks resistance to the classical anthelmintics (benzimidazoles, macrocyclic lactones and cholinergic agonists). Here we review studies on its mode of action which suggest that it acts to inhibit neuronal and muscle activity of nematodes by increasing the opening of calcium-activated potassium (SLO-1) channels.
Keywords: Emodepside, C. elegans, Ascaris suum, SLO-1, voltage-activated potassium currents
1. Introduction
Parasitic nematode infections place a heavy burden on both humans and animals. It is estimated that the global prevalence of parasitic nematode infections in humans is over two billion (de Silva, et al., 2003). These infections are debilitating, produce lost productivity, mental impairment, and poor growth and contribute to poverty. The incidence of human helminthiases is higher in warmer, wetter areas where poor sanitation makes the spread of nematode parasite infections all too easy. In hot dry or very cold climates, the spread of the helminth infection is much slower even if sanitation is limited, because the free living intermediate stages do not survive well. In domestic animals, nematode parasites cause production loss, welfare issues and reduce the food supply. In the absence of effective vaccines and good sanitation to prevent the spread of these parasitic infections, anthelmintic drugs are used for both treatment and prophylaxis in humans and animals. Disturbingly, there are reports of growing resistance to the main groups of anthelmintic drugs in both man and animals. There is evidence of resistance to the benzimidazoles (albendazole), nicotinic agonists (levamisole/pyrantel) and macrocyclic lactones (ivermectin) in domestic animals (Wolstenholme, et al., 2004) and concerns in humans (Geary, et al., 2009). Recently, novel ‘resistance-busting’ anthelmintics (emodepside, a cyclooctadepsipeptide; monepantel, an amino-acetonitrile derivative, and derquantel, a paraherquamide derivative) have been developed. The need for these new anthelmintics and ways to combat resistance to the currently available anthelmintics is urgent. Here we review recent information on the mode of action of emodepside with the intention that this information will facilitate understanding and development of the drug, and perhaps development of additional compounds.
2. Spectrum of action
Sasaki et al. (1992) described the isolation of the cyclooctadepsipeptide PF1022A from cultured Mycelia sterilia, a fungus found on leaves of a flowering shrub (Camellia japonica). Emodepside (Figure 1A) is a semisynthetic analogue of PF1022A that is produced by adding two morpholine rings to the para-position of the two D-phenyllactic acids (Harder et al., 2005) in order to enhance pharmacokinetic properties. Emodepside or PF1022A are effective against: gastro-intestinal nematodes of mice, rats, chickens, sheep, cattle, horse, dogs and cats and Trichonella spiralis (Harder et al., 2003, Martin et al., 1996); and pre-adult stages of the filariae, Acanthocheilonema viteae, Brugia malayi, and Litomosoides sigmodontis. The effects against the adult stages of filaria are species dependent; there is little effect of emodepside against adult B. malayi (Harder et al., 2003).
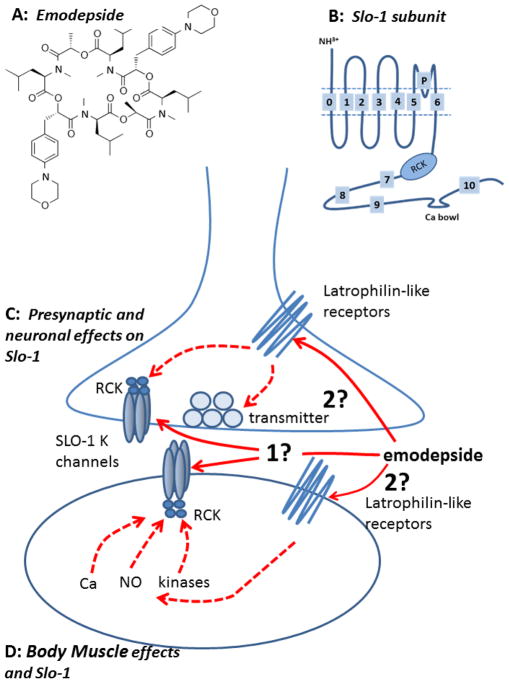
Figure 1.
