Abstract
Background
At present, no medical therapy is known to affect the progression of rheumatic mitral stenosis (MS). We sought to assess the effect of statin treatment on long-term progression of MS in a large population.
Methods and Results
From our 20-year database, we identified all patients with rheumatic MS with ≥2 echocardiographies ≥1 year apart. Exclusion criteria were previous intervention on the mitral valve, more than moderate aortic regurgitation, or symptoms at first examination. The study sample included 315 patients (mean age, 61±12 years; 224 women); 35 patients (11.1%) were treated with statins, and 280 (88.9%) were not. Mean follow-up period was 6.1±4.0 years (range, 1 to 20). The rate of decrease in mitral valve area was significantly lower in the statin group compared with the untreated group (0.027±0.056 versus 0.067±0.082 cm2/y; P=0.005). The annualized change in mean transmitral gradient was lower in statin-treated patients (0.20±0.59 versus 0.58±0.96 mm Hg/y; P=0.023). The prevalence of fast MS progression (annual change in mitral valve area >0.08 cm2) was significantly lower in the statin group (P=0.008). An increase in systolic pulmonary artery pressure of >10 mm Hg was found in 17% of patients in the statin group versus 40% of untreated patients (P=0.045).
Conclusions
Our study shows a significantly slower progression of rheumatic MS in patients treated with statins. These findings could have an important impact in the early medical therapy of patients with rheumatic heart disease.
Keywords: mitral valve, rheumatic heart disease, statins, stenosis
Rheumatic heart disease (RHD) is the major cause of mitral stenosis (MS), especially but not exclusively in developing countries, when one considers the increasing trend to globalization. The prevalence of RHD is estimated worldwide at 15.6 million people,1 and every year there are 470 000 newly diagnosed cases and 233 000 deaths attributable to RHD.2 Rheumatic MS is a gradually progressive disease in which calcification leads to a severe degree of stenosis and affects the therapeutic approach.3 Recent studies showed that calcification is not a passive process but a bone formation process involving expression of osteoblast markers and neoangiogenesis.4–6 In addition, increased plasma levels of C-reactive protein (CRP), proinflammatory cytokines, and circulating adhesion molecules have been demonstrated in patients with chronic rheumatic MS,7–11 and elevated CRP levels seem to predict MS progression.12 If these mechanisms are considered to be similar to those involved in vascular atherosclerosis or aortic valve calcification, they could represent potential targets for pharmacological agents to slow this disease process. Several retrospective and prospective studies have assessed the effect of statins in slowing the progression of calcific aortic stenosis with conflicting results.13–20 A recent study of our group has suggested a potential positive effect of hydroxymethylglutaryl coenzyme-A reductase inhibitors (statins) in reducing the progression of rheumatic aortic stenosis.21 No data are available to date on a possible positive effect of statin therapy in rheumatic mitral valve (MV) disease. The aim of the present study was to assess the effect of statin treatment on long-term progression of rheumatic MS in a large population of patients with RHD.
Methods
Study Sample
From our adult computerized database, we identified all patients with rheumatic MS examined over the last 20 years (between October 1988 and March 2009). Rheumatic etiology of MV involvement was identified with the use of 2 compulsory criteria: the presence of confirmed history of rheumatic fever in the patients’ medical records and the echocardiographic diagnosis of rheumatic MV disease. The echocardiographic diagnosis of rheumatic MV disease was based on the typical aspect of rheumatic MV and subvalvular apparatus involvement (eg, commissural fusion with doming of the MV leaflets in diastole; thickening at the leaflet tips and variable degrees of thickening and/or calcification of the remainder of the leaflets; fusion, shortening, fibrosis, and calcification of the mitral chordae)22 and also on excluding all other possible etiologies of MS (eg, calcific MS with thin and mobile mitral leaflet tips, congenital MS, and various systemic disorders). All patients who had a baseline measured MV area (MVA) and at least 2 echocardiographic studies ≥1 year apart were considered eligible for the study. Exclusion criteria were as follows: previous MV replacement or MV commissurotomy, previous mitral balloon valvuloplasty, more than moderate aortic regurgitation, or symptoms at first examination and lack of detailed information on medical therapy. Demographic, echocardiographic, and clinical data were obtained by reviewing the patient’s medical records. Our group has recently published a study about the role of statins in rheumatic aortic stenosis progression. The actual study sample differs significantly from the previous study; many patients in that study had had a previous intervention on the MV and were not included in the present study. In addition, many patients of the present study did not have aortic valve involvement.
