Abstract
OBJECTIVE
We investigated the effect of early-phase insulin secretion on the incidence of type 2 diabetes in individuals with impaired glucose tolerance (IGT) participating in the Finnish Diabetes Prevention Study (DPS). We examined how a lifestyle intervention affected early-phase insulin secretion (ratio of total insulin area under the curve [AUC] and total glucose AUC [AIGR] from 0 to 30 min) during a 4-year follow-up intervention trial and whether AIGR0–30 response was modified by insulin sensitivity (IS) and obesity.
RESEARCH DESIGN AND METHODS
A total of 443 participants with IGT originally randomized to a lifestyle intervention or control group were studied. IS and AIGR0–30 were estimated from an oral tolerance glucose test administered annually during the 4-year follow-up trial and were related to the risk of diabetes onset over a 6-year follow-up.
RESULTS
Lifestyle intervention resulted in higher IS (P = 0.02) and lower unadjusted AIGR0–30 (P = 0.08) during the 4-year follow-up. A higher IS and a lower BMI during the follow-up were associated with a lower unadjusted AIGR0–30 during the follow-up, independently of study group (P < 0.001). A greater increase in IS on the median cutoff point of a 0.69 increase was associated with higher IS-adjusted AIGR0–30 during the follow-up (P = 0.002). In multivariate models, IS and IS-adjusted AIGR0–30 were both inversely associated with diabetes incidence (P < 0.001). Participants who progressed to type 2 diabetes were more obese and had lower IS and Matsuda IS index-AIGR0–30 than nonprogressors.
CONCLUSIONS
Our results indicate that the reduction in the risk of developing type 2 diabetes after lifestyle intervention is related to the improvement of IS along with weight loss. Improved IS may also have beneficial effects on preservation of β-cell function.
Genetic and environmental factors both contribute to the development of type 2 diabetes (1). Current evidence indicates that an underlying defect in insulin secretion in the presence of insulin resistance leads to the development of diabetes. Impaired glucose tolerance (IGT) is already characterized by impaired first-phase insulin secretion, a determinant for further progression to diabetes (2–5).
Lifestyle changes involving healthy diet, moderate weight loss, and increased physical activity reduce the risk of diabetes (6–8). The extent to which this is due to reduced insulin resistance or improved insulin secretion is not known. Improvements in insulin secretion and insulin sensitivity after 1 year of lifestyle intervention were associated with lower diabetes risk in the Diabetes Prevention Program (DPP) study during a follow-up of 3.2 years (6). In a substudy of the Finnish Diabetes Prevention Study (DPS) in persons with IGT who did not progress to diabetes, insulin secretion measured during a frequently sampled intravenous glucose tolerance test (IVGTT) remained stable for years (9). However, data from long-term intervention trials on the mechanisms that may result in improvement of glucose metabolism and prevention of diabetes associated with healthy lifestyle changes are scarce.
Therefore, we investigated the effect of surrogate indices of early-phase insulin secretion and insulin sensitivity from an oral glucose tolerance test (OGTT) on diabetes incidence in individuals participating in the Finnish DPS. We also evaluated whether insulin secretion response in the OGTT was modified by insulin sensitivity and obesity, and how lifestyle intervention may affect the β-cell function.
RESEARCH DESIGN AND METHODS
Design of the DPS
The DPS was a randomized, controlled, multicenter study in Finland between the years 1993 and 2000 (ClinicalTrials.govNCT00518167) in which 522 individuals with IGT were randomized into an intervention or control group in five centers. The study design and methods of the DPS have been reported in detail elsewhere (8,10). The study protocol was approved by the ethics committee of the National Public Health Institute of Helsinki, Finland, and all of the study participants gave written informed consent.
The main inclusion criteria were BMI >25 kg/m2, age 40–64 years, and IGT based on the mean values of two OGTTs according to the World Health Organization 1985 criteria. Random allocation to one of the two study groups was stratified according to the center, sex, and the 2-h glucose at the screening OGTT. At baseline and at annual visits, individuals completed a medical history questionnaire and underwent a physical examination that included anthropometric measurements and an OGTT.
