Abstract
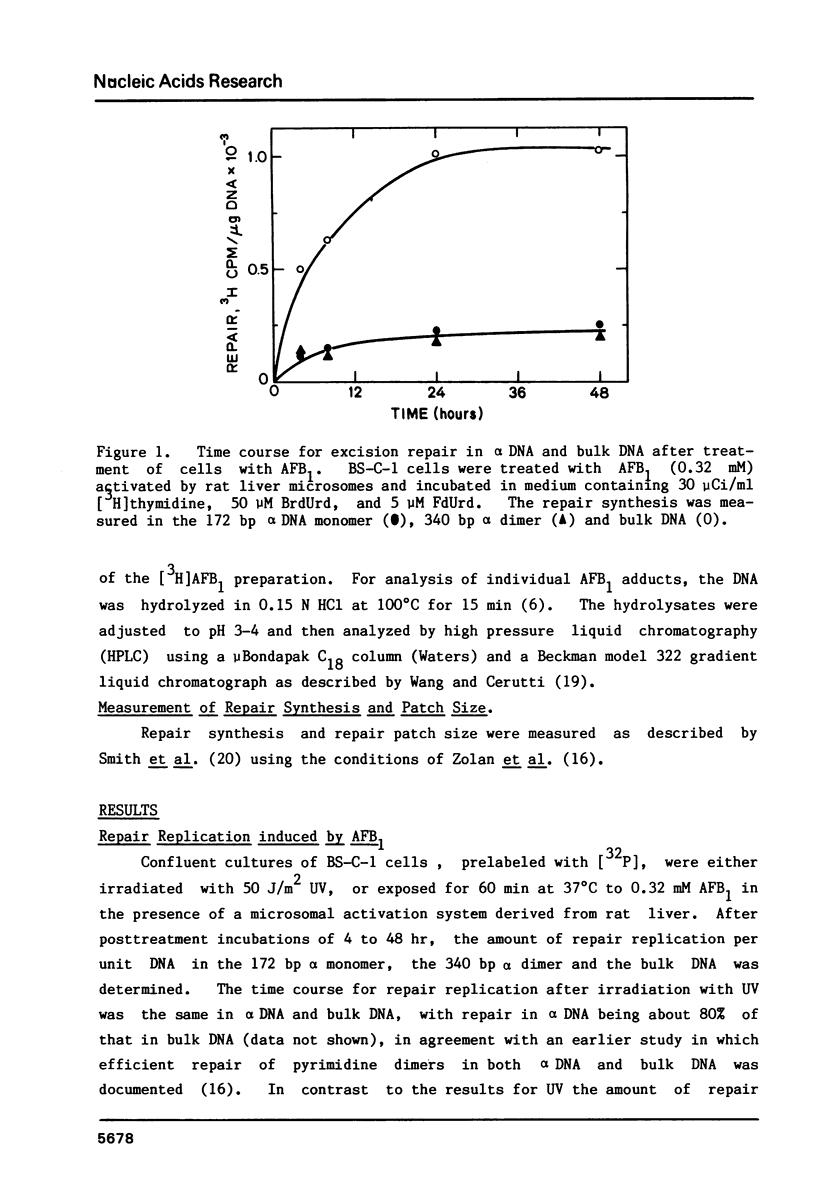
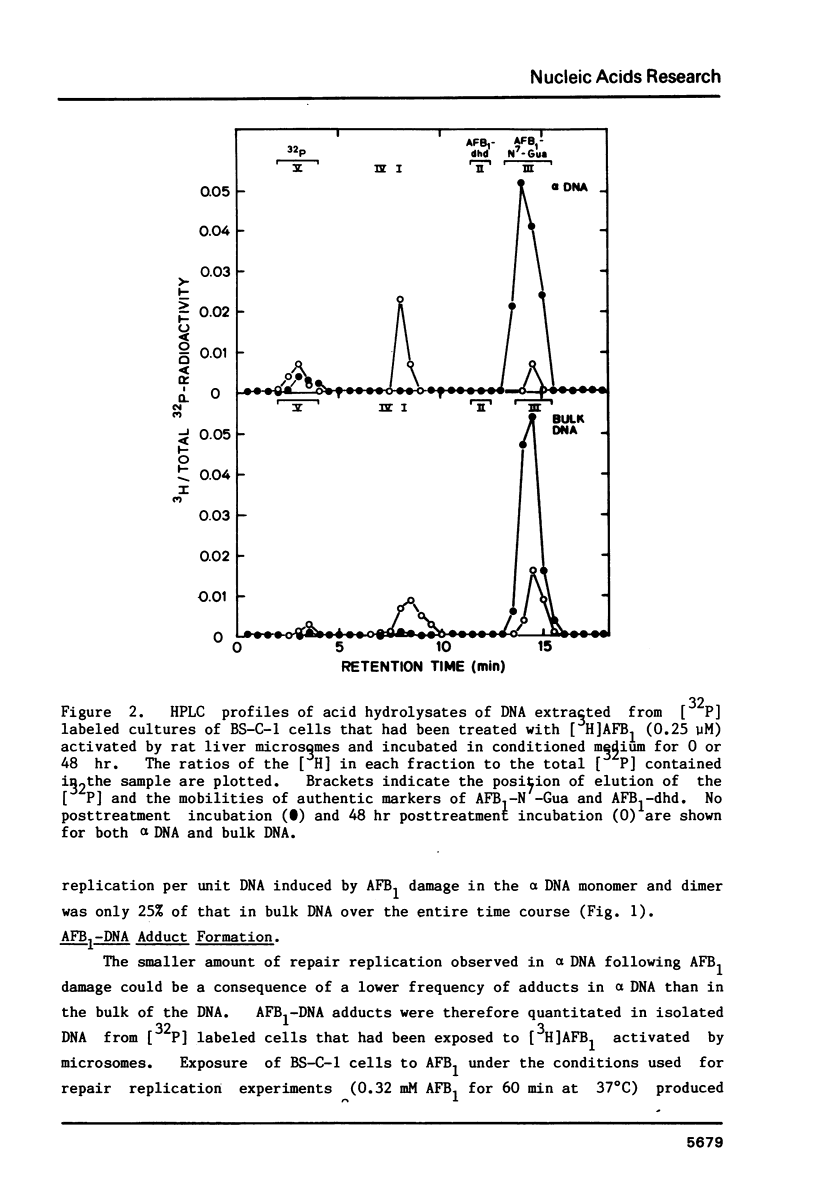
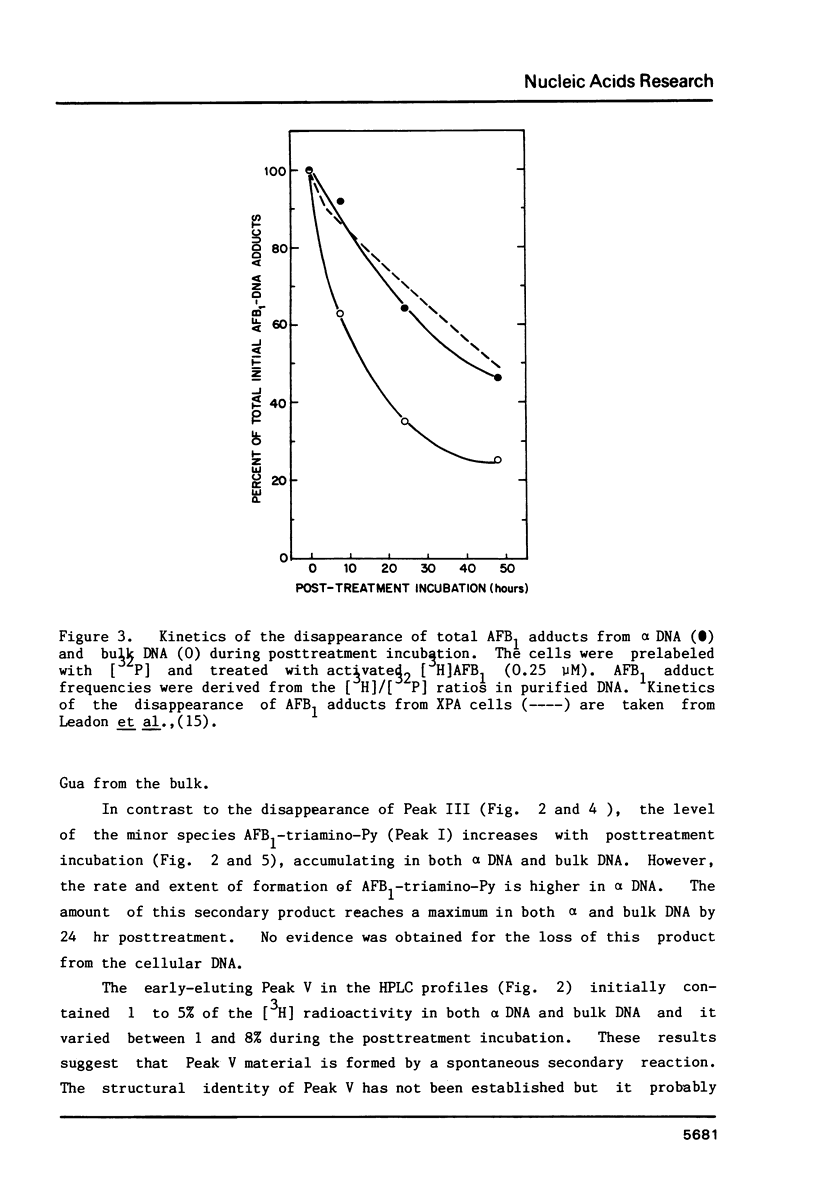
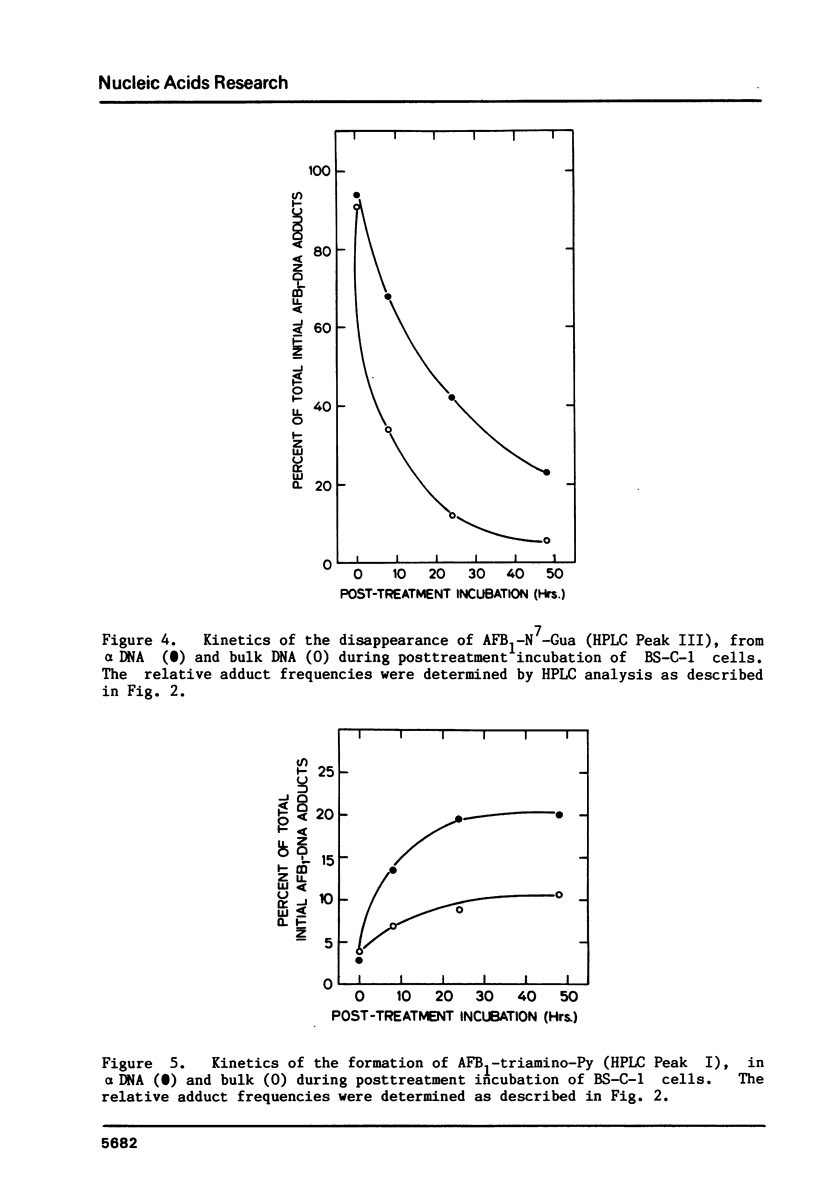
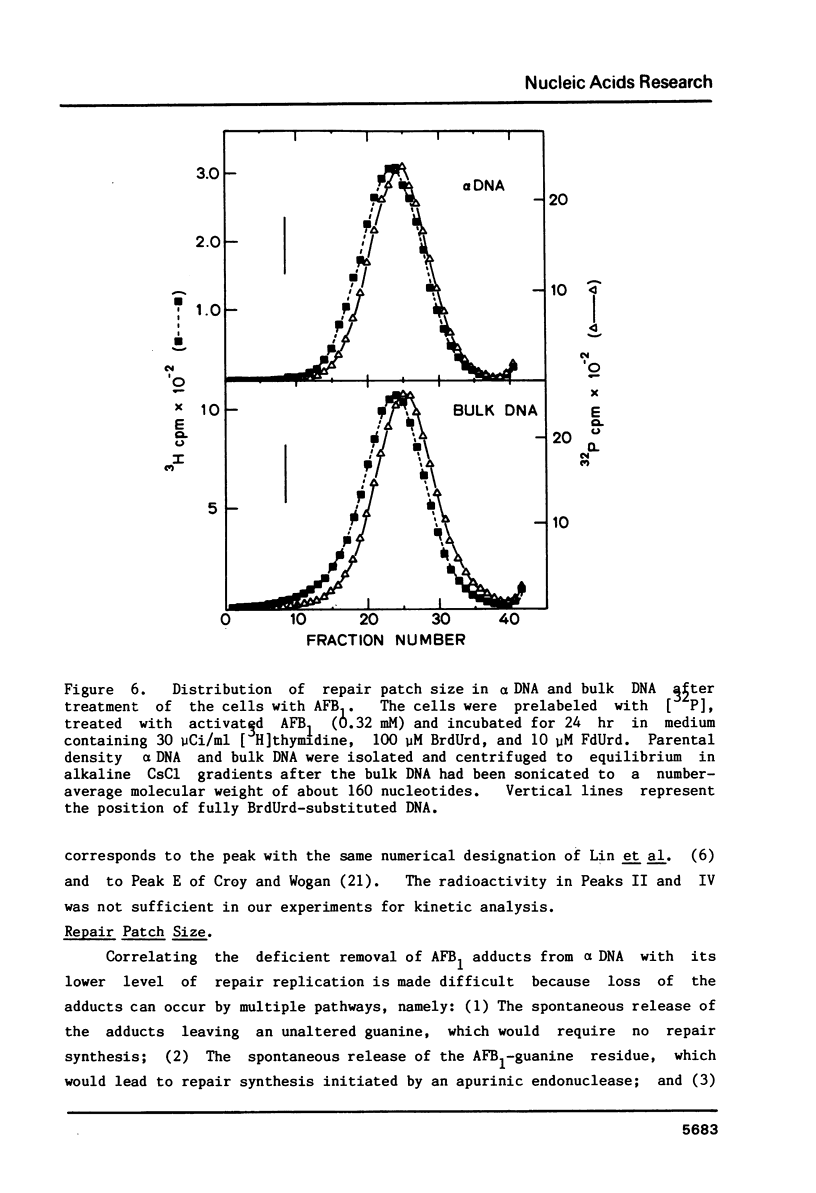
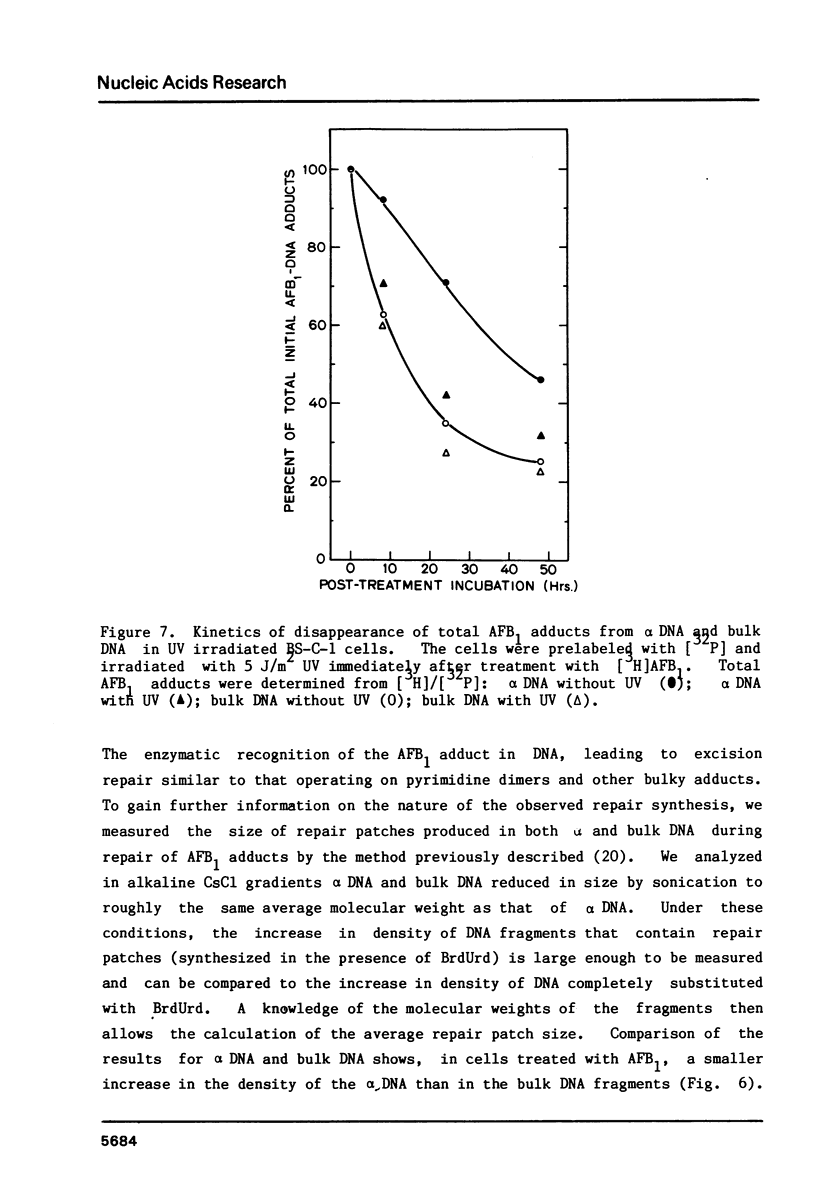
We have investigated the processing of adducts formed by covalent binding of aflatoxin B1 (AFB1) to DNA in confluent cultures of African green monkey cells. Repair synthesis elicited by AFB1 adducts was deficient in alpha DNA sequences compared to that in bulk DNA, although the initial levels of modification were the same for these DNAs. The removal of the primary initial adduct, AFB1-N7-Guanine, was deficient in alpha DNA and the kinetics of its loss resembled those previously reported for removal from total DNA in xeroderma pigmentosum cells of complementation group A. Spontaneous loss of the AFB1 moiety or the concomitant loss of the guanine to yield an apurinic site account for these results. The formation of the more chemically stable secondary product, AFB1-triamino-Pyrimidine, occurred more rapidly and to a greater extent in alpha DNA than in bulk DNA, probably because of slower removal of the primary product. The excision repair patch size for AFB1 adducts in alpha DNA was only 10 nucleotides compared to 20 nucleotides for repair of AFB1 adducts in bulk