Abstract
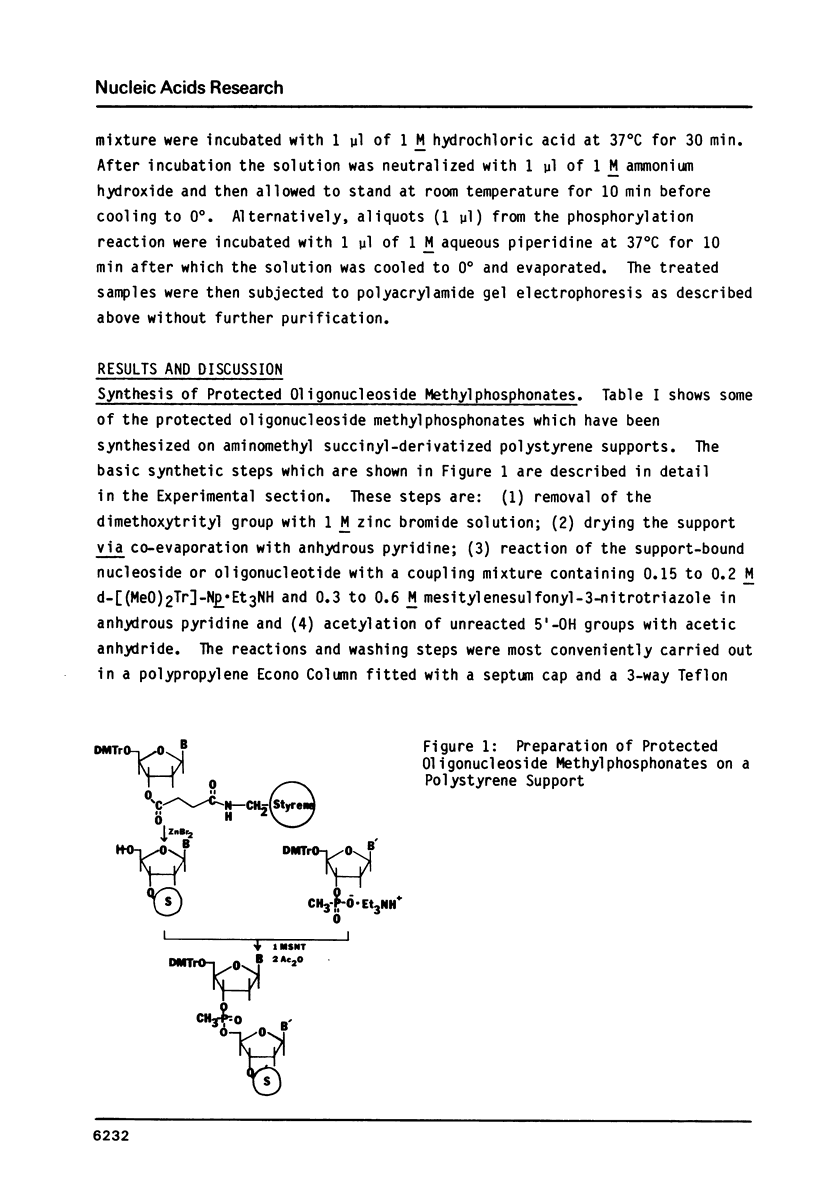
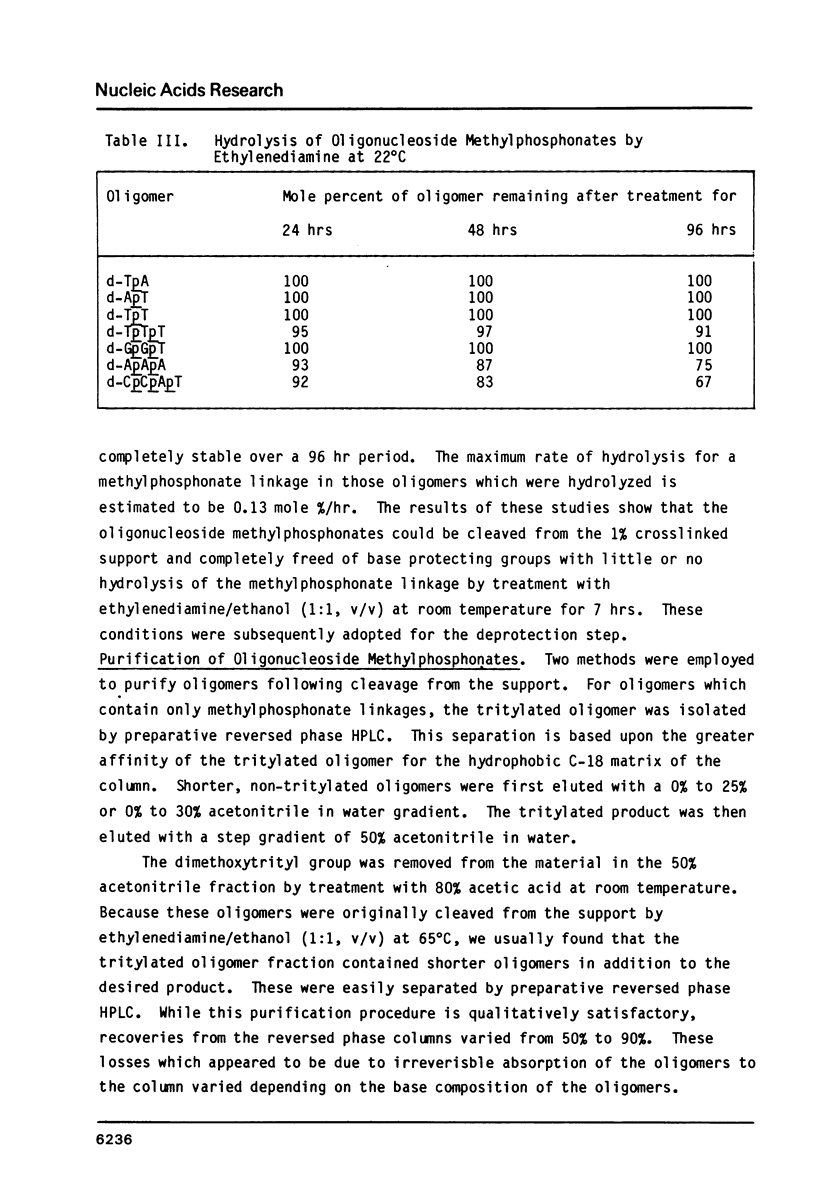
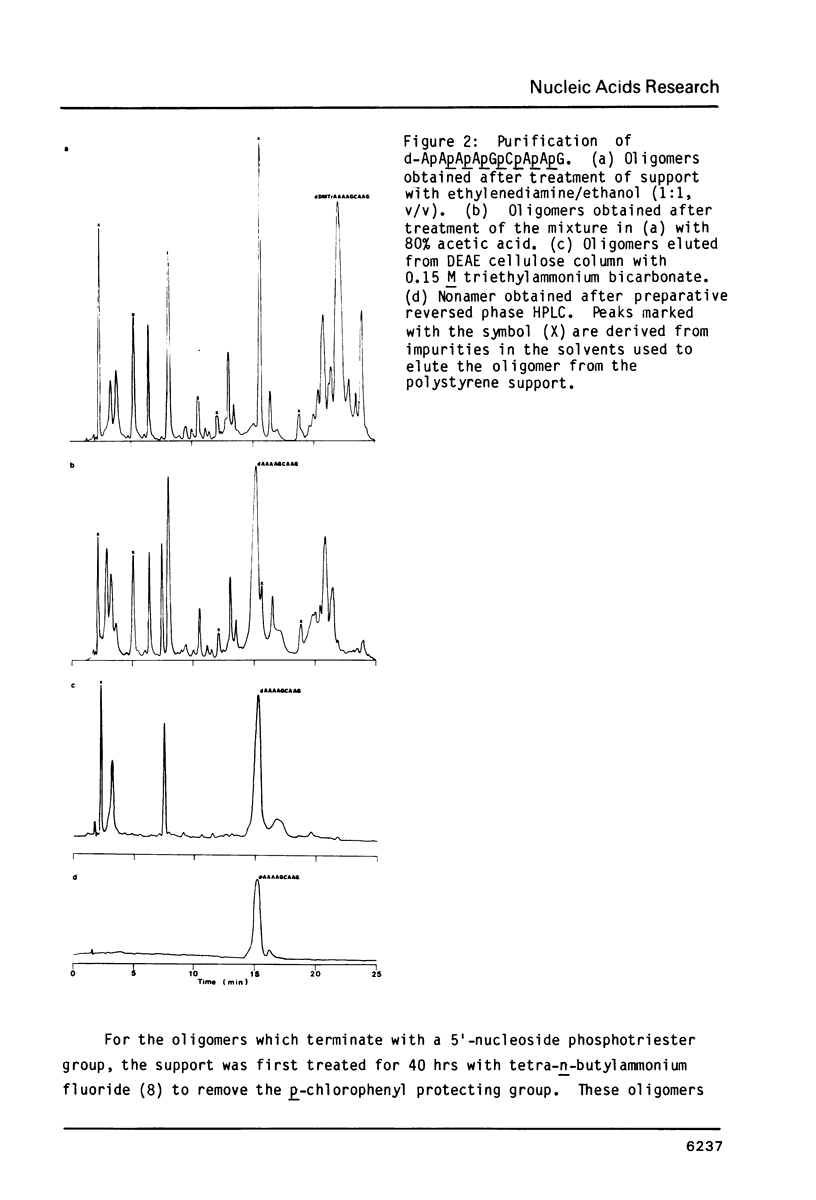
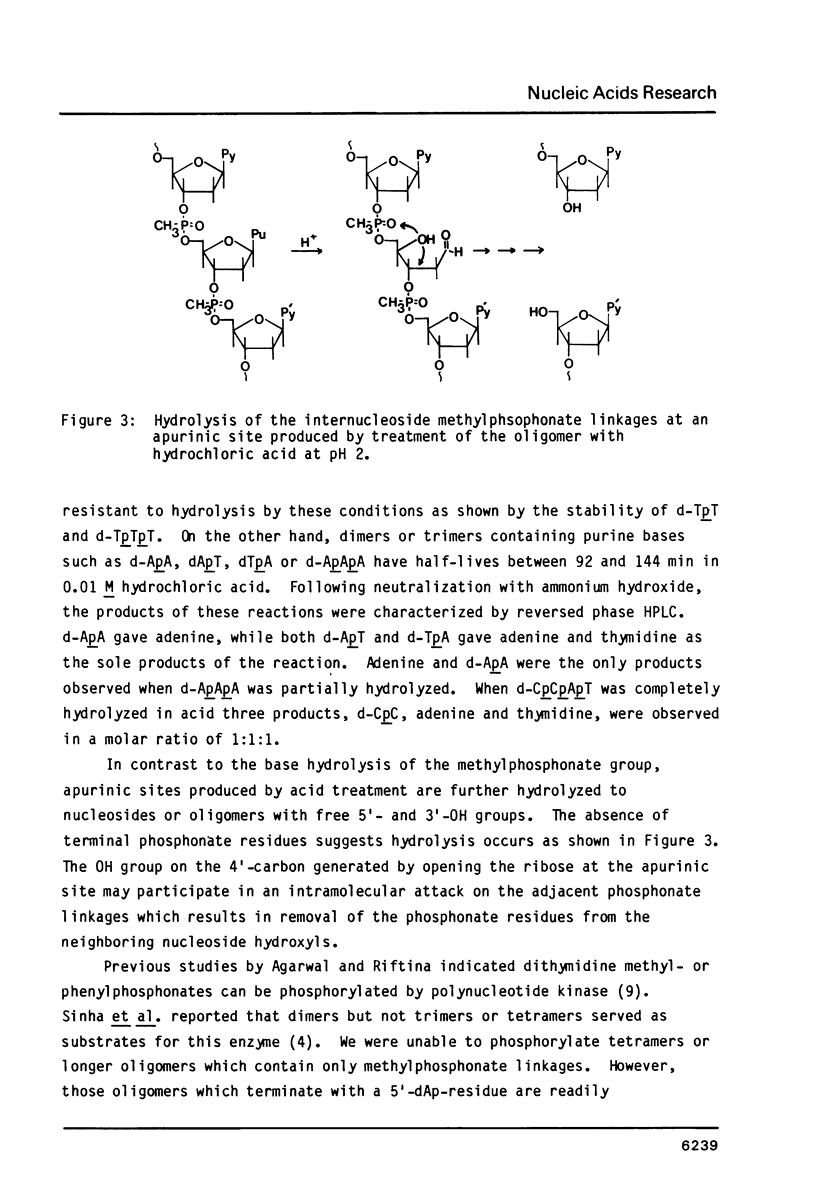
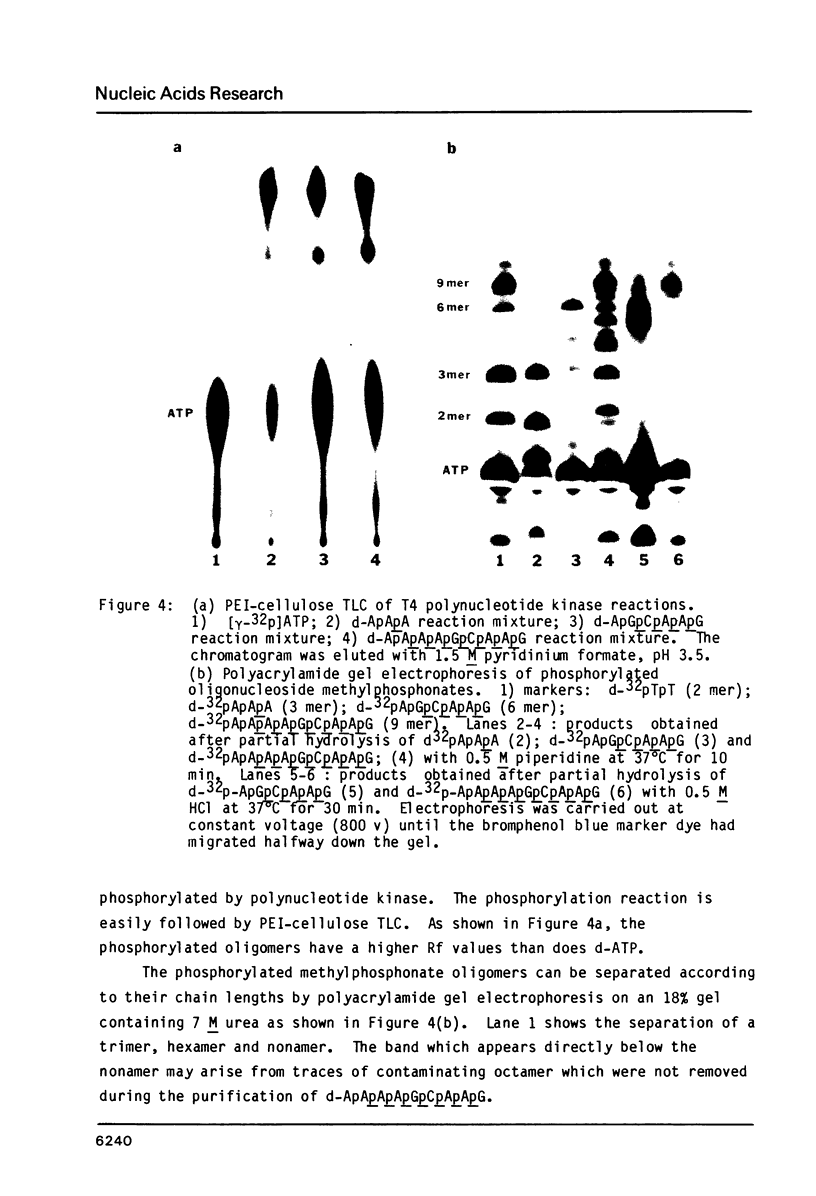
An efficient procedure is described for synthesizing deoxyribonucleoside methylphosphonates on polystyrene polymer supports which involves condensing 5'-dimethoxytrityldeoxynucleoside 3'-methylphosphonates. The oligomers are removed from the support and the base protecting groups hydrolyzed by treatment with ethylenediamine in ethanol, which avoids hydrolysis of the methylphosphonate linkages. Two