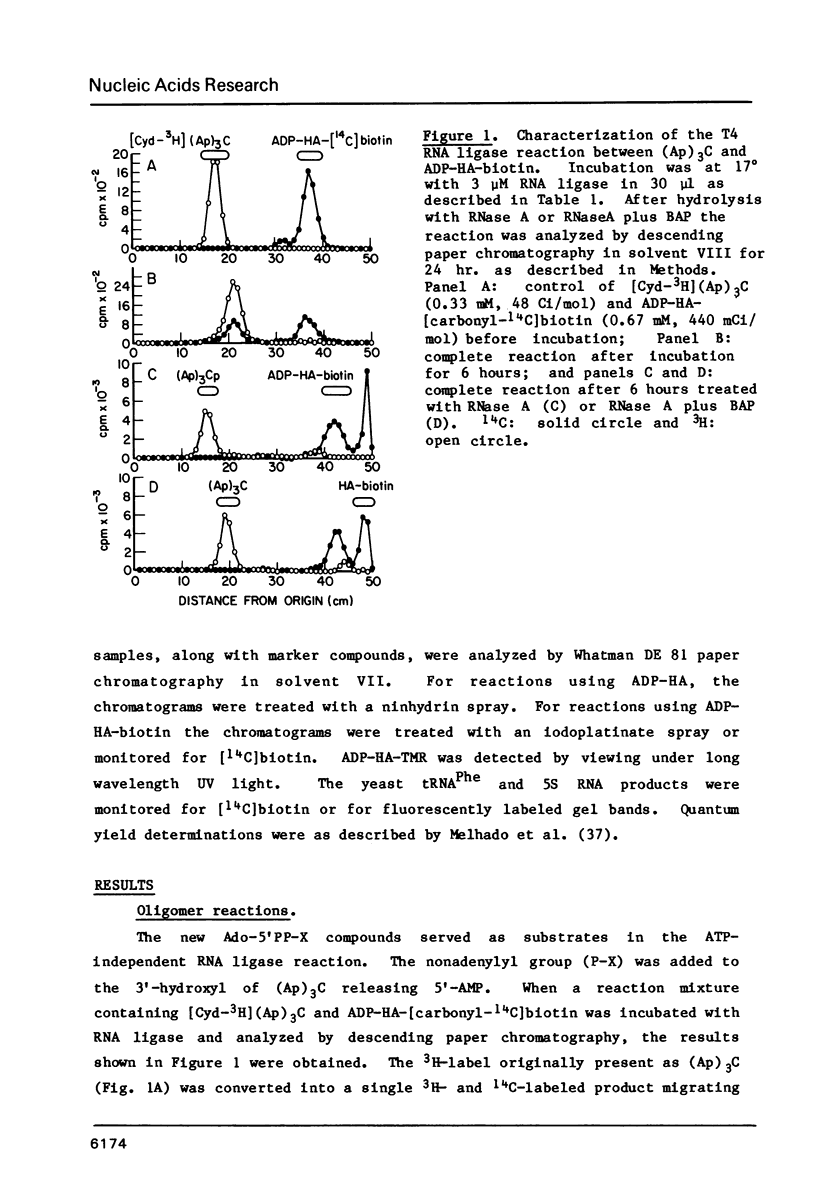
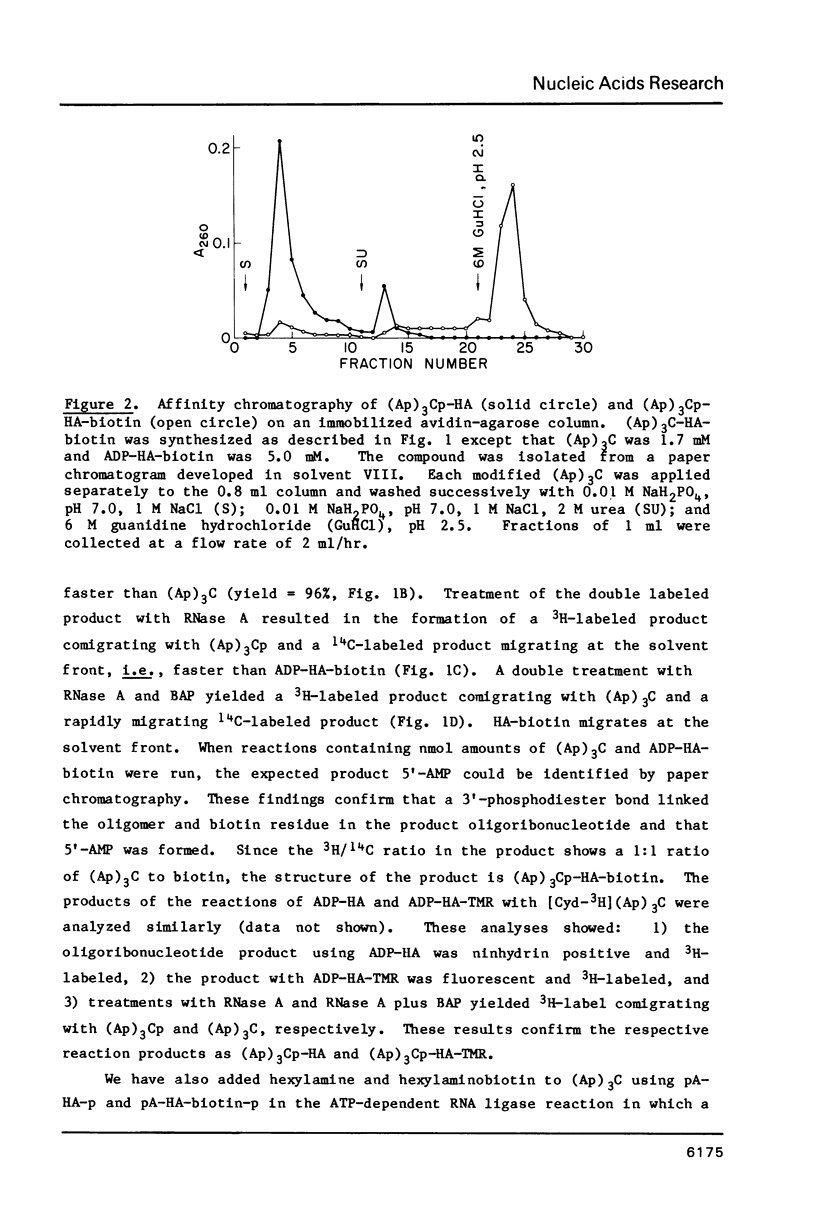
Abstract
Biotin, fluorescein, and tetramethylrhodamine derivatives of P1-(6-aminohex-1-yl)-P2-(5'-adenosine) pyrophosphate were synthesized and used as substrates with T4 RNA ligase. In the absence of ATP, the non-adenylyl portion of these substrates is transferred to the 3'-hydroxyl of an RNA acceptor to form a phosphodiester bond and the AMP portion is released. E. coli and D. melanogaster 5S RNA, yeast tRNAPhe, (Ap)3C, and (Ap)3A serve as acceptors with yields of products varying from 50 to 100%. Biotin-labeled oligonucleotides are bound selectively and quantitatively to avidin-agarose and may be eluted with 6 M guanidine hydrochloride, pH 2.5. Fluorescein and tetramethylrhodamine-labeled oligonucleotides are highly fluorescent and show no quenching due to attachment to the acceptor. The diverse structures of the appended groups and of the chain lengths and compositions of the acceptor RNAs show that T4 RNA ligase will be a useful modification reagent for the addition of various functional groups to the 3'-terminus of RNA molecules.