DNA. Irradiation of cells with low doses of UV prior to or immediately after treatment with AFB1 increased the rate and extent of removal of AFB1 adducts from alpha DNA to the levels found in the bulk DNA, indicating that the formation of pyrimidine dimers or their repair may alter the chromatin structure of alpha DNA sufficiently to facilitate its repair.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey G. S., Nixon J. E., Hendricks J. D., Sinnhuber R. O., Van Holde K. E. Carcinogen aflatoxin B1 is located preferentially in internucleosomal deoxyribonucleic acid following exposure in vivo in rainbow trout. Biochemistry. 1980 Dec 9;19(25):5836–5842. doi: 10.1021/bi00566a027. [DOI] [PubMed] [Google Scholar]
- Brown F. L., Musich P. R., Maio J. J. The repetitive sequence structure of component alpha DNA and its relationship to the nucleosomes of the African green monkey. J Mol Biol. 1979 Jul 15;131(4):777–799. doi: 10.1016/0022-2836(79)90201-8. [DOI] [PubMed] [Google Scholar]
- Cech T., Pardue M. L. Cross-linking of DNA with trimethylpsoralen is a probe for chromatin structure. Cell. 1977 Jul;11(3):631–640. doi: 10.1016/0092-8674(77)90080-0. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Essigmann J. M., Reinhold V. N., Wogan G. N. Identification of the principal aflatoxin B1-DNA adduct formed in vivo in rat liver. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1745–1749. doi: 10.1073/pnas.75.4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy R. G., Wogan G. N. Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Res. 1981 Jan;41(1):197–203. [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Modification of DNA by aflatoxin B1 creates alkali-labile lesions in DNA at positions of guanine and adenine. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4120–4124. doi: 10.1073/pnas.75.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essigmann J. M., Croy R. G., Nadzan A. M., Busby W. F., Jr, Reinhold V. N., Büchi G., Wogan G. N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1870–1874. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittler F., Zachau H. G. Subunit structure of alpha-satellite DNA containing chromatin from African green monkey cells. Nucleic Acids Res. 1979 Sep 11;7(1):1–13. doi: 10.1093/nar/7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis A. A., Snyder R. D., Dunn W. C., Regan J. D. Classification of chemical agents as to their ability to induce long- or short-patch DNA repair in human cells. Mutat Res. 1981 Sep;83(2):159–169. doi: 10.1016/0027-5107(81)90001-4. [DOI] [PubMed] [Google Scholar]
- Garner R. C., Miller E. C., Miller J. A. Liver microsomal metabolism of aflatoxin B 1 to a reactive derivative toxic to Salmonella typhimurium TA 1530. Cancer Res. 1972 Oct;32(10):2058–2066. [PubMed] [Google Scholar]
- Garner R. C., Wright C. M. Induction of mutations in DNA-repair deficient bacteria by a liver microsomal metabolite of aflatoxin B1. Br J Cancer. 1973 Dec;28(6):544–551. doi: 10.1038/bjc.1973.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Hertzog P. J., Lindsay Smith J. R., Garner R. C. A high pressure liquid chromatography study on the removal of DNA-bound aflatoxin B1 in rat liver and in vitro. Carcinogenesis. 1980 Sep;1(9):787–793. doi: 10.1093/carcin/1.9.787. [DOI] [PubMed] [Google Scholar]
- Hertzog P. J., Smith J. R., Garner R. C. Characterisation of the imidazole ring-opened forms of trans-8,9-dihydro-8,9-dihydro-8-(7-guanyl)9-hydroxy aflatoxin B1. Carcinogenesis. 1982;3(6):723–725. doi: 10.1093/carcin/3.6.723. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Cerutti P. A. A rapid and mild procedure for the isolation of DNA from mammalian cells. Anal Biochem. 1982 Mar 1;120(2):282–288. doi: 10.1016/0003-2697(82)90349-9. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Tyrrell R. M., Cerutti P. A. Excision repair of aflatoxin B1-DNA adducts in human fibroblasts. Cancer Res. 1981 Dec;41(12 Pt 1):5125–5129. [PubMed] [Google Scholar]