Summary diagrams of modepside structure, Slo-1 subunit and a model of the mode action of emodepside on nematode body muscle. A: Emodepside (Molecular formula C60H90N6014 Molecular weight 1119.4). B: Diagram of transmembrane structure of one Slo-1 subunit; each Slo-1 K channel is made up of 4 of these subunits. Emodepside may act in part presynaptically on neurons (C) and in part on body muscle (D). Emodepside may act directly on SLO-1 K channels in the muscle or neurons (1) or indirectly by stimulating latrophilin-like receptors and signaling cascades that may involve NO, protein Kinase C and/or calcium. The release of transmitters may also be affected by the activation of neuronal SLO-1 K channels. It is unlikely that emodepside acts at the extracellular surface of the SLO-1 K channel because of the slow time course of its action. It is very lipophilic and could act in the lipid membrane phase on the SLO-1 K channel or move into the cytoplasm and act intracellularly. A SLO-1 K subunit (B) and channel (C & D) is shown composed of 4 subunits along with the ‘RCK’ cytoplasmic regulatory region of the channel.
3.1 Different Mode of Action
Emodepside selectively inhibits body muscle contraction of nematodes (Terada 1992, Willson et al., 2003). Emodepside is effective against nematode isolates that have developed resistance to drugs from the major classes of anthelmintic (Samson-Himmelstjerna von et al., 2005), namely: ivermectin (an allosteric modulator of GluCl channels, Pemberton et al., 2001), levamisole (a nematode selective nAChR agonist, Qian et al., 2006, Qian et al., 2008) and febantel (a selective ligand for nematode β-tubulin, Miro et al., 2006). Because emodepside remains effective against resistant isolates, it suggests that emodepside has a different mode of action.
3.1 Does not act as a GABA agonist or nicotinic antagonist
The earliest studies on the mechanisms of action of the cyclooctadepsipeptides used PF1022A. PF1022A seemed to exert its anthelmintic action on either nematode nerve or muscle rather than on its energy metabolism; low concentrations of PF1022A (<1 μM) inhibited the motility of the nematode parasite, Angiostrongylus cantonensis (Terada, 1992). Although it was reported (Chen et al., 1996) that PF1022A bound to GABA receptors of Ascaris suum muscle, suggesting a direct effect on nematode GABA receptors, direct electrophysiological recording from Ascaris suum muscle found that PF1022A did not act like GABA, nor did it act as a cholinergic antagonist (Martin et al., 1996). Emodepside does not produce an increase in the muscle membrane conductance like GABA or piperazine (Martin, 1982) again showing that these compounds do not act as GABA agonists.
Another possibility (GeBner et al. 1996) was that PF1022A is an ionophore because PF1022, PF1022-001 (antipode of PF1022A), valinomycin, enniatin A1 and beauvericin all have ionophore activities, and may increase bilayer conductivity to monovalent ions (Na+, Li+, K+ and Cs+). However, only PF1022A not PF1022-001 (the antipode), had a potent paralytic effect on A. suum, suggesting that the selective anthelmintic effects of PF1022A was not due to any ion-carrier activity.
3.2 K-dependent hyperpolarization by releasing inhibitory neuropeptides (PF1/PF2)
Willson et al. (2003) tested further effects of emodepside on Ascaris suum muscle contraction and electrophysiology. They observed that 10 μM emodepside had a slower inhibitory action on muscle contraction than GABA and produced a slow hyperpolarization without a detectable change in conductance. The inhibitory neuropeptides, PF1 & PF2, also produce a slow inhibition of contraction of A. suum muscle, similar to emodepside (Fellowes et al., 2000, Willson et al., 2003). PF1 causes a slow, non-reversible, concentration-dependent membrane hyperpolarization that is significantly blocked by 4-aminopyridine (Franks et al., 1994, Verma et al., 2009). When Willson et al. (2003) found that the K channel blocker 4-aminopyridine inhibited the effect of emodepside on membrane potential they reasoned that emodepside may mimic effects of PF1 & PF2 by stimulating the release of inhibitory neuropeptides like PF1 or PF2 which then produce a K-dependent hyperpolarization. Although there is a similarity between effects of PF1 and emodepside effects on the membrane potential their effects are not identical. PF1 and emodepside both increase calcium-dependent voltage-activated K currents in A. suum, but the effects on calcium currents are different: PF1 inhibits voltage-activated Ca2+ currents (Verma et al., 2009); but emodepside has no effect on voltage-activated Ca2+ currents (Buxton et al, 2011). The effects of emodepside are also slower in onset: emodepside inhibits ryanodine-induced spiking more slowly than PF1, probably because of the lack of emodepside effect on the voltage-activated Ca2+ currents (Buxton et al 2011). Thus although there may be some similarities in the effects of emodepside and the inhibitory neuropeptides, the evidence suggests that emodepside does not act by releasing PF1-like neuropeptides.