types of oligomers were synthesized: those containing only methylphosphonate linkages, d-Np(Np)nN, and those which terminate with a 5' nucleotide residue, dNp (Np)nN. The latter oligomers can be phosphorylated by polynucleotide kinase, and are separated by polyacrylamide gel electrophoresis according to their chain length. Piperdine randomly cleaves the oligomer methylphosphonate linkages and generates a series of shorter oligomers whose number corresponds to the length of the original oligomer. Apurinic sites introduced by acid treatment spontaneously hydrolyze to give oligomers which terminate with free 3' and 5' OH groups. These reactions may be used to characterize the oligomers.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ito H., Ike Y., Ikuta S., Itakura K. Solid phase synthesis of polynucleotides. VI. Further studies on polystyrene copolymers for the solid support. Nucleic Acids Res. 1982 Mar 11;10(5):1755–1769. doi: 10.1093/nar/10.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman K., McParland K., Miller P., Ts'o P. O. Selective inhibition of Escherichia coli protein synthesis and growth by nonionic oligonucleotides complementary to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1537–1541. doi: 10.1073/pnas.78.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Cheng D. M., Dreon N., Jayaraman K., Kan L. S., Leutzinger E. E., Pulford S. M., Ts'o P. O. Preparation of a decadeoxyribonucleotide helix for studies by nuclear magnetic resonance. Biochemistry. 1980 Sep 30;19(20):4688–4698. doi: 10.1021/bi00561a023. [DOI] [PubMed] [Google Scholar]
- Miller P. S., McParland K. B., Jayaraman K., Ts'o P. O. Biochemical and biological effects of nonionic nucleic acid methylphosphonates. Biochemistry. 1981 Mar 31;20(7):1874–1880. doi: 10.1021/bi00510a024. [DOI] [PubMed] [Google Scholar]