For this study, analyses were limited to those 443 participants who had at least one measurement of glucose and insulin at 30 min during the 4-year follow-up trial because samples for 30-min insulin and glucose were not collected at baseline (Supplementary Fig. 1). The median length of the study was 4 years (range 1–6). During this 4-year follow-up period, all participants were undergoing the randomized intervention. Participants who developed diabetes discontinued the study (n = 12, n = 14, and n = 19 at years 2, 3, and 4, respectively), and the measurements from their previous annual visits before diagnosis were used for the analyses.
Program for the intervention group
The intervention program has been described previously (8,10). Briefly, the individuals in the intervention group received individually tailored dietary advice aiming at reducing weight and the intake of total and saturated fat and increasing the intake of dietary fiber. Individuals in the intervention group also received individual guidance to increase their level of physical activity. The most intensive period of intervention was during the first year of the study, when the participants showed improvement in main lifestyle indicators (e.g., body weight and glucose and lipid concentrations). The control group received general advice on the benefits of weight reduction, physical activity, and a healthy diet.
Glucose and insulin homeostasis
During 1993 to 1996, a baseline 2-h OGTT was performed (75 g glucose load). In the OGTT performed during follow-up visits starting from the middle of 1996, samples were also taken for 30-min insulin and glucose and for 60-min glucose measurements (Supplementary Fig. 1).
Laboratory determinations
Glucose levels were measured locally by standard methods, and the measurements were standardized by the central laboratory in Helsinki (8). Serum insulin was determined with a radioimmunoassay (Pharmacia, Uppsala, Sweden) that shows 41% cross-reactivity with proinsulin.
Calculations
Glucose area under the curve (AUC) during the OGTT was calculated using the trapezoidal method. As surrogate indices of the first/early-phase insulin secretion and of peripheral insulin sensitivity, the ratio of total insulin AUC and total glucose AUC during the 0–30 min OGTT (AIGR0–30) and the Matsuda index of insulin sensitivity (Matsuda ISI: 10,000/square root of [fasting glucose × fasting insulin × (arithmetic mean of glucose × arithmetic mean insulin both during an OGTT at 0, 30, and 120 min)]) were calculated according to published equations (11,12). These indices were chosen based on a previous large population study conducted by our local collaborators in which Matsuda ISI and AIGR0–30 were considered the best indices of insulin sensitivity and secretion (11). In addition, a frequently sampled IVGTT was performed in a subsample of the DPS, and the insulin sensitivity index (SI) and acute-phase insulin response (AIR) were calculated by the MINMOD Millennium software (9). AIR and SI measured at year 4 were used for validation of the AIGR0–30 (n = 53) and Matsuda ISI (n = 47). The Pearson correlation coefficient (r) of AIGR0–30 with AIR was 0.67 (P < 0.001). Matsuda ISI had a correlation of r = 0.73 (P < 0.001) with SI. We also calculated Matsuda ISI according to the latest publication (13) to assess insulin sensitivity at baseline without using the 30-min glucose and insulin values. Its correlation coefficient was also significant (r = 0.74, P < 0.001) versus SI in IVGTT. Both Matsuda ISIs were strongly correlated at all 4-year follow-up visits (r between 0.94 and 0.95, P < 0.001 for all).
Because of the known nonlinear relationship between insulin secretion and insulin sensitivity, we also adjusted, by regression analysis, the log of AIGR0–30 by the log of the Matsuda ISI at each year of the study to obtain insulin secretion independently of insulin sensitivity (3,4,14,15). We then transformed the adjusted AIGR0–30 back into untransformed values by taking the antilog (14) to obtain Matsuda ISI-adjusted AIGR0–30 (ISI-adjusted AIGR0–30). ISI-adjusted AIGR0–30 was strongly correlated with SI-adjusted AIR (rs = 0.63, P < 0.001). The outcome variables were averaged from the available yearly measurements of each participant during the 4-year follow-up (16). For those individuals developing diabetes during the first 4 years, the measurements at the time of conversion to diabetes and thereafter were excluded.