- Lin J. K., Miller J. A., Miller E. C. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res. 1977 Dec;37(12):4430–4438. [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- McLellan T. Electrophoresis buffers for polyacrylamide gels at various pH. Anal Biochem. 1982 Oct;126(1):94–99. doi: 10.1016/0003-2697(82)90113-0. [DOI] [PubMed] [Google Scholar]
- Muench K. F., Misra R. P., Humayun M. Z. Sequence specificity in aflatoxin B1--DNA interactions. Proc Natl Acad Sci U S A. 1983 Jan;80(1):6–10. doi: 10.1073/pnas.80.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli H. J., Cerutti P. A. Nucleosomal distribution of thymine photodimers following far- and near-ultraviolet irradiation. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1215–1223. doi: 10.1016/0006-291x(82)91098-1. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Rosenberg H., Singer M., Rosenberg M. Highly reiterated sequences of SIMIANSIMIANSIMIANSIMIANSIMIAN. Science. 1978 Apr 28;200(4340):394–402. doi: 10.1126/science.205944. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Smith C. A., Hanawalt P. C. Repair of DNA in human cells after treatment with activated aflatoxin B1. Cancer Res. 1977 Jun;37(6):1786–1793. [PubMed] [Google Scholar]
- Sarasin A., Goze A., Devoret R., Moulé Y. Induced reactivity of UV-damaged phage gamma in E. coli K12 host cells treated with aflatoxin B1 metabolites. Mutat Res. 1977 Feb;42(2):205–214. doi: 10.1016/s0027-5107(77)80024-9. [DOI] [PubMed] [Google Scholar]