3.3 Latrophilin receptors
Immunoscreening with an antibody to PF1022A of an H. contortus cDNA library revealed a G-protein receptor that is latrophilin-like, now designated HC110R, and which has been expressed in HEK293 cells where it lead to PF1022A-dependent gating of calcium (Saeger et al., 2001). The homology of HC110R to mammalian latrophilin receptors which trigger neurotransmitter release, raises the hypothesis that emodepside may act (in part) by stimulating neurotransmitter release producing inhibition of muscle activity. Muhlfeld (Muhlfeld et al., 2009) used surface plasmon resonance to show that the neuropeptides AF1, AF10 and PF2 bind, with low affinities, to HC110-R, implying that these neuropeptides may be putative natural ligands of the latrophilin-like receptor. They did not observe any high affinity binding characteristics with PF1.
C. elegans is sensitive to effects of emodepside (Bull et al., 2007). The effects are: inhibition of movement, inhibition of pharyngeal pumping and inhibition of egg-laying. In 2004, Willson et al. described effects of emodepside (100 nM) on the C. elegans pharynx with the effects of emodepside stimulating exocytosis and eliciting pharyngeal paralysis. The paralysis of the pharynx produced by emodepside depended on the presence of the lathrophillin (LAT-1) receptor with emodepside resistance appearing in lat-1 null mutants. Two genes were recognized that encode latrophilin receptors in C. elegans, lat-1 and lat-2. The role of LAT-1 and LAT-2 in mediating effects of emodepside on feeding and locomotion were investigated with RNAi and null-mutants (Willson et al., 2004; Bull et al., 2007; Guest et al., 2007). The pharyngeal system of lat-1 null-mutants had reduced emodepside sensitivity, but the sensitivity of locomotion to emodepside was unchanged (Guest et al., 2007), suggesting that emodepside has both LAT-1 dependent effects on the pharynx and LAT-independent effects on locomotion.
3.4 SLO-1 as a target for emodepside in C. elegans
Guest et al. (2007) used a mutagenesis screen of C. elegans and found mutant alleles of slo-1 that encode a Ca2+-dependent K channel in C. elegans that were resistant to the effects of emodepside on locomotion. They observed that slo-1 but not slo-2 null-mutants were more resistant to the inhibitory effects of emodepside than lat-1 and lat-2 (latrophilin receptor) double mutants. Guest et al. (2007) proposed that emodepside either directly or indirectly activates SLO-1 that is present in body wall muscle and motor neurons to produce its inhibitory effects in nematodes. Initial slo-1 pharyngeal expression experiments in slo-1 null-mutants, did not detect effects of emodepside on the frequency of pharyngeal pumping. However, Crisford et al. (2011) subsequently described how ectopic over-expression of SLO-1a in C. elegans pharyngeal muscle did, in fact, give rise to sensitivity of the pharyngeal muscle to emodepside. Crisford et al. (2011) also described transgenic experiments in which the C. elegans SLO-1a channel was swapped for KCNMA1, the human orthologue. Interestingly, the sensitivity to emodepside in the rescues depended upon origin of the SLO-1 channel: the human KCNMA1 channel was 10–100 time less sensitive to emodepside than the rescues expressing C. elegans SLO-1a channel. In addition to the heterologous expression of the human KCNMA1 BK channel, expression of the SLO-1 K channels of the nematode parasites, A. caninum and C. oncophora in the C. elegans slo-1 loss of function mutant, NM1968, found that expression restored emodepside sensitivity (Welz et al., 2011). Restoration of the full emodepside sensitivity was also found to depend on the nature of the promoter used: the parasite slo-1 promoter produced only partial recovery of sensitivity compared to full recovery produced by the C. elegans slo-1 promoter. One explanation for this effect of the promoters on emodepside sensitivity suggests that the parasite promoter is less efficient in C. elegans; another explanation is that the parasite promoters used for slo-1 expression might be truncated, thus driving only partial expression activity. A proposed model for the mode of action of emodepside in C. elegans (Welz et al., 2011) is that: emodepside acts directly or indirectly to activate the SLO-1 K channel; the effect on pharyngeal pumping involves latrophilin receptors and SLO-1 on pharyngeal neurons; and the effect on body wall muscle involves SLO-1 in the muscle but not latrophilin receptors.