Full text
PDF








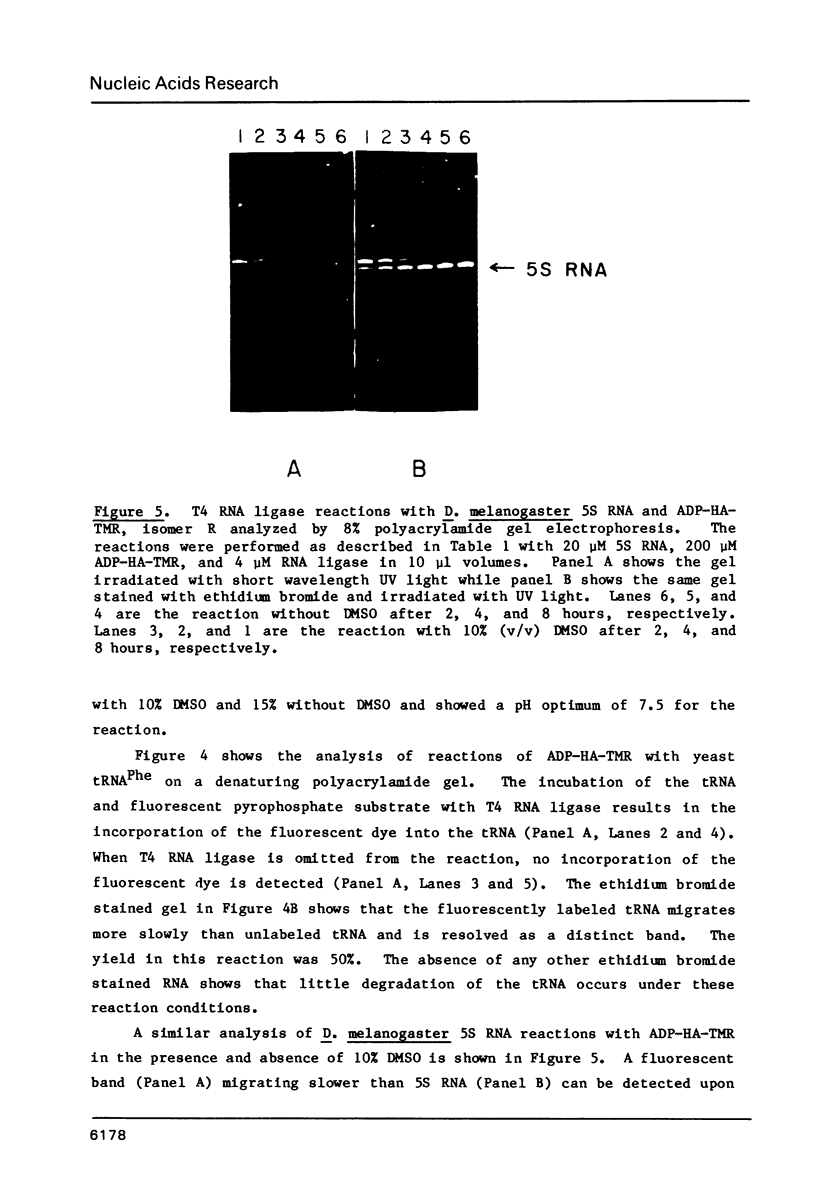
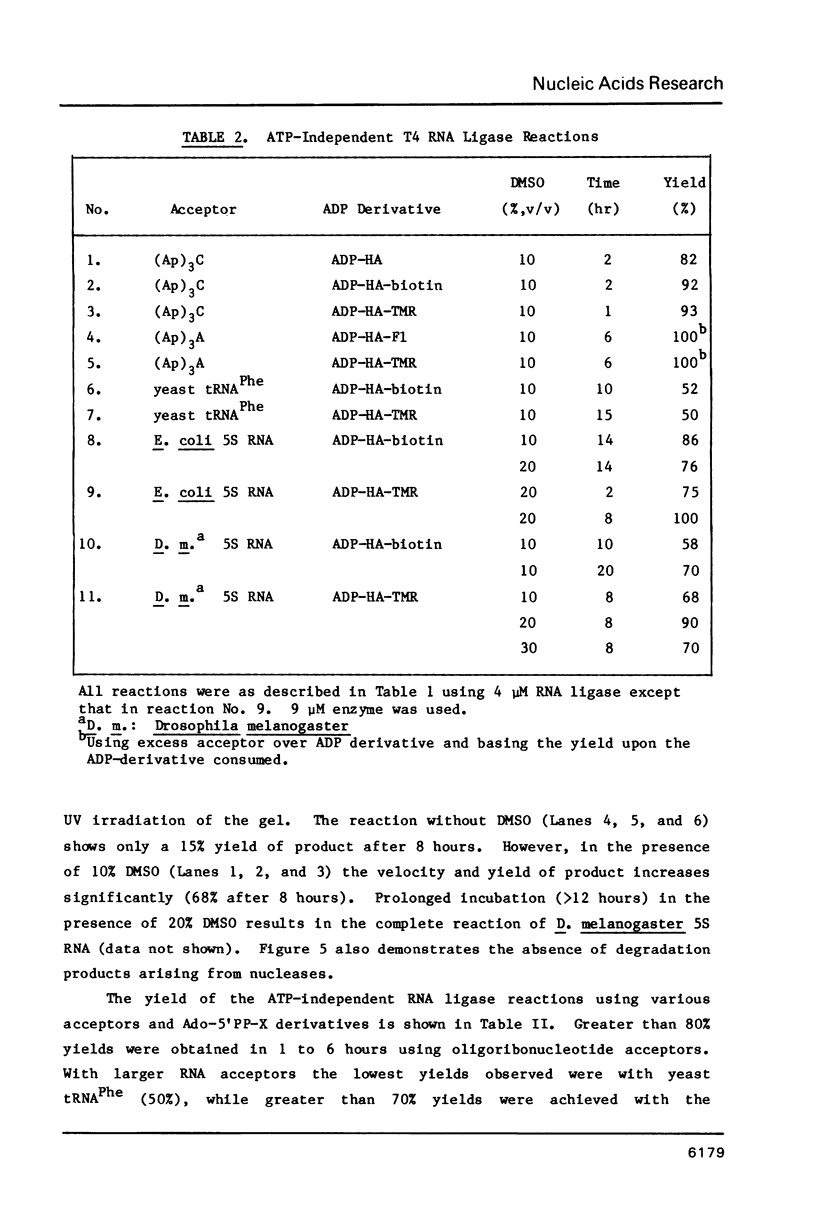





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Barker R., Trayer I. P., Hill R. L. Nucleoside phosphates attached to agarose. Methods Enzymol. 1974;34:479–491. doi: 10.1016/s0076-6879(74)34059-1. [DOI] [PubMed] [Google Scholar]
- Bauman J. G., Wiegant J., van Duijn P. Cytochemical hybridization with fluorochrome-labeled RNA. I. Development of a method using nucleic acids bound to agarose beads as a model. J Histochem Cytochem. 1981 Feb;29(2):227–237. doi: 10.1177/29.2.6166653. [DOI] [PubMed] [Google Scholar]
- Bayer E., Wilchek M. Insolubilized biotin for the purification of avidin. Methods Enzymol. 1974;34:265–267. doi: 10.1016/s0076-6879(74)34023-2. [DOI] [PubMed] [Google Scholar]
- Brennan C. A., Manthey A. E., Gumport R. I. Using T4 RNA ligase with DNA substrates. Methods Enzymol. 1983;100:38–52. doi: 10.1016/0076-6879(83)00044-0. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Angerer L. M., Yen P. H., Hershey N. D., Davidson N. Electron microscopic visualization of tRNA genes with ferritin-avidin: biotin labels. Nucleic Acids Res. 1978 Feb;5(2):363–384. doi: 10.1093/nar/5.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron V., Soltis D., Uhlenbeck O. C. Polynucleotide kinase from a T4 mutant which lacks the 3' phosphatase activity. Nucleic Acids Res. 1978 Mar;5(3):825–833. doi: 10.1093/nar/5.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- England T. E., Gumport R. I., Uhlenbeck O. C. Dinucleoside pyrophosphate are substrates for T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4839–4842. doi: 10.1073/pnas.74.11.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. Enzymatic oligoribonucleotide synthesis with T4 RNA ligase. Biochemistry. 1978 May 30;17(11):2069–2076. doi: 10.1021/bi00604a008. [DOI] [PubMed] [Google Scholar]