Clinical Data
The following clinical data were collected: age, gender, history of smoking, hypertension, diabetes mellitus, hypercholesterolemia, prior evidence of coronary artery disease (CAD) (history of myocardial infarction or coronary revascularization or documented CAD by coronary angiography). Information regarding the use of statin treatment (type of drug, dose, and treatment duration) was obtained. The available medical records did not indicate the number of rheumatic fever attacks/rheumatic carditis events before the first echocardiographic examination and the previous antibiotic prophylaxis treatment and duration. No patient was on antibiotic prophylaxis at the time of the echocardiographic examinations.
Echocardiographic Examination
Echocardiography was performed with commercially available ultrasound systems. MVA was assessed by both the pressure half-time method23 and direct planimetry24 whenever possible. To determine MVA by the pressure half-time method, transmitral inflow velocities were recorded by continuous-wave Doppler echocardiography from the apical 4-chamber view, and valve areas were calculated as an average of 3 beats in sinus rhythm and of 5 beats in atrial fibrillation. Mean gradient across the MV was measured.25 The Wilkins score was calculated as described previously.26 Presence of mitral and aortic regurgitation was evaluated qualitatively, and the severity of regurgitation was estimated by a semiquantitative evaluation: mild, moderate, and severe, according to the width and depth of the regurgitant jet.3,27 Left atrial (LA) dimension was reported as anteroposterior diameter measured in the parasternal long-axis view. Right ventricular systolic pressure was estimated from tricuspid regurgitation with the use of the modified Bernoulli equation25 and addition of 5 to 15 mm Hg for the estimated right atrial pressure. In the absence of pulmonary stenosis, systolic pulmonary artery pressure was considered equal with right ventricular systolic pressure. All echocardiograms were analyzed by an investigator blinded to the medical treatment of patients.
The study sample was divided into 2 subgroups according to statin treatment (statin and nonstatin group, respectively). Primary end point was the annual change in MVA, referred to as rate of MS progression (cm2/y; calculated by dividing the difference between the last and the first measurements by the time between examinations). Fast MS progression was defined as an annual change in MVA >0.08 cm2/y, corresponding to the 75th percentile of annualized MVA change reported in our entire study sample. Secondary end points were the pattern of fast MS progression and changes in mitral regurgitation severity. The progression of pulmonary hypertension was defined as an increase in systolic pulmonary artery pressure >10 mm Hg during follow-up. Annualized changes in mean transmitral gradient (mm Hg/y) and LA dimension were also calculated.
Statistical Analysis
Continuous variables were expressed as mean±SD and categorical variables as percentages. The χ2 test was used for the comparison of dichotomous variables and the Student t test for continuous variables. Wilcoxon rank sum test was used for comparison of nominal variables. To identify parameters that could predict MS progression rate, single-variable correlation analysis and multivariable linear regression analysis were performed. A P value <0.05 was considered statistically significant. The analyses were performed with the use of SPSS for Windows, version 13.0 (SPSS, Inc, Chicago, Ill).
Results
At baseline, 736 patients had rheumatic MS and the MVA measured (the query of the database was for rheumatic MS on native valves with MVA measured); 266 patients were excluded because they had only 1 echocardiographic examination or an echocardiographic follow-up examination at <1 year. From the remaining 470 patients, we excluded 12 patients for the presence of more than moderate aortic regurgitation, 81 patients for the lack of detailed information on medical therapy, and 62 patients for previous mitral commissurotomy or balloon valvuloplasty, and the final study sample consisted of 315 patients (mean age, 61±12 years; 224 women). Mean follow-up period was 6.1±4.0 years. Of all patients, 35 (11.1%) were receiving statin treatment, and 280 (88.9%) were not. Statins employed and mean daily dosages were as follows: simvastatin (18 patients, 16±8 mg), atorvastatin (10 patients, 16±5 mg), pravastatin (4 patients, 30±12 mg), and rosuvastatin (3 patients, 10±0 mg). There were no significant differences in age, gender distribution, and follow-up period between the 2 subgroups of patients. Patients in the statin group, as expected, had more frequently hypercholesterolemia (89% versus 14%; P<0.001), hypertension (49% versus 28%; P=0.018), and previous evidence of CAD (71% versus 41%; P=0.001) (Table 1). Baseline echocardiographic characteristics of the entire study sample and of the 2 groups are shown in Table 2. MVA assessment at baseline and at follow-up was available by the pressure half-time method in all patients and by planimetry in 101 patients. In those 101 patients, the correlation between the 2 methods was very high (r=0.90, P<0.001), and the Bland-Altman analysis28 confirmed a good correlation between these 2 methods of MVA estimation in our study sample. Therefore, for all analyses in the study, we used MVA assessment by the pressure half-time method.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients in the Entire Study Sample and in the 2 Subgroups of Patients
| Variable | All Patients (n=315) |
Statin Group (n=35) |
Nonstatin Group (n=280) |
P* |
|---|---|---|---|---|
| Age, y | 61±12 | 60±9 | 61±13 | 0.66 |
| Female, n (%) | 224 (71) | 23 (66) | 201 (72) | 0.64 |
| Atrial fibrillation, n (%) | 119 (38) | 9 (28) | 110 (39) | 0.17 |
| Hypercholesterolemia, n (%) | 70 (22) | 31 (89) | 39 (14) | <0.001 |
| Hypertension, n (%) | 94 (30) | 17 (49) | 77 (28) | 0.018 |
| Diabetes mellitus, n (%) | 48 (15) | 5 (14) | 43 (15) | 0.93 |
| CAD, n (%) | 139 (44) | 25 (71) | 114 (41) | 0.001 |
| Follow-up, y | 6.1±4.0 | 7.1±4.2 | 5.9±4 | 0.097 |
P value for comparisons between statin group and nonstatin group.