4. Properties of SLO-1 K channels
Two similar, SLO-1 and SLO-2 types of K channel (Lim et al., 1999, Wang et al., 2001, Jospin et al., 2002) have been identified in C. elegans. Although the proteins of these channels have some similar motif sequences, the channels are very different in their regulation by intracellular ions. SLO-2 is regulated by intracellular Na+ as well as Cl−; SLO-1 is regulated primarily by intracellular Ca2+ (Jospin et al. 2002). We focus here more on the SLO-1 ion-channels, as it is a putative site of action of emodepside. The SLO-1 K channels have large (~200 pS) conductances and are sometimes called ‘big’ potassium (BK) channels, maxi-K channels or SLO family channels. The SLO-1 K channel of vertebrates is composed of 4 α-subunits; homologous subunits of C. elegans and A. suum are found and like vertebrate SLO-1 α-subunits have seven (S0—S6) transmembrane regions, a P-loop between S5 and S6, a large intracellular domain (S7-S-10) and a well conserved ‘calcium bowl’ between domains S9 and S10 (see Figure 1B–D & 2). In addition, the regulator of the K channel conductance (RCK) domains (S7, S8) contains high and low affinity calcium binding sites. The SLO-1 α-subunits show alternative splicing, producing channels with different calcium sensitivities; in C. elegans there are at least 3 splice variants (SLO-1a, SLO-1b and SLO-1c; Wang et al., 2001). Each α-subunit of the channel has at least two high affinity calcium binding sites and one low calcium/magnesium binding sites. The channel also combines with secondary, regulatory β-subunits (Knaus et al., 1994) in vertebrates but these subunits have not yet been identified in C. elegans. However, Drosophila, which also lack β subunits, has Slob proteins, which appear to carry out similar functions to the vertebrate β-subunits and these may be present in nematodes (Claridge-Chang et al., 2001).
Figure 2.
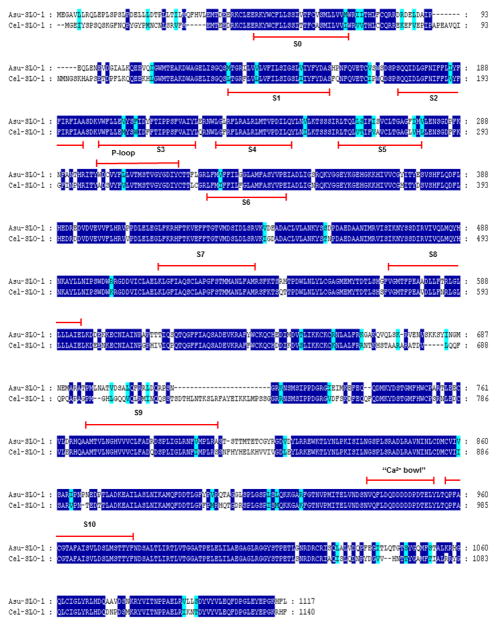
SLO-1 A. suum and C. elegans and alignments of deduced amino-acid sequences. Identical amino-acids between C. elegans and A. suum sequences are shaded in dark blue and distinct aminoacids sharing similar physico-chemical properties are shaded in light blue. Predicted signal peptide sequences are shaded in grey. The transmembrane domains (TM) are noted below the sequences. Comparison of Asu-SLO-1 (Genbank accession n° ACC68842.1) and Cel-SLO-1 (Genbank accession n° NP_001024259.1) showing the 7 TM domains (S0 – S6), P-loop, S7 – S10 intracellular domains and “Ca2+ bowl”. Domain annotation corresponds to A. suum SLO-1. Figure, modified from Buxton et al., 2011.
The suggested function of the SLO-1 K channels is that they adjust the resting membrane potential of electrically excitable cells and adjust the level of excitability, up or down, and so affect the response to other inputs. In addition to opening of the channel being regulated (Figure 1C & D) by membrane potential and calcium, magnesium, NO, CO, arachidonic acid, prostaglandins and phosphorylation by cAMP-dependent protein kinase A, diacylgycerol/Ca2+-dependent protein kinase C, and cyclic GMP-dependent protein kinase G can affect channel opening (Salkoff et al., 2006; Ghatta et al., 2006). These kinases allow SLO-1 to be coupled to multiple and quite diverse signaling cascades permitting different ways of adjusting the excitability of the cells. The effect of different nematode neuropeptides which can affect cAMP cGMP levels, (e.g. AF1, AP2, PF1 & PF2) could then affect SLO-1 channels (Verma et al., 2007, Verma et al., 2009).