- Gumport R. I., Hinton D. M., Pyle V. S., Richardson R. W. T4 RNA ligase as a nucleic acid synthesis and modification reagent. Nucleic Acids Symp Ser. 1980;(7):167–171. [PubMed] [Google Scholar]
- Hecht S. M., Alford B. L., Kuroda Y., Kitano S. "Chemical aminoacylation" of tRNA's. J Biol Chem. 1978 Jul 10;253(13):4517–4520. [PubMed] [Google Scholar]
- Hinton D. M., Baez J. A., Gumport R. I. T4 RNA Ligase joins 2'-deoxyribonucleoside 3',5'-bisphosphates to oligodeoxyribonucleotides. Biochemistry. 1978 Nov 28;17(24):5091–5097. doi: 10.1021/bi00617a004. [DOI] [PubMed] [Google Scholar]
- Hinton D. M., Gumport R. I. The synthesis of oligodeoxyribonucleotides using RNA ligase. Nucleic Acids Res. 1979 Sep 25;7(2):453–464. doi: 10.1093/nar/7.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasiewicz M. L., Schoenberg D. R., Mueller G. C. Selective retrieval of biotin-labeled cells using immobilized avidin. Exp Cell Res. 1976 Jun;100(1):213–217. doi: 10.1016/0014-4827(76)90344-x. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelicke A., Sprinzl M., von der Haar F., Khwaja T. A., Cramer F. Structural studies on phenylalanine transfer ribonucleic acid from yeast with the spectroscopic label formycin. Eur J Biochem. 1974 Apr 16;43(3):617–625. doi: 10.1111/j.1432-1033.1974.tb03449.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Miyake T., Nagao K., Uemura H., Nishikawa S., Sugiura M., Ikehara M. Elongation of oligonucleotides in the 3'-direction with activated mononucleotides and their analogs using RNA ligase. Nucleic Acids Res. 1980 Feb 11;8(3):601–610. doi: 10.1093/nar/8.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Uemura H., Doi T., Miyake T., Nishikawa S., Ikehara M. A new method for 3'-labelling of polyribonucleotides by phosphorylation with RNA ligase and its application to the 3'-modification for joining reactions. Nucleic Acids Res. 1979 Feb;6(2):443–454. doi: 10.1093/nar/6.