Table 2.
Baseline Echocardiographic Characteristics of Patients in the Entire Study Sample and in the 2 Subgroups of Patients
| Variable | All Patients (n=315) |
Statin Group (n=35) |
Nonstatin Group (n=280) |
P* |
|---|---|---|---|---|
| MVA, cm2 | 1.55±0.43 | 1.66±0.32 | 1.53±0.44 | 0.092 |
| Mean mitral gradient, mm Hg |
6.35±2.75 | 6.24±2.56 | 6.36±2.78 | 0.81 |
| Mitral regurgitation degree, 0/1/2/3 |
14/184/100/17 | 0/22/12/1 | 14/162/88/16 | 0.82 |
| sPAP, mm Hg† | 35±11 | 32±8 | 36±11 | 0.086 |
| LA dimension, cm | 5.2±1.2 | 4.9±0.8 | 5.2±1.3 | 0.18 |
| Left ventricular ejection fraction, % |
60±7 | 59±6 | 60±7 | 0.42 |
| Wilkins score | 5.9±2.0 | 5.6±1.9 | 5.9±2.0 | 0.40 |
Continuous variables are expressed as mean±SD; nominal variables are expressed as numbers. sPAP indicates systolic pulmonary arterial pressure.
P value for comparisons between statin group and nonstatin group.
sPAP was available in 224 patients (200 patients in the nonstatin group and 24 patients in the statin group).
MVA and mean transmitral gradient values at baseline were similar in patients in the statin and nonstatin groups; the initial severity of mitral regurgitation was also similar in the 2 subgroups (Table 2).
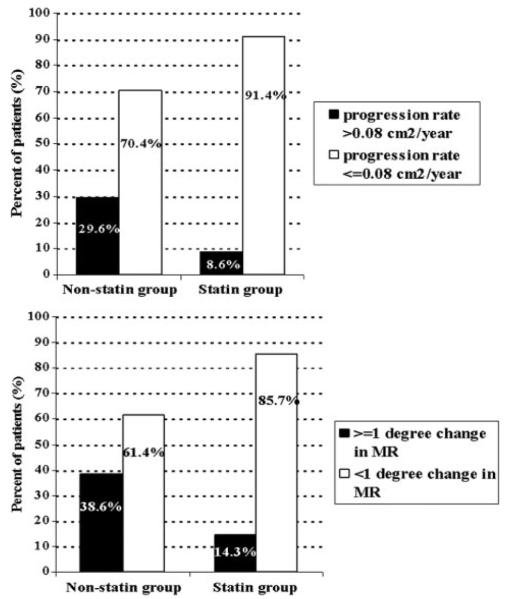
The mean rate of MS progression in the entire study sample was 0.062±0.079 cm2/y. At subgroup analysis (Table 3), the rate of MS progression, expressed as the rate of decrease in MVA, was significantly lower in statin-treated patients (0.027±0.056 versus 0.067±0.082 cm2; P=0.005). An annual change in MVA >0.08 cm2/y, referred to as fast MS progression, was found in 3 patients (8.6%) treated with statins and in 83 untreated patients (29.6%) (P=0.008) (Figure). Similarly, the annualized change in mean transmitral gradient was lower in statin-treated patients (0.20±0.59 versus 0.58±0.96 mm Hg/y; P=0.023).
Table 3.