4.1 SLO-1 as a target for emodepside in A. suum
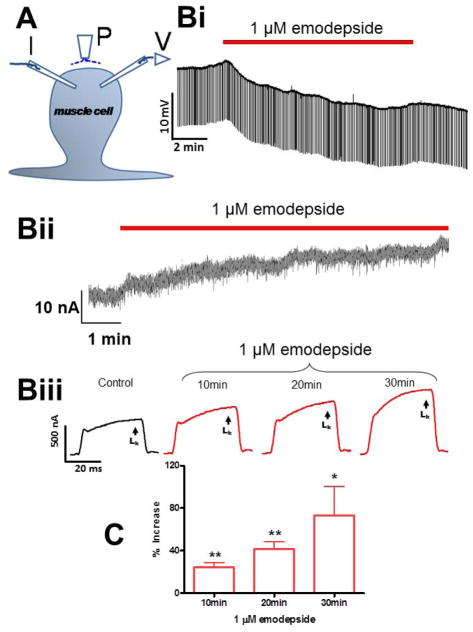
Willson et al. (2003) described the muscle relaxation and inhibitor effect of emodepside on A. suum body muscle and that emodepside produced a Ca2+-dependent hyperpolarization. It was suggested that emodepside may act at the neuromuscular junction to stimulate release of an inhibitory neuropeptide similar in action to PF1 or PF2. Voltage-clamp experiments have now allowed the effect of PF1, increasing the opening of Ca2+-dependent voltage-activated K channels and decreasing the calcium currents present in A. suum muscle to be observed (Verma et al., 2009). Buxton et al., (2011) found Asc-lat-1 and Asu-slo-1, evolutionarily conserved homologues of the lat-1 and slo-1 genes, to be expressed in adult A. suum body muscle flaps. They showed, using the same voltage-clamp techniques, that emodepside activates SLO-1-like K channels like PF1 (Figure 3) but unlike PF1, emodepside does not decrease calcium currents. These voltage-activated K channels were Ca2+-dependent and inhibited by 5 mM 4-aminopyridine. The membrane hyperpolarization and increase in voltage-activated K current produced by emodepside (Figure 3) are very slow in onset and increase over a period of more than 10 minutes (Buxton et al. 2011); the speed of onset is slower than the onset of the effect of the inhibitory action of PF1. The slow onset effect of emodepside might be due to the very lipophilic nature of emodepside and a membrane partitioning effect. It may also be because the effects of emodepside are indirect and produced by activation of a slow signaling cascade. The effect of emodepside is potentiated by sodium nitroprusside (a NO donor) and PMA (a protein kinase C activator), antagonized by iNOS inhibitors (with NNLA) and antagonized by inhibition of protein kinase C (staurosporine, Buxton et al., 2011). Interestingly, these signaling molecules are known activators of SLO-1 in other cells (Bolotina et al., 1994, Holden-Dye et al., 2007, Mistry and Garland, 1998, Wang et al., 1999) and therefore encourage the view that emodepside could act through either or both of these signaling cascades and the signaling cascades may be in series or parallel (Figure 1C & D). A number of studies on the mammalian orthologues of SLO-1 show that they are directly and alternately regulated by complex, multiple signaling cascades, involving NO and diacylglyerol or PKC activation (Ghatta et al., 2006, Salkoff et al., 2006).
Figure 3.
Electrophysiological techniques (two micropipette current-clamp and voltage-clamps) for recording from Ascaris suum. (A) A. suum muscle bag showing the current (I) and voltage (V) micropipettes in the bag, and the perfusion needle (P). (Bi) Representative current-clamp traces showing the slow hyperpolarizing membrane potential during and after 10 min application of 1 μM emodepside. Note that the trace does not get thinner because the membrane resistance does not change detectably. (Bii) Outward current response to 1 μM emodepside at higher time resolution. Holding potential −35 mV. Notice that emodepside produces a gradually increasing current after a delay of some 30 seconds. The response does not plateau in the time period of this recording. (B III) voltage-clamp traces of control K current and the time-dependent effects of 1 μM emodepside on the K currents, all to a step potential of 0 mV from a holding potential of −35 mV. (C) Bar chart (mean ± s.e.) of 1 μM emodepside effect on steady state (LK) currents. Comparison was made between the control 0 mV step current at 30 – 40 ms and the corresponding current increased by emodepside at 10, 20 and 30 min. Emodepside increased LK currents at 10 min (p < 0.01, n = 4, paired t-test), 20 min (p < 0.01, n = 4, paired t-test) and 30 min (p < 0.05, n = 4, paired t-test). Figure, modified from Buxton et al. (2011).