Statistical analyses
Variables with a non-normal right-skewed distribution were log transformed for statistical analyses and given as geometric mean with a 95% CI. To test the association of the BMI and Matsuda ISI with insulin secretion at the 4-year follow-up and to test whether there was a group effect, univariate general linear models adjusted for age and sex were constructed. The association of insulin secretion and insulin sensitivity during the first 4 years of follow-up with the risk of incident diabetes during a mean of 6 years of follow-up was assessed by Cox proportional hazards regression models adjusted for age, sex, and study group (intervention or control). For those participants who developed diabetes during the first 4 years, the measurements taken at the year of diagnosis were excluded. Univariate general linear modeling was used for comparisons between progressor and nonprogressors to type 2 diabetes during a mean of 6 years of follow-up for the main variables measured during the 4-year follow-up. A value of P < 0.05 was considered statistically significant. Analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL).
RESULTS
Participants’ characteristics
Individuals in the intervention and control groups included in this analysis had similar body weight, BMI, age, sex distribution, and glucose and insulin levels at baseline (Supplementary Table 1). The decrease in fasting and 2-h glucose, body weight, and BMI were already larger in the intervention group than in the control group after the first year of intervention (Supplementary Table 2) and were in line with the results reported for the entire DPS population (n = 522) (8,10). Insulin sensitivity tended to increase more in the intervention than in the control group (P = 0.07). When divided by the median cutoff point for the change in Matsuda ISI (0.69), the proportion of participants in the higher (mean change, 2.13) and in the lower (−0.41) ranges was significantly different between the intervention (58% and 42%, respectively) and the control (41% and 59%, respectively) groups (P = 0.002).
Fasting and postload glucose and insulin values and BMI during the 4-year follow-up
Overall, belonging to the intervention group was associated with a better glucose and insulin profile during the follow-up (Supplementary Table 3). Participants in the intervention group had lower body weight and BMI during the follow-up than those in the control group, but the difference between the groups was not significant (P = 0.18 and P = 0.25, respectively).
Effect of lifestyle intervention on insulin secretion and insulin sensitivity during the 4-year follow-up
During the follow-up, although the average value (geometric mean [95% CI]) for the unadjusted AIGR0–30 tended to be lower in the intervention than in the control group (30.9 [28.7–33.0] vs. 33.0 [30.8–35.3], P = 0.08), the average Matsuda ISI was significantly higher in the intervention group than in the control group (4.24 [3.99–4.49] vs. 3.85 [3.60–4.11], P = 0.02). However, ISI-adjusted AIGR0–30 was not different between the study groups (29.2 [27.9–30.5] vs. 29.5 [28.1–30.9], P = 0.82).
Associations of BMI and insulin sensitivity with early-phase insulin secretion during the 4-year follow-up in the combined intervention and control groups
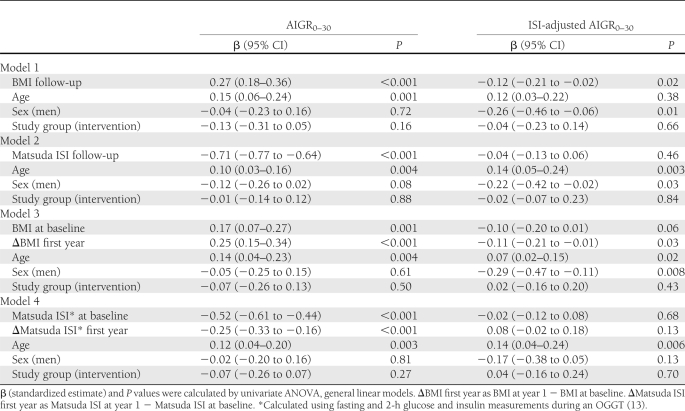
In different models, BMI was directly associated (β = 0.27; P < 0.001) and Matsuda ISI was strongly (β = −0.71; P < 0.001) inversely associated with AIGR0–30 during the follow-up, and both attenuated the group effect on AIGR0–30 (Table 1). BMI was inversely associated with ISI-adjusted AIGR0–30 during this period (β = −0.12; P = 0.03). In models where Matsuda ISI was placed as the dependent variable, higher BMI was inversely associated with Matsuda ISI (β = −0.49; P < 0.001) independently of study group, and BMI also attenuated the effect of lifestyle intervention on Matsuda ISI (P = 0.07 for the group effect).