Outcomes in Patients Treated With Statins vs Untreated Patients
| Outcome | Statin Group (n=35) |
Nonstatin Group (n=280) |
P |
|---|---|---|---|
| MS progression rate, cm2/y | 0.027±0.056 | 0.067±0.080 | 0.005 |
| Annualized change in mean transmitral gradient, mm Hg/y |
0.20±0.59 | 0.58±0.96 | 0.023 |
| Fast MS progression (>0.08 cm2/y), n (%) |
3 (8.6) | 83 (29.6) | 0.008 |
| ≥1 degree change in mitral regurgitation severity |
5 (14.3) | 108 (38.6) | 0.008 |
| Change in mitral regurgitation severity, −2/−1/0/1/2/3 |
0/2/28/4/1 | 1/25/146/98/9/1 | 0.038 |
| Change in sPAP, mm Hg | 5±12 | 10±14 | 0.095 |
| Increase of >10 mm Hg in sPAP, n (%) |
4 (17) | 80 (40) | 0.045 |
| Wilkins score at last echocardiogram |
6.0±1.9 | 6.8±2.1 | 0.033 |
| Change in LA dimension, cm/y |
0.14±0.27 | 0.12±0.28 | 0.69 |
Continuous variables are expressed as mean±SD; nominal variables are expressed as numbers or numbers and percentages. sPAP indicates systolic pulmonary artery pressure.
Figure.
Rheumatic MV involvement: prevalence of fast MS progression pattern and of change in mitral regurgitation severity in statin-treated versus untreated patients.
During follow-up, worsening of mitral regurgitation severity was more important in the nonstatin group (P=0.038) (Table 3), and, when a ≥1 degree worsening of mitral regurgitation was considered, significantly fewer patients treated with a statin reached this end point (P=0.008). Only 17% of patients in the statin group presented an increase in systolic pulmonary artery pressure of >10 mm Hg during follow-up versus 40% in the nonstatin group (P=0.045). The annualized change of LA diameter was not different in the 2 groups (Table 3).
During follow-up, no patient in the study sample was identified as experiencing recurrences of rheumatic fever and/or diagnosed streptococcal infection, as assessed by a review of all of their medical records.
On single-variable analysis, statin treatment, presence of CAD, baseline MVA, baseline mean transmitral gradient, and baseline LA dimension were significantly correlated with MS progression rate. On multivariable regression analysis (including the parameters significantly correlated to MS progression rate on single-variable analysis), baseline MVA, baseline LA dimension, and statin treatment emerged as independent predictors of MS progression rate (Table 4).
Table 4.
Single-Variable and Multivariable Analysis of Predictors of MS Progression Rate
| Single-Variable Analysis |
Multivariable Analysis |
|||
|---|---|---|---|---|
| Parameter | Pearson Coefficient |
P | Standardized β Coefficient |
P |
| Age | −0.002 | 0.97 | ||
| Gender | 0.05 | 0.38 | ||
| Baseline MVA | −0.24 | <0.001 | −0.33 | <0.001 |
| Baseline mean mitral gradient |
0.18 | 0.001 | −0.01 | 0.87 |
| Baseline mitral regurgitation degree |
−0.06 | 0.29 | ||
| LA dimension | −0.15 | 0.007 | −0.25 | <0.001 |
| Hypercholesterolemia | 0.06 | 0.29 | ||
| Hypertension | 0.07 | 0.22 | ||
| CAD | 0.15 | 0.009 | 0.10 | 0.064 |
| Statin treatment | 0.16 | 0.005 | 0.14 | 0.010 |
Discussion
This is, to our knowledge, the first study that seeks to assess the potential role of statin treatment in slowing the progression of rheumatic MS. Although the global prevalence of RHD seems to be decreasing, this condition remains an important cause of morbidity and mortality in some areas of the world. Up to 1% of schoolchildren in Africa, Asia, the Eastern Mediterranean region, and Latin America show signs of RHD,2 and it is possible that this prevalence could be even higher because the estimates are not necessarily established with the use of echocardiographic studies.29 Currently, the treatment of chronic RHD is directed mainly at secondary prevention of acute rheumatic carditis and symptomatic medical treatment, percutaneous balloon valvuloplasty, or surgical valvotomy/MV replacement when significant valve disease develops.3 There is no specific treatment aimed at preventing the progression of valvular damage.