Figure 2 shows alignments of C. elegans and A. suum SLO-1 (Genbank accession n° ACC68842.1). The Asu-SLO-1 sequence encodes a protein of 1117 amino-acids that has 78% identity and 87% similarity to the Cel-SLO-1 sequence. The SLO-1 sequences have seven transmembrane-spanning domains (S0 – S6), a P-loop, four hydrophobic intracellular segments, (S7 – S10) and a “Ca2+ bowl” that typifies a large conductance calcium-sensitive potassium channels, (Ghatta, et al., 2006; Lim, et al., 1999; Schreiber and Salkoff, 1997; Wallner, et al., 1996; Wei, et al., 1996).
5. Conclusion
Emodepside is a broad spectrum anthelmintic that has a mode of action different from the other classical groups of anthelmintic and is not expected to show cross-resistance with them. It has an inhibitory effect on motility, pharyngeal pumping and egg laying of nematodes and its mode of action involves increased opening of a SLO-1 K channel. In rescue experiments done by Crisford et al. (2011), there is evidence of direct action of emodepside on the SLO-1 K channel. The very slow time-dependent effect (Buxton et al., 2011) suggests that the site of action is not an extracellular domain of the SLO-1 K channel. The slow time course is consistent with a site of action within the membrane or intracellular domain.
Emodepside is a broad spectrum cyclooctadepsipeptide anthelmintic drug.
It has a mode of action different from the other classical groups of anthelmintics.
We review its mode of action that show it inhibits neuronal and muscle activity.
Rescue experiments suggest emodepside opens nematode Ca-dependent SLO-1 K channels.
Its slow time-dependent action suggests it does not act in the extracellular domain of these channel.
Acknowledgments
The research project culminating in this paper was funded by National Institute of Allergy and Infectious Diseases (NIH) grant 2R56AI047194-11 RJM, the Iowa Center for Advanced Neurotoxicity (ICAN) seed grant to APR and by the French National Institute for Agricultural Research to CLC, CN and JC. SKB is the grateful recipient of a scientific Chateaubriand doctoral fellowship granted by the Office for Science and Technology of the Embassy of France in the USA and from the “Santé, Sciences, Technologies” doctoral school Tours University. CLC held a 2011 Fellowship award from the OECD’s Cooperative Research Programme “Biological Resource Management for Sustainable Agricultural Systems”. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health or other funding bodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 2.Bull K, Cook A, Hopper NA, Harder A, Holden-Dye L, Walker RJ. Effects of the novel anthelmintic emodepside on the locomotion, egg-laying behaviour and development of Caenorhabditis elegans. Int J Parasitol. 2007;37:627–636. doi: 10.1016/j.ijpara.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Buxton SK, Neveu C, Charvet CL, Robertson AP, Martin RJ. On the Mode of Action of Emodepside: Slow Effects on Membrane Potential and Voltage-Activated Currents in Ascaris suum. British Journal of Pharmacology. 2011 doi: 10.1111/j.1476-5381.2011.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Terada M, Cheng JT. Characterization of subtypes of gamma-aminobutyric acid receptors in an Ascaris muscle preparation by binding assay and binding of PF1022A, a new anthelmintic, on the receptors. Parasitol Res. 1996;82:97–101. doi: 10.1007/s004360050077. [DOI] [PubMed] [Google Scholar]
- 5.Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 2001;32:657–671. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- 6.Crisford C, Marray C, O’Connor V, Edwards RJ, Kruger N, Samson-Himmelstjerna Gv, Harder A, Walker RJ, Holden-Dye L. Selective Toxicity of the Anthelmintic Emodepside Revealed by Heterologous Expression of Human KCNMA1 in Caenorhabditis elegans. Molecular Pharmacology. 2011 doi: 10.1124/mol.111.071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Fellowes RA, Maule AG, Marks NJ, Geary TG, Thompson DP, Halton DW. Nematode neuropeptide modulation of the vagina vera of Ascaris suum: in vitro effects of PF1, PF2, PF4, AF3 and AF4. Parasitology. 2000;120:79–89. doi: 10.1017/s0031182099005260. [DOI] [PubMed] [Google Scholar]