Table 1.
Associations of BMI and Matsuda ISI with AIGR0–30 and with ISI-adjusted AIGR0–30 during the 4-year follow-up in participants from the Finnish DPS
Impact of changes in BMI and insulin sensitivity after the first year of intervention on the insulin secretion during the subsequent 4-year follow-up
A greater decrease in BMI or a greater increase in Matsuda ISI after the first year of intervention (the most intensive period of the study) was associated with lower AIGR0–30 at the subsequent follow-up (P < 0.001) and again attenuated the group effect on AIGR0–30 during the follow-up (Table 1). A greater decrease in BMI during this period had a weaker effect on ISI-adjusted AIGR0–30 (P = 0.03) than in AIGR0–30. In similar models, being in the group of participants with a greater increase in Matsuda ISI based on the median cutoff (0.69) was associated with higher ISI-adjusted AIGR0–30 during the follow-up than those with less improvement in Matsuda ISI (β = 0.29, P = 0.002).
Early-phase insulin secretion and glucose response in the combined intervention and control groups during the 4-year follow-up
During the follow-up, Matsuda ISI and ISI-adjusted AIGR0–30 were independently associated with lower concentrations of fasting glucose, 2-h glucose, and glucose AUC0–120 in models adjusted for age, sex, and study group (P < 0.001, Supplementary Table 4).
Early-phase insulin secretion and insulin sensitivity and development of diabetes
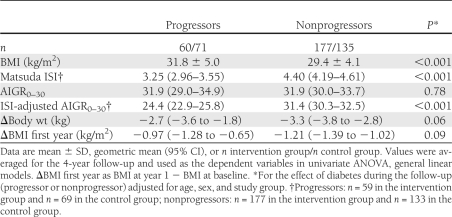
During a mean follow-up of 6 years (range 1–10), the number of diabetes cases was 71 in the control and 60 in the intervention groups. After taking into account the effects of the study group, age, and sex, participants who progressed from IGT to diabetes compared with those who did not progress to diabetes during the mean 6-year follow-up had lower Matsuda ISI and ISI-adjusted AIGR0–30 and higher BMI on average during the 4-year follow-up study, but AIGR0–30 was identical in both groups (Table 2). Participants who developed diabetes also reduced less their body weight and BMI during the first year of the trial (Table 2).
Table 2.
BMI, AIGR0–30, Matsuda ISI, and ISI-adjusted AIGR0–30 during the 4-year follow-up and body weight and BMI change during the first year of the intervention study in participants of the Finnish DPS who did or did not progress from IGT to type 2 diabetes after a mean follow-up of 6 years
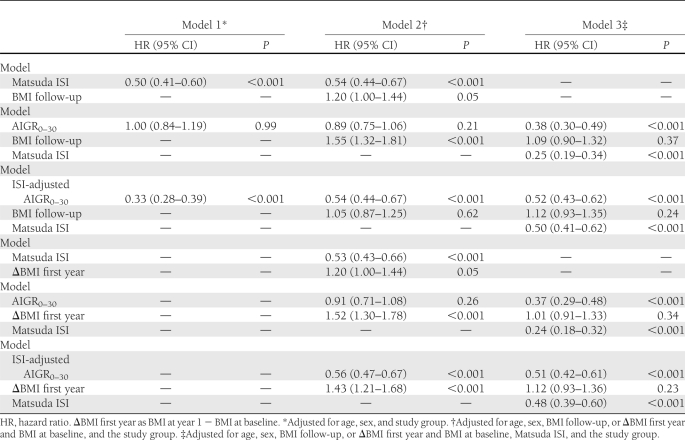
Cox regression analyses showed that average Matsuda ISI and ISI-adjusted AIGR0–30 during the follow-up were inversely associated with the incidence of diabetes during the mean 6-year follow-up (Table 3). Although average AIGR 0–30 during the 4-year follow-up alone did not predict diabetes, when Matsuda ISI was included in the model, lower insulin secretion and insulin sensitivity both predicted progression to diabetes. Higher BMI during the follow-up or lower BMI change during the first year of the lifestyle intervention study alone or independently of AIGR0–30 predicted diabetes. This association was no longer significant when Matsuda ISI or ISI-adjusted AIGR0–30 was included in the model.