The most frequent rheumatic valve involvement is the MV; an echocardiographic screening performed in children from geographic areas with high prevalence of RHD showed an extremely high prevalence of MV involvement (87.3% in Cambodia and 98.4% in Mozambique).29 On the other hand, rheumatic etiology is still the most frequent cause of MS worldwide. The pathogenic mechanisms leading to rheumatic valve disease consist initially of antigenic mimicity of the M protein antigen found in both heart structures and group A hemolytic Streptococcus, which results in an autoimmune reaction of the heart in response to streptococcal infection.30 The rheumatic process leads to inflammation in all layers of the heart. However, the disease primarily affects the endocardium, leading to inflammation, valvulitis, and scarring of the cardiac valves. After a rheumatic attack, the alterations of the valve progress slowly, driven by hemodynamic stress on the injured valve and by repeated acute episodes of rheumatic fever.12,30 MS develops and progresses in time from leaflet thickening, commissural fusion, and chordal shortening and fusion to ultimately form calcification of the valve and subvalvular apparatus. The factors that determine the rate of MS progression could be related to a continuing rheumatic process with either repetitive rheumatic insults or an ongoing inflammatory process but also to trauma caused by the continuing turbulence of the blood flow through a deformed valve orifice, analogous to the mechanism suggested for calcific aortic stenosis.31,32 The progression of rheumatic MS over long periods of time is generally slow but extremely variable. The rate of rheumatic MS progression in different studies assessing the natural history of rheumatic MS was 0.09±0.13 cm2/y,30 0.09±0.21 cm2/y,33 or 0.06±0.04 cm2/y.32 In our study, we found a rate of rheumatic MS progression of 0.062±0.079 cm2/y in the entire study sample and a significantly slower progression rate (0.027±0.056 cm2/y) in patients treated with statins compared with those untreated. The individual or cutoff parameters that may predict the rate of decline in MVA in the individual patient are controversial. One study describes a higher initial MV score and transmitral gradient as predictors of more rapid progression,33 but another study showed that no single value for initial MVA, echocardiographic score, gradient, or combination of such values could separate the groups with and without progression or allow confident prediction of changes in valve area in any individual patient.31 In addition, we found a significant difference between the 2 groups regarding the proportions of patients with fast MS progression, with fewer patients experiencing a fast MS progression pattern in the statin group.
Valve calcification is an important determinant of disease progression and patients’ outcome, orienting the therapeutic approach (eg, the feasibility of percutaneous balloon dilatation).3,26 Recent studies have tried to elucidate the cellular mechanisms responsible for calcification in the cardiac valves, with either nonrheumatic or rheumatic etiology. It has been suggested that the mechanism for valvular calcification is similar to skeletal bone formation and that calcification occurs in areas of neoangiogenesis, which is stimulated by an active inflammatory process.4–6,34 The cellular differences between the rheumatic and degenerative lesions are the intense inflammatory infiltrate and the demonstration of vascular endothelial growth factor in areas of neoangiogenesis found in the rheumatic valves compared with the degenerative valves.6 There is an increasing body of evidence that inflammation is an ongoing process in patients with chronic rheumatic valve disease. Elevated CRP levels, circulating adhesion molecules, and different proinflammatory cytokines as well as oxidative stress have been demonstrated in these patients.7–11 Moreover, there is some evidence that increased levels of CRP could be associated with rheumatic MS progression.12
In view of all of this evidence, the idea of a therapy that could target some of the mechanisms of the calcification process and the ongoing inflammation has emerged. Therefore, a number of experimental studies35–39 as well as retro-spective and prospective clinical trials have tested the role of statins in influencing the valvular calcification process in calcific aortic stenosis. Some of the experimental studies35,37 have shown a reduction in the atherosclerotic bone-forming lesions, whereas the clinical studies have had conflicting results,13–20 influenced by the characteristics of populations studied and by the stage of valvular disease at which the therapeutic intervention was applied. In the first prospective randomized study (Scottish Aortic Stenosis and Lipid Lowering Trial [SALTIRE]) testing the effects of statins in aortic valve disease,18 the intensive lipid-lowering therapy did not halt the progression of calcific aortic stenosis or induce its regression; a potential reason is the fact that patients received the therapy late in the course of the disease process. Experimental data show that the earlier in the disease process that the statin therapy is initiated, the greater is the potential for slowing the progression of this disease.38,40 The Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial19 initiated therapy earlier in the disease process, but the progression of aortic stenosis was not influenced. In the Rosuvastatin Affecting Aortic Valve Endothelium (RAAVE) study, prospective treatment of moderate aortic stenosis with rosuvastatin targeting serum low-density lipoprotein slowed progression of echocardiographic measures of aortic stenosis severity and improved inflammatory biomarkers, providing the first clinical evidence for targeted therapy in asymptomatic patients with moderate to severe aortic stenosis.17
The only study that tested the role of statins in rheumatic valve disease21 showed that statin treatment could reduce the progression of rheumatic aortic stenosis. In the present study, we analyzed the effects of statin treatment in slowing the progression of rheumatic MS, targeting these pathophysiological mechanisms involved in the development of rheumatic valve involvement. We found a significantly slower progression of rheumatic MS in patients treated with statins compared with untreated patients. In addition, there was also a significant effect in preventing the worsening of mitral regurgitation, indicating a positive effect of statin treatment on slowing the progression of rheumatic MV disease. The hemodynamic consequences of MS on pulmonary circulation are well known, and some studies have described the progression of right heart disease associated with MS: progression of pulmonary hypertension and tricuspid regurgitation, gradual increase in right heart size, and slight decrease in right ventricular function over time.31 In our study, the slower progression of MV disease in the statin group was associated with a slower progression of pulmonary hypertension.
