Abstract
The plasticity region of Helicobacter pylori is a large chromosomal segment including isolate-specific open reading frames with characteristics of pathogenicity islands. It remains unclear whether genes in the plasticity region play a role in the pathogenesis of gastric mucosal inflammation and gastroduodenal disease. Our aim was to assess the role of selected genes in the plasticity region in relation to risk of H. pylori-related disease and the severity of gastric mucosal damage. We used PCR to study the relation of disease outcome and mucosal damage with four genes in the H. pylori plasticity region (jhp0940, jhp0945, jhp0947, and jhp0949) from isolates obtained from both Western (n = 296) and East Asian (n = 217) patients. The prevalence of jhp0945, jhp0947, and jhp0949 differed significantly between Western and East Asian isolates. In Western isolates, the presence of jhp0945 was significantly associated with gastric ulcer, duodenal ulcer, and gastric cancer (odds ratios [95% confidence intervals]: 2.27 [1.04 to 4.98], 1.86 [1.03 to 3.34], and 1.92 [1.03 to 3.56], respectively). jhp0940-positive Western isolates were significantly associated with absence of gastric ulcer or duodenal ulcer (0.21 [0.05 to 0.94] and 0.31 [0.12 to 0.78], respectively). No significant difference was observed between inflammatory cell infiltration or atrophy and the presence or absence of plasticity region genes. The outcome of H. pylori infections varies widely geographically. These data suggest a possible role for difference in the prevalence of plasticity region genes in the geographic variation in H. pylori-related diseases.
INTRODUCTION
Helicobacter pylori infection of human gastric mucosa results in chronic gastritis which may eventuate in gastric ulcer or gastric cancer. Gastric cancer is generally thought to arise through a series of steps in which progressive mucosal damage ultimately results in mucosal atrophy (28). The presence of H. pylori-induced gastric mucosal atrophy varies among different populations, and this variation in outcome has been associated with differences in H. pylori virulence factors, host genetics, and/or environment factors (7, 25, 26, 29, 34).
A number of important H. pylori virulence factors have been identified, such as the cag pathogenicity island (PAI) and VacA (3). Putative H. pylori virulence genes have been classified into three general types: (i) isolate-specific genes (e.g., cagA, dupA, and plasticity region genes), (ii) the virulent gene with different genotypic gene activities (e.g., vacA, cagA repeat region, and hopQ), and (iii) the phase-variable genes (e.g., oipA and babA) (31). The presence, absence, and activity of these different virulence factors have been related to the severity of gastric mucosal injury and inflammation and thus to the risk of development of different gastroduodenal diseases (3, 4, 26, 29, 32–34).
By definition, strain-specific genes are present in only some H. pylori isolates. The cag PAI, which encodes a type IV secretion system that delivers the CagA protein into host gastric epithelial cells (5, 27), is the best-studied virulence factor. However, many strain-specific genes lie outside the cag PAI; up to 50% of the strain-specific genes transferred from other species are located in the plasticity region (20). For example, H. pylori strain J99, isolated in the United States in 1994 from patients with a duodenal ulcer, has 48 genes in the plasticity region (i.e., jhp0914 to jhp0961) (1, 2). Each H. pylori isolate shows variability in gene content such that different components of the plasticity region may be responsible for differences in virulence potential (1, 2). The prevalence of genes jhp0914 to jhp0961 ranges from 13% to 100%, with only three genes being present within more than 90% of H. pylori (i.e., jhp0915 [100%], jhp0955 [94%], and jhp0957 [94%]). Genes found in less than 20% include jhp0914 (17%), jhp0925 (19%), jhp0926 (19%), and jhp0959 (13%) (11, 21, 31). It remains unknown whether strain-specific genes or combinations of strain-specific genes influence the severity of gastric mucosal inflammation and the risk of different H. pylori-related diseases. In addition, the biological functions of most open reading frames (ORFs) in the plasticity region remain unknown. Recently jhp0940, jhp0945, jhp0947, and jhp0949 in H. pylori obtained in the West have been reported to be associated with an increased risk of gastroduodenal disease and an increase in inflammatory cytokines (e.g., interleukin 8 [IL-8], IL-12, and tumor necrosis factor alpha [TNF-α]) (6, 15–17, 19, 20, 23). In previous reports, however, the role of selected genes in the plasticity region in relation to the risk of H. pylori-related disease and the severity of gastric mucosal damage was controversial and unclear. Moreover, there were no reports comparing H. pylori obtained from different geographic populations. Here, we used more than 500 Western and East Asian H. pylori isolates to examine the prevalence of jhp0940, jhp0945, jhp0947, and jhp0949 and their relation to mucosal inflammation and H. pylori-related disease.