Table 3.
Hazard ratios for the risk of developing type 2 diabetes during a mean of 6-year follow-up according to a 1-SD change in AIGR0–30, Matsuda ISI, and ISI-adjusted AIGR0–30 at the 4-year follow-up in participants from the Finnish DPS
Of note, in similar models assessing the regression from IGT to normal glucose tolerance (NGT) during a mean 6-year follow-up (Supplementary Table 5), a 1-SD increase in ISI-adjusted AIGR0–30 was associated with a 1.3 increase in the regression from IGT to NGT (P = 0.002). AIGR0–30 and Matsuda ISI alone were not associated with the regression from IGT to NGT (P = 0.29 and P = 0.14, respectively), but when they were entered in the same model, a 1-SD increase in AIGR0–30 and Matsuda ISI was associated with a 1.5 increase in the regression from IGT to NGT (P = 0.001), independently also of BMI.
CONCLUSIONS
Nonpharmacologic lifestyle intervention in high-risk individuals prevented or at least postponed the onset of type 2 diabetes in the Finnish DPS. Participants in the intervention group showed greater reductions in fasting and postchallenge glucose levels and in body weight compared with those in the control group (8,10).
In the current study, we demonstrated that higher insulin sensitivity estimated as Matsuda ISI and higher insulin sensitivity-adjusted insulin secretion (ISI-adjusted AIGR0–30) during the 4-year follow-up study were both associated with lower diabetes incidence during a mean follow-up of 6 years. Regression to NGT was more strongly associated with higher ISI-adjusted AIGR0–30 than insulin sensitivity. Individuals who developed type 2 diabetes reduced less BMI during the first year of the intervention, were more obese, and had lower Matsuda ISI and ISI-adjusted AIGR0–30 values during the study than the individuals who did not develop type 2 diabetes.
We found that AIGR0–30 per se did not predict diabetes during the mean 6-year follow-up, unless Matsuda ISI contribution was taken into consideration in the models. In contrast, low Matsuda ISI and ISI-adjusted AIGR0–30 were both associated with a higher risk of developing diabetes, independently of BMI. Previous prospective studies have shown that insulin resistance and impaired early-phase insulin secretion predicted the conversion from IGT to diabetes (2–5,17,18). Moreover, in insulin-resistant states, improvement of insulin resistance protected from diabetes and was associated with lower endogenous insulin requirement and preservation of β-cell function (19). Therefore, some improvement in insulin secretion adjusted for insulin sensitivity seems to be possible in the IGT phase, resulting in better β-cell function and possibly lowering the risk of developing diabetes. Altogether, these findings clearly demonstrate the importance of insulin secretion in the development of type 2 diabetes and that the effect of insulin sensitivity on insulin secretion needs to be taken into account due to their complex interaction.
In line with published observations, we observed that insulin sensitivity, which was higher in the intervention than in the control group during the 4-year follow-up, was inversely associated with the risk of developing type 2 diabetes (3,5,17) and also inversely associated with BMI during this same interval. In previous findings from a subsample of individuals participating in the DPS, the improvement in insulin sensitivity between baseline and the fourth year of the study was strongly correlated with the magnitude of weight loss (9). These findings were independent of randomization group, and the associations found could be partly explained, for example, by a possible decrease in nonesterified fatty acid release that accompanies loss of body mass (20,21).
Although at first glance paradoxical, the lower AIGR0–30 observed in the lifestyle intervention group can be explained by the improvement in Matsuda ISI, which remained higher during the follow-up in this group, and reductions in body weight and BMI in the intervention arm during the first year of the study compared with the control group. Our study, along with other studies but with shorter follow-up duration, shows that a lower BMI and better insulin sensitivity are associated with a decrease in the demand of insulin in obese, insulin-resistant, and glucose-intolerant individuals (22–24).