These results confirm the potentially beneficial effects of statin treatment in slowing the progression of rheumatic valve disease.21 This positive effect could be related to both their lipid- and nonlipid-lowering effects, including anti-inflammatory properties,41 especially through CRP lowering,42,43 improvement in endothelial function,44 and reduction of oxidative processes.42 In addition, impacts on the lipid effects of activation of the low-density lipoprotein receptor-related protein 5–mediated bone formation and cellular proliferation may play a role.45,46 Most of the additional nonlipid effects, referred to as pleiotropic effects, are a direct result of the downstream effect of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A, the rate-limiting enzyme in the synthesis of cholesterol.47 Future prospective clinical trials are necessary to understand the role of statins in the treatment of RHD.47,48
Study Limitations
The main limitation of our study is the fact that it is a retrospective, observational, nonrandomized study with its inherent limitations. Patients were not randomly assigned to the statin or nonstatin group; this classification was done on the basis of the treatment patients were receiving according to their medical records. One reason for the patients in the statin group to receive statin treatment is the greater prevalence of hypercholesterolemia, hypertension, and CAD, which made the 2 groups somewhat dissimilar. This stems from the retrospective nature of this study. For the lack of complete MVA measurements (also a consequence of the retrospective nature of the study), we analyzed only MVA and mean transmitral gradient to assess MS progression. The severity of mitral regurgitation was not always assessed with all of the established criteria3 during the long period of >20 years, but for each moment of time a semiquantitative assessment validated in our laboratory was used. LA size was assessed only with the use of the anteroposterior diameter from the parasternal long-axis view because at the time of the first echocardiographic studies, little was known about the value of measuring its area or volume.
Even if a patient was considered to be statin-treated provided that the information about statin treatment was present in serial medical records during the entire follow-up period, we cannot ascertain perfect compliance with the statin treatment.
Lacking information about the number of rheumatic fever attacks/rheumatic carditis events, the time interval between the acute rheumatic fever diagnosis and the first echocardiographic examination and the previous antibiotic prophylaxis treatment and duration is another limitation of this retrospective study. Although data about recurrences of rheumatic fever and prophylaxis are important factors in analyzing the progression of rheumatic MV disease, their significance in our study sample may be less important because this is a cohort of relatively old patients (mean age, 61±12 years) living in a nonendemic area for rheumatic fever.2 Future studies addressing the effect of medical therapy such as statins in slowing the progression of rheumatic valve disease should assess this effect in addition to that of antibiotic prophylaxis.
Another limitation is the lack of low-density lipoprotein and CRP levels (these data were not available at a time when such parameters were not assessed routinely). However, this only limits further pathophysiological insights into the possible mechanisms of statin benefit in patients with MS but does not influence the main study finding.
Conclusions
The effect of statin treatment in slowing the progression of rheumatic MV disease is assessed for the first time in the present study. The results show a significantly slower progression of rheumatic MV disease in patients treated with statins. If confirmed by prospective randomized studies, these findings could have an important impact on the early medical therapy of patients with RHD.
CLINICAL PERSPECTIVE.
This is the first study to assess the role of statin treatment in slowing the progression of rheumatic mitral stenosis (MS). Three hundred fifteen patients with rheumatic MS, either statin treated (35 patients, 11.1%) or untreated (280 patients, 88.9%), were followed for 6.1±4.0 years in this retrospective study. We found a significantly slower progression of MS, as assessed by decrease in mitral valve area, in patients treated with statins compared with untreated patients (0.027±0.056 versus 0.067±0.082 cm2/y; P=0.005). The increase in mean transmitral gradient was also lower in statin-treated patients (0.20±0.59 versus 0.58±0.96 mm Hg/y; P=0.023). The prevalence of fast MS progression (annual change in mitral valve area >0.08 cm2) was significantly lower in the statin group (P=0.008). In addition, there was a significant effect of statin treatment in reducing progression of mitral regurgitation during follow-up (P=0.038). These results indicate a possible benefit of statin treatment in slowing the progression of rheumatic mitral valve disease. Although the global prevalence of rheumatic valve disease seems to be decreasing, this condition remains an important cause of morbidity and mortality in many areas of the world. A medical treatment effective in slowing the progression of rheumatic mitral valve disease would have a critical role in delaying mitral valve interventions (eg, percutaneous or surgical). This could be particularly important in areas where access to such interventions is inadequate. If confirmed by prospective randomized controlled trials, these results may have important therapeutic, social, and economic implications.
Footnotes
Disclosures Dr Rajamannan is an inventor on a patent owned by the Mayo Clinic entitled “Method for Slowing Heart Valve Degeneration.” Dr Rajamannan does not receive any royalties from this patent. The other authors have no potential conflicts of interest to disclose.