MATERIALS AND METHODS
Patients.
H. pylori isolates were obtained from infected patients in Western countries (United States [n = 202] and Colombia [n = 94]) and East Asian countries (South Korea [n = 105] and Japan [n = 112]) in the isolate collection period of 2002 to 2005 (Table 1). The study population consisted of patients with gastric ulcers (GU) (n = 81), duodenal ulcers (DU) (n = 112), gastric cancer (n = 123), or gastritis alone (n = 197). Gastritis was endoscopically and pathologically defined as H. pylori gastritis without peptic ulcers or gastric cancer. Informed consent had been obtained from all patients under protocols approved by the local hospital's ethics committee.
Table 1.
Demographic characteristics of H. pylori-positive Western and East Asian patients enrolled in this study
| Characteristic | Results for isolates from: |
P valueb | ||||
|---|---|---|---|---|---|---|
| United States | Colombia | South Korea | Japan | Total | ||
| No. of patients | 202 | 94 | 105 | 112 | 513 | |
| Age (mean ± SD) | 50.8 ± 1.0 | 56.8 ± 1.5 | 47.6 ± 1.2 | 58.9 ± 1.3 | 52.5 ± 0.7 | <0.01 |
| Sex (% male) | 84 | 59 | 76 | 61 | 71 | <0.01 |
| Diseasea | ||||||
| Gastritis | 106 (52.5%) | 32 (34.0%) | 32 (30.5%) | 27 (24.1%) | 197 (38.4%) | <0.01 |
| GU | 32 (15.8%) | 0 (0.0%) | 19 (18.1%) | 30 (26.8%) | 81 (15.8%) | |
| DU | 43 (21.3%) | 25 (26.6%) | 18 (17.1%) | 26 (23.2%) | 112 (21.8%) | |
| GC | 21 (10.4%) | 37 (39.4%) | 36 (34.3%) | 29 (25.9%) | 123 (24.0%) | |
Abbreviations: GU, gastric ulcer; DU, duodenal ulcer; GC, gastric cancer.
P values were from analyzed demographic data from four different countries by one-way ANOVA for age and Fisher's exact test for sex and disease.
jhp0940, jhp0945, jhp0947, and jhp0949 status by PCR.
H. pylori taken from each patient by endoscopy was grown at 37°C on brain heart infusion (BHI) (BD, Sparks, MD) plates containing 7% horse blood (Cocalico Biological, Inc., Reamstown, PA) in a microaerobic condition. Chromosomal DNA of H. pylori was isolated from confluent plate cultures expanded from a single colony using the QIAamp tissue kit (Qiagen Inc., Santa Clarita, CA) according to the manufacturer's instructions. Genotypes of vacA signal (s), middle (m), and intermediate (i) regions and cagA status were determined by PCR as described previously (3, 13, 32). We also checked the status of H. pylori DNA by checking H. pylori-specific 16S rRNA as controls by PCR. The jhp0940, jhp0945, jhp0947, and jhp0949 status were determined by PCR methods using primer pairs shown in Table 2 (6, 16). The PCR conditions were 95°C for 5 min, then 35 cycles of 95°C for 30 s, 48°C for 45 s, and 72°C for 30 s, and finally 72°C for 5 min. When a PCR using two different primer pairs was positive, the status of jhp0949 was determined as positive.
Table 2.