We could not find any difference between the intervention and control groups concerning ISI-adjusted AIGR0–30 that could indicate a direct beneficial effect of lifestyle intervention on β-cell function. Excluding data from the year diabetes was diagnosed may have underestimated the effect of lifestyle intervention. Nevertheless, higher ISI-adjusted AIGR0–30 during the follow-up was associated with a lower risk of developing diabetes and a higher chance of regressing from IGT to NGT. Moreover, participants who had higher increase in Matsuda ISI during the most intensive period of the intervention trial, and who mostly belonged to the intervention group by study design, had higher ISI-adjusted AIGR0–30 during the follow-up. Therefore, weight loss achieved with the lifestyle intervention may be a mediating factor on preserving β-cell function by improving insulin sensitivity and perhaps by avoiding lipotoxicity resulting for example, from ectopic fat accumulation, higher release of nonesterified fatty acid, and activation of inflammatory cascades, all factors related to obesity (20,21). Overall, our findings emphasize the importance of targeting reduction in BMI to improve insulin sensitivity and preserve insulin secretion capacity to prevent or postpone the conversion from IGT to diabetes.
A major limitation of our study was that we could not estimate early-phase insulin secretion and sensitivity from OGTT at baseline before the intervention began. Nonetheless, the main clinical and metabolic features related to insulin and glucose metabolism, such as BMI, age, sex proportion, glucose, and insulin parameters did not differ between the intervention and control groups. Therefore, the differences between groups reported in this study are likely to be a reflection of the intervention itself. Of note, diabetes incidence was similarly lower in the original intervention group as in the population included in this study. Insulin sensitivity and insulin secretion were not measured by the hyperinsulinemic-euglycemic clamp or the IVGTT. We did not use the oral glucose minimal model indices for estimating insulin secretion (25), which also includes the incretin response, because we did not measure C-peptide. However, we used an IVGTT for validation of the indices used in the current study in a subsample of the DPS. In DPS, early-phase insulin secretion (AIR) has high repeatability, which, as the insulin sensitivity-adjusted AIR, was also associated with diabetes risk (16). In a similar population, AIGR0–30 and Matsuda ISI were considered the best indices of insulin sensitivity and secretion (11). The averaged values of measures of obesity, insulin sensitivity, and insulin secretion during the 4-year follow-up were used for the analyses. The main changes in lifestyle and body weight occurred during the first year of the study and were largely maintained thereafter. To decrease the variability of the crude measurements and increase the statistical power, we therefore averaged values (16).
Strengths of the current study include the well-characterized and homogenous study population (obese individuals with IGT) and yearly measurements during a relative long period of follow-up of a large and carefully conducted lifestyle intervention study population.
Our results indicate that the reduction in the risk of developing type 2 diabetes after lifestyle intervention is related to the improvement of insulin sensitivity. The weight loss achieved with the lifestyle intervention, which also improved insulin sensitivity, might have beneficial effects on better preservation of β-cell function. Because weight loss results from joint effects of changes in diet and physical activity and is possibly modified by genetic factors, the interplay between their effects on insulin secretion and risk of developing type 2 diabetes requires further and more detailed investigation.
Supplementary Material
Acknowledgments
This study was supported by the Academy of Finland (117844, 40758, 211497, and 118590 to M.U.; 38387 and 46558 to J.T.; 206310 and 73566 to S.K.-K.; 128315 and 129330 to J.L.; and 131593 to V.D.F.d.M.), the Juho Vainio Foundation (to J.L.), the Novo Nordisk Foundation (to J.L.), the EVO fund of Kuopio University Hospital (5106, 5168, and 5254 to M.U.), and the Ministry of Education of Finland, The Finnish Diabetes Research Foundation, Sigrid Juselius Foundation, North-Savo Finnish Cultural Foundation, and Nordic Centre of Excellence (NCoE) on “Systems biology in controlled dietary interventions and cohort studies” (SYSDIET; project number 070014).