- 9.Franks C, Holden-Dye L, Williams R, Pang F, Walker R. A nematode FMRFamide-like peptide, SDPNFLRFamide (PF1), relaxes the dorsal muscle strip preparation of Ascaris suum. Parasitology. 1994;108:229–236. doi: 10.1017/s0031182000068335. [DOI] [PubMed] [Google Scholar]
- 10.Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, de Silva NR, Olliaro PL, Lazdins-Helds JK, Engels DA, Bundy DA. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol. 2009;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 11.GeBner G, Meder S, Rink T, Boheim G, Harder A, Jeschke P, Scherkenbeck J, Londershausen M. Ionophore and Anthelmintic Activity of PF1022A, a Cyclooctadepsipeptide, are not related. Pestic Sci. 1996;48:399–407. [Google Scholar]
- 12.Ghatta S, Nimmagadda D, Xu X, O’Rourke ST. Large-conductance, calcium-activated potassium channels: Structural and functional implications. Pharmacology & Therapeutics. 2006;110:103–116. doi: 10.1016/j.pharmthera.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Guest M, Bull K, Walker RJ, Amliwala K, O’Connor V, Harder A, Holden-Dye L, Hopper NA. The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int J Parasitol. 2007;37:1577–1588. doi: 10.1016/j.ijpara.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Harder A, Holden-Dye L, Walker R, Wunderlich F. Mechanisms of action of emodepside. Parasitol Res. 2005;97:S1–S10. doi: 10.1007/s00436-005-1438-z. [DOI] [PubMed] [Google Scholar]
- 15.Harder A, Schmitt-Wrede HP, Krucken J, Marinovski P, Wunderlich F, Willson J, Amliwala K, Holden-Dye L, Walker R. Cyclooctadepsipeptides--an anthelmintically active class of compounds exhibiting a novel mode of action. Int J Antimicrob Agents. 2003;22:318–331. doi: 10.1016/s0924-8579(03)00219-x. [DOI] [PubMed] [Google Scholar]
- 16.Harder A, Schmitt-Wrede HP, Krucken J, Marinovski P, Wunderlich F, Willson J, Amliwala K, Holden-Dye L, Walker R. Cyclooctadepsipeptides--an anthelmintically active class of compounds exhibiting a novel mode of action. Int J Antimicrob Agents. 2003;22:318–331. doi: 10.1016/s0924-8579(03)00219-x. [DOI] [PubMed] [Google Scholar]
- 17.Holden-Dye L, O’Connor V, Hopper NA, Walker RJ, Harder A, Bull K, Guest M. SLO, SLO, quick, quick, slow: calcium-activated potassium channels as regulators of Caenorhabditis elegans behaviour and targets for anthelmintics. Invert Neurosci. 2007;7:199–208. doi: 10.1007/s10158-007-0057-z. [DOI] [PubMed] [Google Scholar]
- 18.Jospin M, Mariol MC, Segalat L, Allard B. Characterization of K(+) currents using an in situ patch clamp technique in body wall muscle cells from Caenorhabditis elegans. J Physiol. 2002;544:373–384. doi: 10.1113/jphysiol.2002.022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem. 1994;269:3921–3924. [PubMed] [Google Scholar]
- 20.Lim HH, Park BJ, Choi HS, Park CS, Eom SH, Ahnn J. Identification and characterization of a putative C. elegans potassium channel gene (Ce-slo-2) distantly related to Ca(2+)-activated K(+) channels. Gene. 1999;240:35–43. doi: 10.1016/s0378-1119(99)00398-4. [DOI] [PubMed] [Google Scholar]
- 21.Martin RJ. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle. British Journal of Pharmacology. 1982;77:255–265. doi: 10.1111/j.1476-5381.1982.tb09294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin RJ, Harder A, Londershausen M, Jeschke P. Anthelmintic Actions of the Cyclic Depsipeptide PF1022A and its Electrophysiological Effects on Muscle Cells of Ascaris suum. Pestic Sci. 1996;48:343–349. [Google Scholar]
- 23.Miro G, Mateo M, Montoya A, Vela E, Calonge R. Survey of intestinal parasites in stay dogs in the Madrid area and comparison of the efficacy of three anthelmintics in naturally infected dogs. Parasitol Res. 2006;100:317–320. doi: 10.1007/s00436-006-0258-0. [DOI] [PubMed] [Google Scholar]
- 24.Mistry DK, Garland CJ. Nitric Oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. British Journal of Pharmacology. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muhlfeld S, Schmitt-Wrede HP, Harder A, Wunderlich F. FMRFamide-like neuropeptides as putative ligands of the latrophilin-like HC110-R from Haemonchus contortus. Molecular & Biochemical Parasitology. 2009;164:162–164. doi: 10.1016/j.molbiopara.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Pemberton D, Franks C, Walker R, Holden-Dye L. Characterization of Glutamate-Gated Chloride Channels in the Pharynx of Wild-Type and Mutant Caenorhabditis elegans Delineates the Role of the Subunit GluCl-alpha2 in the Function of the Native Receptor. Molecular Pharmacology. 2001;59:1037–1043. doi: 10.1124/mol.59.5.1037. [DOI] [PubMed] [Google Scholar]