Primer pairs of four kinds of plasticity regions
| Gene and primer type | Sequence | Reference |
|---|---|---|
| jhp0940 | ||
| Forward | 5′-GAA ATG TCC TAT ACC AAT GG | 16 |
| Reverse | 5′-CCT AAG TAG TGC ATC AAG G | |
| jhp0945 | ||
| Forward | 5′-ACT CCA GCC AGT ATT GTA AA-3′ | 6 |
| Reverse | 5′-TTC TTG CGA GTT AGG ATT GG-3′ | |
| jhp0947 | ||
| Forward | 5′-GAT AAT CCT ACG CAG AAC G-3′ | 16 |
| Reverse | 5′-GCT AAA GTC ATT TGG CTG TC-3′ | |
| jhp0949 | ||
| Forward | 5′-ATA GGA GTG GGT GCT TAC TT-3′ | 6 |
| Reverse | 5′-AGC AAC AAC AAA GGC ATG TA-3′ | |
| Forward | 5′-TTC AAA AAG TCC CCG AAA TG-3′ | This study |
| Reverse | 5′-GGA TGT CCT GGC ATG TCT CT-3′ |
When the gene status was considered negative by PCR, we further confirmed the results using dot blot analyses. A total of 500 ng of total DNA was added to 100 μl of TE buffer and mixed with 100 μl of a denaturing buffer (0.5 M NaOH, 1.5 M NaCl). The denatured DNA was transferred to a Hybond-N+ membrane (Amersham, GE Healthcare) by means of a Bio-Dot microfiltration apparatus (Bio-Rad Laboratories, Inc.). DNA of H. pylori J99 and human DNA were also transferred to the membrane as positive and negative controls, respectively. The membranes were hybridized at 42°C overnight in plastic bags containing ECL Gold hybridization buffer supplemented with 5% (wt/vol) blocking agent and 0.5 M NaCl. The membranes were washed three times in primary washing buffer (0.5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] [pH 7.0], 0.4% sodium dodecyl sulfate) at room temperature for 15 min and three times in secondary washing buffer (2× SSC) at room temperature for 15 min. Finally, the membranes were exposed to Hyperfilm ECL film (Amersham, GE Healthcare). If PCR results yielded negative results but dot blot testing showed a positive blot, we considered the samples positive.
Histology.
Gastric biopsy specimens had been taken from the antrum (pyloric gland area) and the body (fundic gland area). The biopsy specimens were fixed in 10% buffered formalin and embedded in paraffin, and the paraffin was cut into sequential 4-μm sections. The H. pylori density, activity of gastritis (neutrophil infiltration), and atrophy were graded from 0 (absent/normal) to 5 (maximal) as previously described (8). The score is presented as the mean scores of biopsy samples from the corpus and antral areas.
Data analysis.
Statistical differences in demographic characteristics, the status of four genes in the plasticity region (jhp0940, jhp0945, jhp0947, and jhp0949), and histological scoring among the different geographic groups were determined by one-way analysis of variance (ANOVA) or the chi-square test. A multiple linear regression analysis, where age, sex, bacterial factors, and clinical outcome were explanatory variables, was performed to determine which factor(s) was related to severity of histology. A P value of less than 0.05 was accepted as statistically significant. Calculations were carried out using statistical software StatView 5.0 (SAS Institute Inc., Cary, NC).
RESULTS
The status of jhp0940, jhp0945, jhp0947, and jhp0949 was determined from 513 H. pylori isolates, including 296 isolates from Western countries and 217 isolates from East Asian countries (Table 1). The mean age of subjects, the sex ratio, and the population of H. pylori-related diseases differed significantly among different countries and between areas (P < 0.01) (Table 1).
The prevalence of jhp0940, jhp0945, jhp0947, and jhp0949.
The prevalence rates of jhp0940, jhp0945, jhp0947, and jhp0949 in patients with H. pylori were 19.8% (102/513), 39.6% (203/513), 27.3% (140/513), and 52.8% (271/513), respectively. All samples considered negative by PCR were confirmed as negative by dot blot hybridization. When we combined the results from South Korea and Japan into East Asian isolates and from the United States and Colombia into Western isolates, there was no statistical difference in the prevalence of jhp0940 between East Asian and Western isolates (17.2% and 23.5%; P = 0.078). In contrast, the prevalence of jhp0945 and jhp0947 in East Asian isolates was significantly lower in Western isolates and that of jhp0949 was significantly higher in East Asian isolates compared to Western isolates (Table 3). The prevalence of jhp0947 in East Asian isolates was only 5.5%.
Table 3.