No potential conflicts of interest relevant to this article were reported.
V.D.F.d.M. analyzed the data, wrote, reviewed, and edited the manuscript, and is the guarantor of the work. J.L. and S.K.-K. participated in data collection, contributed to discussion, and reviewed and edited the manuscript. J.E. and M.L. contributed to discussion and reviewed and edited the manuscript. P.I.-P. and J.S. participated in data collection and reviewed and edited the manuscript. J.T. is a principal investigator of the DPS, contributed to discussion, and reviewed and edited the manuscript. M.U. is a principal investigator of the DPS, participated in data collection, contributed to discussion, and reviewed and edited the manuscript.
This study was presented as an oral communication at the 29th International Symposium on Diabetes and Nutrition, Rome, Italy, 30 June–2 July 2011.
Footnotes
Clinical trial reg. no. NCT00518167, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1272/-/DC1.
References
- 1.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 3.Kitabchi AE, Temprosa M, Knowler WC, et al. ; Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Festa A, Williams K, D’Agostino R, Jr, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 2006;55:1114–1120 [DOI] [PubMed] [Google Scholar]
- 5.Snehalatha C, Mary S, Selvam S, et al. Changes in insulin secretion and insulin sensitivity in relation to the glycemic outcomes in subjects with impaired glucose tolerance in the Indian Diabetes Prevention Programme-1 (IDPP-1). Diabetes Care 2009;32:1796–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 8.Tuomilehto J, Lindström J, Eriksson JG, et al. ; Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 9.Uusitupa M, Lindi V, Louheranta A, Salopuro T, Lindström J, Tuomilehto J. Long-term improvement in insulin sensitivity by changing lifestyles of persons with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes 2003;52:2532–2538 [DOI] [PubMed] [Google Scholar]
- 10.Lindström J, Louheranta A, Mannelin M, et al. ; Finnish Diabetes Prevention Study Group The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 11.Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009;58:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care 2010;33:e93. [DOI] [PubMed] [Google Scholar]
- 14.Laaksonen DE, Niskanen L, Mykkänen H, et al. Long-term repeatability of measures of early insulin secretion derived from an intravenous glucose tolerance test and conversion from impaired glucose tolerance to diabetes. Ann Med 2008;40:303–311 [DOI] [PubMed] [Google Scholar]
- 15.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 16.Laaksonen DE, Lindström J, Lakka TA, et al. ; Finnish Diabetes Prevention Study Physical activity in the prevention of type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes 2005;54:158–165 [DOI] [PubMed] [Google Scholar]
- 17.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin secretion and increased insulin resistance are independently related to the 7-year risk of NIDDM in Mexican-Americans. Diabetes 1995;44:1386–1391 [DOI] [PubMed] [Google Scholar]
- 18.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 2001;24:89–94 [DOI] [PubMed] [Google Scholar]
- 19.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 20.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 21.Li N, Frigerio F, Maechler P. The sensitivity of pancreatic beta-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem Soc Trans 2008;36:930–934 [DOI] [PubMed] [Google Scholar]
- 22.Laakso M, Uusitupa M, Takala J, Majander H, Reijonen T, Penttilä I. Effects of hypocaloric diet and insulin therapy on metabolic control and mechanisms of hyperglycemia in obese non-insulin-dependent diabetic subjects. Metabolism 1988;37:1092–1100 [DOI] [PubMed] [Google Scholar]
- 23.Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab 2004;89:2704–2710 [DOI] [PubMed] [Google Scholar]
- 24.Carr DB, Utzschneider KM, Boyko EJ, et al. A reduced-fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra-abdominal fat and improves insulin sensitivity but not beta-cell function. Diabetes 2005;54:340–347 [DOI] [PubMed] [Google Scholar]
- 25.Manco M, Panunzi S, Macfarlane DP, et al. ; Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) Consortium One-hour plasma glucose identifies insulin resistance and beta-cell dysfunction in individuals with normal glucose tolerance: cross-sectional data from the Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) study. Diabetes Care 2010;33:2090–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.