- 27.Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L- and B-subtypes of nematode nAChR resolved at the single-channel in Ascaris suum. The FASEB Journal. 2006;20:E2108–2116. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- 28.Qian H, Robertson AP, Powell-Coffman JA, Martin RJ. Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. The FASEB Journal. 2008;22:3247–3254. doi: 10.1096/fj.08-110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeger B, Schmitt-Wrede HP, Dehnhardt M, Benten WP, Krucken J, Harder A, Von Samson-Himmelstjerna G, Wiegand H, Wunderlich F. Latrophilin-like receptor from the parasitic nematode Haemonchus contortus as target for the anthelmintic depsipeptide PF1022A. FASEB J. 2001;15:1332–1334. doi: 10.1096/fj.00-0664fje. [DOI] [PubMed] [Google Scholar]
- 30.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nature Reviews Neuroscience. 2006;5:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 31.Samson-Himmelstjerna von G, Harder A, Sangster NC, Coles GC. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology. 2005;130:343–347. doi: 10.1017/s0031182004006523. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki T, Takagi M, Yaguchi T, Miyadoh S, Okada T, Koyama M. A new anthelmintic cyclodepsipeptide, PF1022A. J Antibiot. 1992;45:692–697. doi: 10.7164/antibiotics.45.692. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J. 1997;73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terada M. Neuropharmacological mechanism of action of PF1022A, an antinematode anthelmintic with a new structure of cyclic depsipeptide, on Angiostrongylus cantonensis and isolated frog rectus. Jpn J Parasitol. 1992;41:108–107. [Google Scholar]
- 35.Verma S, Robertson AP, Martin RJ. The nematode neuropeptide, AF2 (KHEYLRF-NH(2)), increases voltage-activated calcium currents in Ascaris suum muscle. Br J Pharmacol. 2007;151:888–899. doi: 10.1038/sj.bjp.0707296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma S, Robertson AP, Martin RJ. Effects of SDPNFLRF-amide (PF1) on voltage-activated currents in Ascaris suum muscle. International Journal for Parasitology. 2009;39:315–326. doi: 10.1016/j.ijpara.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma S, Robertson AP, Martin RJ. Effects of SDPNFLRF-amide (PF1) on voltage-activated currents in Ascaris suum muscle. Int J Parasitol. 2009;39:315–326. doi: 10.1016/j.ijpara.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallner M, Meera P, Toro L. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca(2+)-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci U S A. 1996;93:14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Zhou Y, Wen H, Levitan IB. Simultaneous binding of two protein kinases to a calcium-dependent potassium channel. J Neurosci. 1999;19:1–7. doi: 10.1523/JNEUROSCI.19-10-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang ZW, Saifee O, Nonet ML, Salkoff L. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron. 2001;32:867–881. doi: 10.1016/s0896-6273(01)00522-0. [DOI] [PubMed] [Google Scholar]
- 41.Wei A, Jegla T, Salkoff L. Eight potassium channel families revealed by the C. elegans genome project. Neuropharmacology. 1996;35:805–829. doi: 10.1016/0028-3908(96)00126-8. [DOI] [PubMed] [Google Scholar]
- 42.Welz C, Harder A, Schnieder T, Hoglund J, von Samson-Himmelstjerna G. Putative G protein-coupled receptors in parasitic nematodes--potential targets for the new anthelmintic class cyclooctadepsipeptides? Parasitol Res. 2005;97(Suppl 1):S22–32. doi: 10.1007/s00436-005-1441-4. [DOI] [PubMed] [Google Scholar]
- 43.Willson J, Amliwala K, Harder A, Holden-Dye L, Walker RJ. The effect of the anthelmintic emodepside at the neuromuscular junction of the parasitic nematode Ascaris suum. Parasitology. 2003;126:79–86. doi: 10.1017/s0031182002002639. [DOI] [PubMed] [Google Scholar]
- 44.Willson J, Amliwala K, Harder A, Holden-Dye L, Walker RJ. The effect of the anthelmintic emodepside at the neuromuscular junction of the parasitic nematode Ascaris suum. Parasitology. 2003;126:79–86. doi: 10.1017/s0031182002002639. [DOI] [PubMed] [Google Scholar]
- 45.Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]