Prevalence of different virulence factors in different ethnic groups
| Virulence factor or region | Genotype | No. (%) found |
P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Western countries |
East Asian countries |
Total (n = 513) | |||||||
| USA (n = 202) | Colombia (n = 94) | Total (n = 296) | South Korea (n = 105) | Japan (n = 112) | Total (n = 217) | ||||
| 35 (17.3%) | 16 (17.0%) | 51 (17.2%) | 27 (25.7%) | 24 (21.4%) | 51 (23.5%) | 102 (19.9%) | 0.12 | ||
| jhp0945 | Present | 96 (47.5%) | 46 (48.9%) | 142 (48.0%) | 30 (28.6%) | 31 (27.7%) | 61 (28.1%) | 203 (39.6%) | <0.01 |
| jhp0947 | Present | 76 (37.6%) | 52 (55.3%) | 128 (43.2%) | 9 (8.6%) | 3 (2.7%) | 12 (5.5%) | 140 (27.3%) | <0.01 |
| jhp0949 | Present | 101 (50.0%) | 42 (44.7%) | 143 (48.3%) | 51 (48.6%) | 77 (68.8%) | 128 (59.0%) | 271 (52.8%) | 0.02 |
| cagA | Present | 169 (83.7%) | 75 (79.8%) | 244 (82.4%) | 102 (97.1%) | 112 (100%) | 214 (98.6%) | 458 (89.3%) | <0.01 |
| vacA s region | s1 | 167 (82.7%) | 72 (76.6%) | 239 (80.7%) | 105 (100%) | 112 (100%) | 217 (100%) | 456 (88.9%) | <0.01 |
| vacA m region | m1 | 130 (64.4%) | 63 (67.0%) | 193 (65.2%) | 91 (86.7%) | 109 (97.3%) | 200 (92.2%) | 393 (76.6%) | < 0.01 |
| vacA i region | i1 | 153 (75.7%) | 67 (71.3%) | 220 (74.3%) | 102 (97.1%) | 110 (98.2%) | 212 (97.7%) | 432 (84.2%) | < 0.01 |
P values were from analyzed demographic data for Western countries versus East Asian countries.
The prevalences of cagA and vacA s1, m1 and i1 regions in H. pylori isolated from patients with gastritis were similar: 89.3% (458/513), 88.9% (456/513), 76.6% (393/513) and 84.2% (432/513), respectively (Table 3). The prevalence of vacA s1, m1 and i1 genotypes combined with cagA-positive status in East Asian isolates was significantly greater than that in Western isolates (P = 0.001, 0.001, and 0.001, respectively) (Table 3).
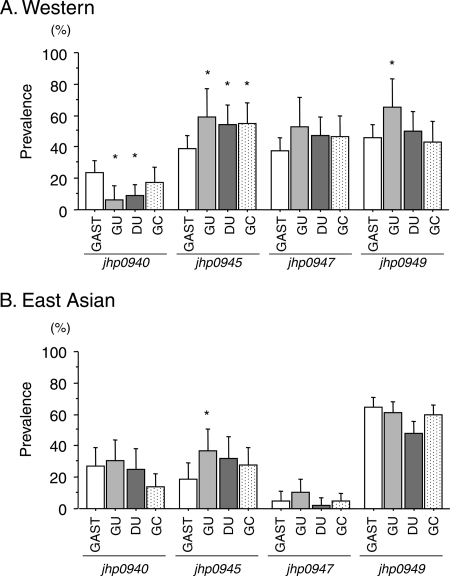
The positive rates of jhp0940 in isolates from patients with duodenal ulcer (15.2%) and gastric cancer (15.4%) were significantly lower than those from gastritis-only patients (24.9%, P = 0.047 and 0.048, respectively). In contrast, the prevalence of jhp0945 was similar in gastric ulcer and duodenal ulcer isolates (45.7% and 45.5%, respectively) and was significantly higher than that in gastritis-only patients (33.0%; P = 0.046 for gastric ulcer and 0.029 for duodenal ulcer, respectively).
