Abstract
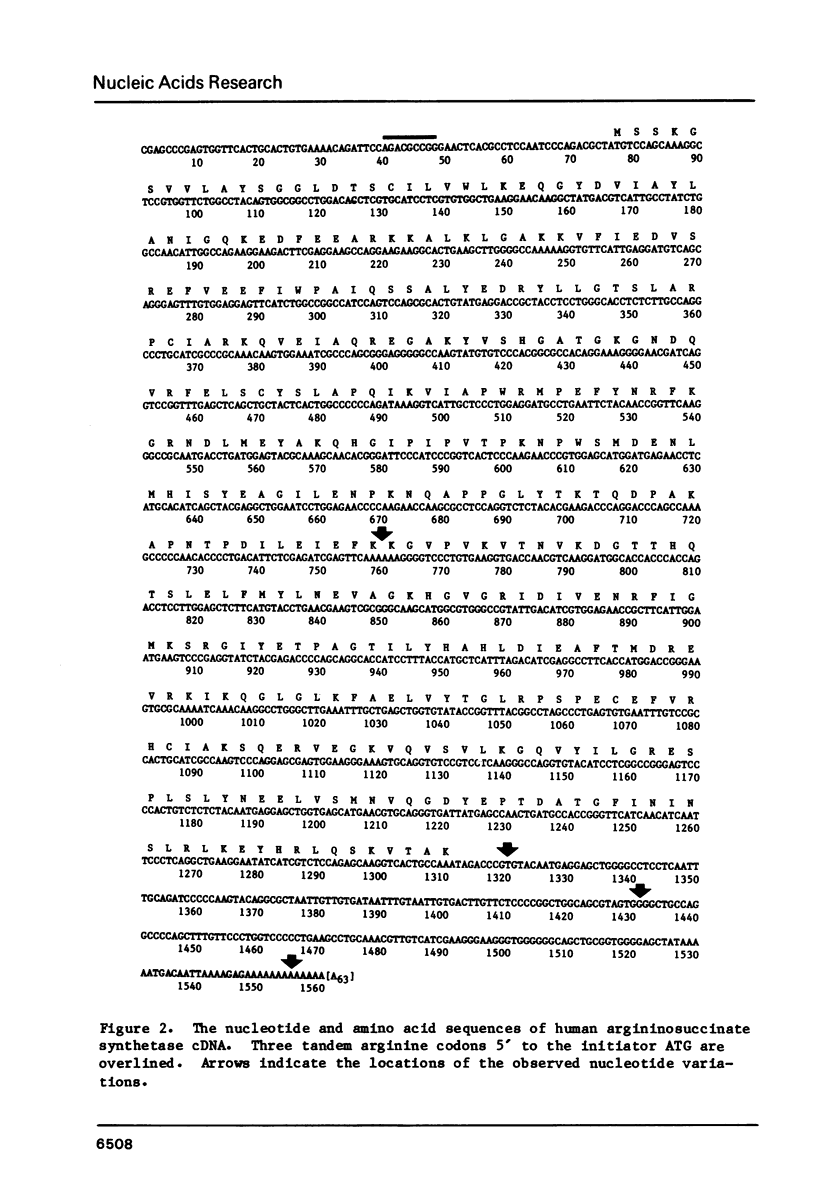
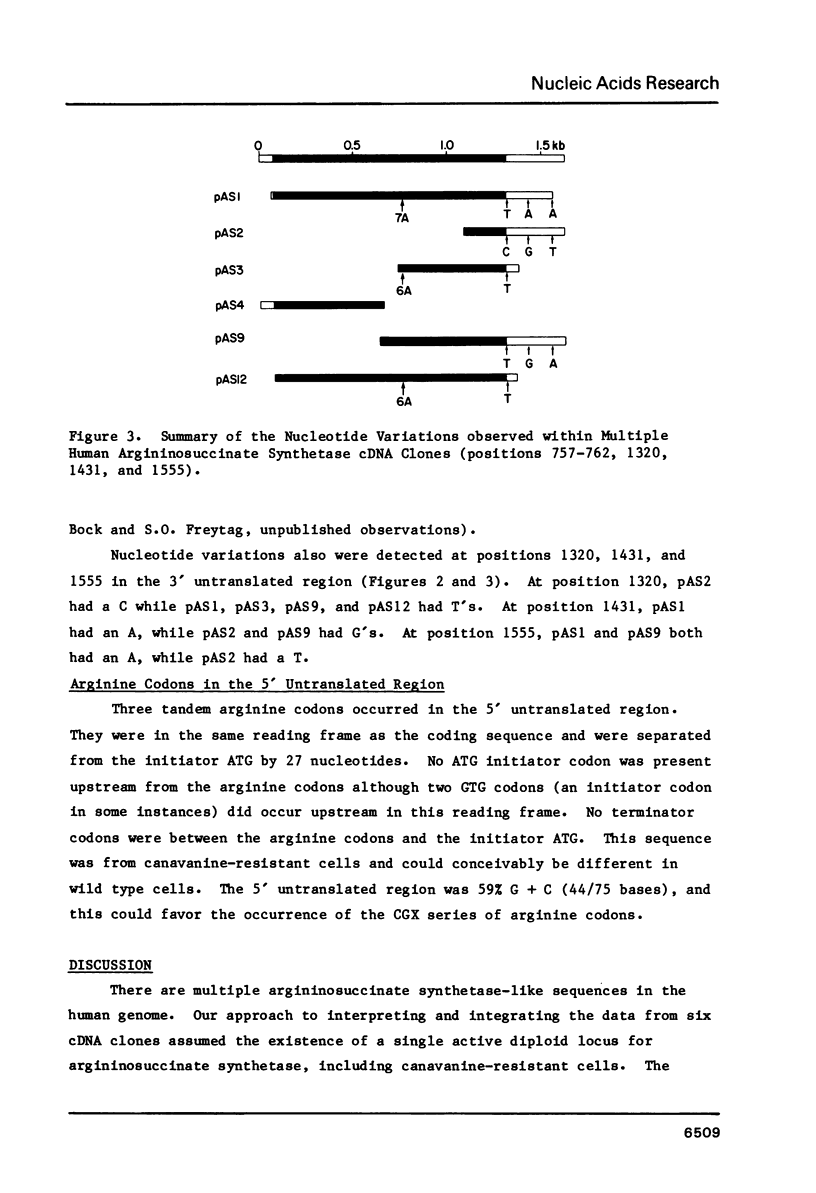
The nucleotide sequence for human argininosuccinate synthetase cDNA was determined by analysis of six clones isolated from a single experiment. The sequence covered 1623 nucleotides including 76 bases of poly(A) and contained a 1236 nucleotide open reading frame encoding a protein of 46,434 daltons. In one cDNA isolate, a cloning artifact or perhaps RNA polymerase error involving addition of an A in a region of six A's within the coding sequence was documented. Single base variations in the 3' untranslated region were examined in detail since detection of DNA polymorphisms in the cDNAs could imply over-expression of both alleles at the active locus in canavanine-resistant cells, i.e. a trans-acting mechanism for enzyme overproduction. However, the sequence from five cDNAs suggested some single base artifacts, and DNA polymorphism remains uncertain. The occurrence of three tandem arginine codons in the 5' untranslated region of the cDNA suggested the possibility of an interaction of arginyl-tRNA with mRNA to regulate RNA processing or half-life as a mechanism for arginine-mediated repression.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Hay N., Skolnik-David H., Aloni Y. Attenuation in the control of SV40 gene expression. Cell. 1982 May;29(1):183–193. doi: 10.1016/0092-8674(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Hudson L. D., Erbe R. W., Jacoby L. B. Expression of the human argininosuccinate synthetase gene in hamster transferents. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4234–4238. doi: 10.1073/pnas.77.7.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby L. B. Canavanine-resistant variants of human lymphoblasts. Somatic Cell Genet. 