- Singer D. S. Arrangement of a highly repeated DNA sequence in the genome and chromatin of the African green monkey. J Biol Chem. 1979 Jun 25;254(12):5506–5514. [PubMed] [Google Scholar]
- Snyder R. D., Regan J. D. DNA repair in normal human and xeroderma pigmentosum group A fibroblasts following treatment with various methanesulfonates and the demonstration of a long-patch (u.v.-like) repair component. Carcinogenesis. 1982;3(1):7–14. doi: 10.1093/carcin/3.1.7. [DOI] [PubMed] [Google Scholar]
- Stich H. F., Laishes B. A. The response of Xeroderma pigmentosum cells and controls to the activated mycotoxins, aflatoxins and sterigmatocystin. Int J Cancer. 1975 Aug 15;16(2):266–274. doi: 10.1002/ijc.2910160209. [DOI] [PubMed] [Google Scholar]
- Swenson D. H., Lin J. K., Miller E. C., Miller J. A. Aflatoxin B1-2,3-oxide as a probable intermediate in the covalent binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal RNA in vivo. Cancer Res. 1977 Jan;37(1):172–181. [PubMed] [Google Scholar]
- Walker I. G., Th'ng J. P. Excision-repair patch size in DNA from human KB cells treated with UV-light, or methyl methanesulfonate. Mutat Res. 1982 Oct;105(4):277–285. doi: 10.1016/0165-7992(82)90042-2. [DOI] [PubMed] [Google Scholar]