When the results were divided into two groups, the prevalence rates of jhp0940, jhp0945, jhp0947, and jhp0949 in Western isolates isolated from gastritis-only patients were 23.9%, 39.1%, 37.7%, and 45.7%, respectively (Fig. 1). The positive rates of jhp0940 in patients with gastric ulcer and duodenal ulcer were significantly lower than that from gastritis patients (12.5% and 8.8%, respectively; P = 0.029 and 0.009, respectively). In contrast, the prevalence rates of jhp0945 in gastric ulcer, duodenal ulcer, and gastric cancer patients were similar (i.e., 59.4%, 54.4%, and 55.2%, respectively) and significantly higher than in gastritis patients (39.1%, P = 0.037, 0.038, and 0.039, respectively). However, there were no significant differences in the prevalence of jhp0947 and jhp0949 between patients with gastritis and those with H. pylori-related diseases.
Fig 1.
Prevalence of jhp0940, jhp0945, jhp0947 and jhp0949 status in patients with gastritis (GAST), gastric ulcer (GU), duodenal ulcer (DU), and gastric cancer (GC) in Western (A) and East Asian (B) regions.
In East Asia, although the presence of jhp0945 was significantly higher in patients with gastric ulcer (36.7%) than that in gastritis patients (18.6%, P = 0.035), there were no significant associations between the development of gastroduodenal diseases and the status of jhp0940, jhp0945, and jhp0949 (Fig. 1). The prevalence of jhp0947 in East Asian isolates was less than 10% irrespective of different diseases, with no significant differences.
Relation between plasticity region gene status and other virulence factors.
The statuses of jhp0940, jhp0945, and jhp0949 in Western isolates were significantly associated with each other (P = 0.001 [jhp0940 versus jhp0945], 0.006 [jhp0940 versus jhp0949], and 0.006 [jhp0945 versus jhp0949] (Table 4). However, the majority of East Asian H. pylori isolates were jhp0947 negative and there were no significant correlations among jhp0940, jhp0945, jhp0947, and jhp0949 statuses. Moreover, the status of these four plasticity region genes had no associations with vacA s1, m1, and i1 genotypes and cagA-positive status in either Western or East Asian isolates (Table 4).
Table 4.
Relationship among different H. pylori virulence factors and plasticity regions
| Region and factor | Relationshipa | |||
|---|---|---|---|---|
| Western | jhp0940 | jhp0945 | jhp0947 | jhp0949 |
| jhp0940 | 0.185* | 0.078 | 0.160* | |
| jhp0945 | <0.001 | 0.161* | ||
| jhp0947 | 0.344* | |||
| jhp0949 | ||||
| vacA s1 | 0.003 | 0.027 | 0.009 | 0.044 |
| vacA m1 | 0.016 | 0.073 | 0.031 | 0.072 |
| vacA i1 | 0.027 | 0.017 | 0.006 | 0.033 |
| cagA | 0.007 | 0.061 | 0.041 | 0.014 |
| East Asian | jhp0940 | jhp0945 | jhp0947 | jhp0949 |
| jhp0940 | 0.109 | 0.095 | 0.024 | |
| jhp0945 | 0.115 | |||
| jhp0947 | 0.038 | |||
| jhp0949 | ||||
| vacA s1 | ||||
| vacA m1 | 0.113 | 0.009 | 0.012 | 0.071 |
| vacA i1 | 0.054 | 0.054 | 0.042 | 0.184 |
| cagA | 0.068 | 0.114 | 0.123 | 0.142 |
The ϕ coefficient value as association between the two factors was analyzed by Fisher's exact test. *, P < 0.05.
As far as combination of the four plasticity region genes was concerned, the majority genotype in East Asian isolates and Western isolates was that of the genes lacking jhp0940-jhp0945-jhp0947-jhp0949 (the −/−/−/− genotype) (n = 47, 21.7% in the East Asian region and n = 76, 25.7% in the Western region) (Table 5). In contrast, the all-four-positive genotype (+/+/+/+ genotype) in East Asian and Western isolates was present only in 0.5% (n = 1) and 2.4% (n = 7) (Table 5); a mosaic pattern was more common, such as the −/+/−/+ and +/+/−/+ genotypes in 90 isolates (n = 57 [26.3%] in the East Asian region and n = 33 [11.1%] in the Western region) (Table 5).
Table 5.