1978 Mar;4(2):221–231. doi: 10.1007/BF01538986. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Calvo J. M. Alternative secondary structures of leader RNAs and the regulation of the trp, phe, his, thr, and leu operons. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6186–6190. doi: 10.1073/pnas.76.12.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Ratner S. Argininosuccinate synthetase of bovine liver: chemical and physical properties. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5197–5199. doi: 10.1073/pnas.79.17.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T. ENZYMES OF ARGININE METABOLISM IN MAMMALIAN CELL CULTURE. I. REPRESSION OF ARGININOSUCCINATE SYNTHETASE AND ARGININOSUCCINASE. J Biol Chem. 1964 Jan;239:136–145. [PubMed] [Google Scholar]
- Su T. S., Beaudet A. L., O'Brien W. E. Abnormal mRNA for argininosuccinate synthetase in citrullinaemia. Nature. 1983 Feb 10;301(5900):533–534. doi: 10.1038/301533a0. [DOI] [PubMed] [Google Scholar]
- Su T. S., Beaudet A. L., O'Brien W. E. Increased translatable messenger ribonucleic acid for argininosuccinate synthetase in canavanine-resistant human cells. Biochemistry. 1981 May 12;20(10):2956–2960. doi: 10.1021/bi00513a037. [DOI] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., Beaudet A. L., O'Brien W. E. Molecular analysis of argininosuccinate synthetase deficiency in human fibroblasts. J Clin Invest. 1982 Dec;70(6):1334–1339. doi: 10.1172/JCI110736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., O'Brien W. E., Beaudet A. L. Cloning of cDNA for argininosuccinate synthetase mRNA and study of enzyme overproduction in a human cell line. J Biol Chem. 1981 Nov 25;256(22):11826–11831. [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]