References
- 1.Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155–168. doi: 10.1016/S0140-6736(05)66874-2. [DOI] [PubMed] [Google Scholar]
- 2.Rheumatic Fever and Rheumatic Heart Disease: Report of WHO Expert Consultation, Geneva, 29 October--1 November 2001. World Health Organization; Geneva, Switzerland: 2004. Technical Report Series 923. [Google Scholar]
- 3.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A, Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology. ESC Committee for Practice Guidelines Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 4.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohler ER, III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 6.Rajamannan NM, Nealis TB, Subramaniam M, Pandya S, Stock SR, Ignatiev CI, Sebo TJ, Rosengart TK, Edwards WD, McCarthy PM, Bonow RO, Spelsberg TC. Calcified rheumatic valve neoangiogenesis is associated with vascular endothelial growth factor expression and osteoblast-like bone formation. Circulation. 2005;111:3296–3301. doi: 10.1161/CIRCULATIONAHA.104.473165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu-Braga YY, Hayashi SY, Schafranski M, Messias-Reason IJ. Further evidence of inflammation in chronic rheumatic valve disease (CRVD): high levels of advanced oxidation protein products (AOPP) and high sensitive C-reactive protein. Int J Cardiol. 2006;109:275–276. doi: 10.1016/j.ijcard.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Gölbasi Z, Uçar O, Keles T, Sahin A, Cagli K, Camsari A, Diker E, Aydogdu S. Increased levels of high sensitive C-reactive protein in patients with chronic rheumatic valve disease evidence of ongoing inflammation. Eur J Heart Fail. 2002;4:593–595. doi: 10.1016/s1388-9842(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 9.Krasuski RA, Bush A, Kay JE, Mayes CE, Jr, Wang A, Fleming J, Pierce C, Kisslo KB, Harrison JK, Bashore TM. C-reactive protein elevation independently influences the procedural success of percutaneous balloon mitral valve commissurotomy. Am Heart J. 2003;146:1099–1104. doi: 10.1016/S0002-8703(03)00506-4. [DOI] [PubMed] [Google Scholar]
- 10.Guilherme L, Cury P, Demarchi LMF, Coelho V, Abel L, Lopez AP, Oshiro SE, Aliotti S, Cunha-Neto E, Pomerantzeff PMA, Tanaka AC, Kalil J. Rheumatic heart disease: proinflammatory cytokines play a role in the progression and maintenance of valvular lesions. Am J Pathol. 2004;165:1583–1591. doi: 10.1016/S0002-9440(10)63415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yetkin E, Erbay AR, Ileri M, Turhan H, Balci M, Cehreli S, Yetkin G, Demirkan D. Levels of circulating adhesion molecules in rheumatic mitral stenosis. Am J Cardiol. 2001;88:1209–1211. doi: 10.1016/s0002-9149(01)02067-7. [DOI] [PubMed] [Google Scholar]
- 12.Alyan O, Metin F, Kacmaz F, Ozdemir O, Maden O, Topaloglu S, Demir AD, Karahan Z, Karadede A, Ilkay E. High levels of high sensitivity C-reactive protein predict the progression of chronic rheumatic mitral stenosis. J Thromb Thrombolysis. 2009;28:63–69. doi: 10.1007/s11239-008-0245-7. [DOI] [PubMed] [Google Scholar]
- 13.Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 14.Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–1730. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 15.Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme A reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–2209. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- 16.Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 17.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 19.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf K, Holme I, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Skjærpe T, Wachtell K, Willenheimer R, for the SEAS Investigators Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1–14. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 20.Antonini-Canterin F, Hirsu M, Popescu BA, Leiballi E, Piazza R, Pavan D, Ginghină C, Nicolosi GL. Stage-related effect of statin treatment on the progression of aortic valve sclerosis and stenosis: a long-term follow-up study in 1046 patients. Am J Cardiol. 2008;102:738–742. doi: 10.1016/j.amjcard.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 21.Antonini-Canterin F, Leiballi E, Enache R, Popescu BA, Roçsca M, Cervesato E, Piazza R, Ginghin̆ C, Nicolosi GL. Hydroxymethylglutaryl coenzyme-A reductase inhibitors delay the progression of rheumatic aortic valve stenosis: a long-term echocardiographic study. J Am Coll Cardiol. 2009;53:1874–1879. doi: 10.1016/j.jacc.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 22.Otto CM. Textbook of Clinical Echocardiography. 4th ed Saunders Elsevier; Philadelphia, Pa: 2009. p. 277. [Google Scholar]