- Wang T. C., Cerutti P. A. Effect of formation and removal of aflatoxin B1:DNA adducts in 10T 1/2 mouse embryo fibroblasts on cell viability. Cancer Res. 1980 Aug;40(8 Pt 1):2904–2909. [PubMed] [Google Scholar]
- Wang T. C., Cerutti P. A. Formation and removal of aflatoxin B1-induced DNA lesions in epithelioid human lung cells. Cancer Res. 1979 Dec;39(12):5165–5170. [PubMed] [Google Scholar]
- Wang T. V., Cerutti P. Spontaneous reactions of aflatoxin B1 modified deoxyribonucleic acid in vitro. Biochemistry. 1980 Apr 15;19(8):1692–1698. doi: 10.1021/bi00549a027. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Wieshahn G. P., Hyde J. E., Hearst J. E. The photoaddition of trimethylpsoralen to Drosophila melanogaster nuclei: a probe for chromatin substructure. Biochemistry. 1977 Mar 8;16(5):925–932. doi: 10.1021/bi00624a018. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Cortopassi G. A., Smith C. A., Hanawalt P. C. Deficient repair of chemical adducts in alpha DNA of monkey cells. Cell. 1982 Mar;28(3):613–619. doi: 10.1016/0092-8674(82)90216-1. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Smith C. A., Calvin N. M., Hanawalt P. C. Rearrangement of mammalian chromatin structure following excision repair. Nature. 1982 Sep 30;299(5882):462–464. doi: 10.1038/299462a0. [DOI] [PubMed] [Google Scholar]