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profy A. T., Lo K. M., Usher D. A. Synthesis of 2'(3')-O-DL-alanyl hexainosinic acid using T4 RNA ligase: suppression of the enzymic reverse transfer reaction by alkaline phosphatase. Nucleic Acids Res. 1983 Mar 11;11(5):1617–1632. doi: 10.1093/nar/11.5.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunell A. A photographic method to quantitate DNA in gel electrophoresis. Methods Enzymol. 1980;65(1):353–358. doi: 10.1016/s0076-6879(80)65045-9. [DOI] [PubMed] [Google Scholar]
- Schreiber J. P., Hsiung N., Cantor C. R. Fluorescence studies of the accessibility of the 3' ends of the ribosomal RNAs in Escherichia coli ribosomes and subunits. Nucleic Acids Res. 1979 Jan;6(1):181–193. doi: 10.1093/nar/6.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler-Meilicke M., Stöffler G., Odom O. W., Zinn A., Kramer G., Hardesty B. Localization of 3' ends of 5S and 23S rRNAs in reconstituted subunits of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5538–5542. doi: 10.1073/pnas.78.9.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Elson D. A photoaffinity labelling study of the messenger RNA-binding region of Escherichia coli ribosomes. Nucleic Acids Res. 1978 Sep;5(9):3389–3407. doi: 10.1093/nar/5.9.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R., Small D. P., Bottomley R. C. Preparation of adenosine nucleotide derivatives suitable for affinity chromatography. Biochem J. 1974 Jun;139(3):609–623. doi: 10.1042/bj1390609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Cameron V. Equimolar addition of oligoribonucleotides with T4 RNA ligase. Nucleic Acids Res. 1977 Jan;4(1):85–98. doi: 10.1093/nar/4.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Söll D. Covalent attachment of fluorescent groups to the 5'-end of transfer RNA. Arch Biochem Biophys. 1973 Mar;155(1):70–81. doi: 10.1016/s0003-9861(73)80010-4. [DOI] [PubMed] [Google Scholar]