- 23.Hatle L, Angelsen B, Tromsdal A. Noninvasive assessment of atrioventricular pressure half-time by Doppler ultrasound. Circulation. 1979;60:1096–1104. doi: 10.1161/01.cir.60.5.1096. [DOI] [PubMed] [Google Scholar]
- 24.Henry WL, Griffith JM, Michaelis LL, McIntosh CL, Morrow AG, Epstein SE. Measurement of mitral valve orifice area in patients with mitral valve disease by real-time, two-dimensional echocardiography. Circulation. 1975;51:827–836. doi: 10.1161/01.cir.51.5.827. [DOI] [PubMed] [Google Scholar]
- 25.Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 26.Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dall’Aglio V, D’Angelo G, Moro E, Nicolosi GL, Burelli C, Zardo F, Cervesato E, Zanuttini D. Interobserver and echo-angio variability of two-dimensional colour Doppler evaluation of aortic and mitral regurgitation. Eur Heart J. 1989;10:334–340. doi: 10.1093/oxfordjournals.eurheartj.a059490. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 29.Marijon E, Ou P, Celermajer DS, Ferriera B, Mocumbi AO, Jani D, Parquet C, Jacob S, Sidi D, Jouven X. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–476. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 30.Carabello BA. Modern management of mitral stenosis. Circulation. 2005;112:432–437. doi: 10.1161/CIRCULATIONAHA.104.532498. [DOI] [PubMed] [Google Scholar]
- 31.Sagie A, Freitas N, Padial LR, Leavitt M, Morris E, Weyman AE, Levine RA. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol. 1996;28:472–479. doi: 10.1016/0735-1097(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 32.Faletra F, De Chiara F, Crivellaro W, Mantero A, Corno R, Brusoni B. Echocardiographic follow-up in patients with mild to moderate mitral stenosis: is a yearly examination justified? Am J Cardiol. 1996;78:1450–1452. doi: 10.1016/s0002-9149(97)89300-9. [DOI] [PubMed] [Google Scholar]
- 33.Gordon SPF, Douglas PS, Come PC, Manning WJ. Two-dimensional and Doppler echocardiographic determinants of the natural history of mitral valve narrowing in patients with rheumatic mitral stenosis: implications for follow-up. J Am Col Cardiol. 1992;19:968–973. doi: 10.1016/0735-1097(92)90280-z. [DOI] [PubMed] [Google Scholar]
- 34.Steiner I, Kašparová P, Kohout A, Dominik J. Bone formation in cardiac valves: a histopathological study of 128 cases. Virchows Arch. 2007;450:653–657. doi: 10.1007/s00428-007-0430-7. [DOI] [PubMed] [Google Scholar]
- 35.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I547–I552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 36.Osman L, Chester AH, Amrani M, Yacoub MH, Smolenski RT. A novel role of extracellular nucleotides in valve calcification: a potential target for atorvastatin. Circulation. 2006;114:I566–I572. doi: 10.1161/CIRCULATIONAHA.105.001214. [DOI] [PubMed] [Google Scholar]
- 37.Arishiro K, Hoshiga M, Negoro N, Jin D, Takai S, Miyazaki M, Ishihara T, Hanafusa T. Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J Am Coll Cardiol. 2007;49:1482–1489. doi: 10.1016/j.jacc.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 38.Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005;91:806–810. doi: 10.1136/hrt.2003.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makkena B, Salti H, Subramaniam M, Thennapan S, Bonow RH, Caira F, Bonow RO, Spelsberg TC, Rajamannan NM. Atorvastatin decreases cellular proliferation and bone matrix expression in the hypercholesterolemic mitral valve. J Am Coll Cardiol. 2005;45:631–633. doi: 10.1016/j.jacc.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–2665. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation. 2005;111:2280–2281. doi: 10.1161/01.CIR.0000167560.93138.E7. [DOI] [PubMed] [Google Scholar]
- 42.Davignon J, Leiter LA. Ongoing clinical trial of the pleiotropic effects of statins. Vasc Health Risk Manag. 2005;I:29–40. doi: 10.2147/vhrm.1.1.29.58937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effects of hydroxymethyl glutaryl coenzyme A reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–1935. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 44.Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112:I229–I234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Yehuda O, DeMaria AN. Statins in rheumatic heart disease: taking the bite out? J Am Coll Cardol. 2009;53:1880–1882. doi: 10.1016/j.jacc.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 48.Rajamannan NM, Antonini-Canterin F, Moura L, Zamorano JL, Rosenhek RA, Best PJM, Lloyd MA, Rocha-Gonçalves F, Chandra S, Alfieri O, Lancellotti P, Tornos P, Baliga RR, Wang A, Bashore T, Ramakrishnan S, Spargias K, Shuvy M, Beeri R, Lotan C, Al Suwaidi J, Bahl V, Pierard LA, Maurer G, Nicolosi GL, Rahimtoola SH, Chopra HK, Pandian NG. Medical therapy for rheumatic heart disease: is it time to be proactive rather than reactive? Indian Heart J. 2009;61:14–23. [PMC free article] [PubMed] [Google Scholar]