Combination of jhp0940, jhp0945, jhp0947, and jhp0949 status
| Genotype pattern |
No. (%) of isolates with pattern |
||||
|---|---|---|---|---|---|
| jhp0940 | jhp0945 | jhp0947 | jhp0949 | East Asian | Western |
| − | − | − | − | 47 (21.7%) | 76 (25.7%) |
| − | − | − | + | 65 (30.0%) | 15 (5.1%) |
| − | − | + | − | 0 (0%) | 11 (3.7%) |
| − | + | − | − | 18 (8.3%) | 26 (8.8%) |
| + | − | − | − | 16 (7.4%) | 14 (4.7%) |
| − | − | + | + | 4 (1.8%) | 29 (9.8%) |
| − | + | + | − | 2 (0.9%) | 14 (4.7%) |
| + | + | − | − | 4 (1.8%) | 7 (2.4%) |
| − | + | + | + | 2 (0.9%) | 60 (20.3%) |
| + | + | + | − | 1 (0.5%) | 4 (1.4%) |
| + | + | + | + | 1 (0.5%) | 7 (2.4%) |
| + | − | − | + | 22 (10.1%) | 6 (2.0%) |
| + | − | + | − | 1 (0.5%) | 1 (0.3%) |
| + | − | + | + | 1 (0.5%) | 2 (0.7%) |
| + | + | − | + | 5 (2.3%) | 11 (3.7%) |
| − | + | − | + | 28 (12.9%) | 13 (4.4%) |
Histology.
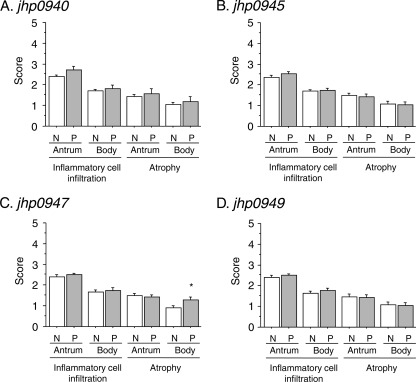
Although the jhp0947 status was associated with a significantly increased risk of corpus atrophy, most of the parameters of inflammatory cell infiltration and mucosal atrophy were not different irrespective of different gastric locations or plasticity region gene status among Western patients (Fig. 2).
Fig 2.
Scores of gastric mucosal atrophy and inflammation cell infiltration in gastric antrum and corpus between jhp0940, jhp0945, jhp0947, and jhp0949 status in Western isolates. *, P < 0.05 compared with negative strain. Abbreviations: N, plasticity region gene-negative strain; P, plasticity region-positive strain.
The gastric mucosa in patients with gastric cancer and gastric ulcer is generally atrophic and that of those with duodenal ulcer generally shows enhanced antral inflammation such that inclusion of these patients in the histological analyses might have introduced a bias; we therefore evaluated the histological analyses using gastritis-only cases, and we found no significant differences (data not shown).
Influence of jhp0940, jhp0945, jhp0947, and jhp0949 status and clinical outcomes.
In East Asia, univariant analysis showed that the jhp0945 status was associated with an increased a risk of gastric ulcer (odds ratio [OR], 2.58; 95% confidence interval [CI], 1.06 to 6.27) (Table 6). However, in other parameters, there were no significant associations between clinical outcomes and jhp0940, jhp0945, jhp0947, and jhp0949 status among East Asian isolates (Table 6).
Table 6.
Age- and sex-adjusted risk for peptic ulcer and gastric cancer in relation to H. pylori virulence factors
| Virulence factor for region | Gastric ulcer |
Duodenal ulcer |
Gastric cancer |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Westerna | |||||||||
| jhp0940 | 0.21 | 0.05–0.94 | 0.04 | 0.31 | 0.12–0.78 | 0.01 | 0.66 | 0.30–1.45 | 0.31 |
| jhp0945 | 2.27 | 1.04–4.98 | 0.04 | 1.86 | 1.03–3.34 | 0.04 | 1.92 | 1.03–3.56 | 0.04 |
| jhp0947 | 1.87 | 0.86–4.07 | 0.11 | 1.47 | 0.82–2.65 | 0.20 | 1.44 | 0.78–2.68 | 0.25 |
| jhp0949 | 2.27 | 1.02–5.07 | 0.05 | 1.19 | 0.67–2.13 | 0.56 | 0.09 | 0.49–1.67 | 0.74 |
| East Asianb | |||||||||
| jhp0940 | 1.24 | 0.53–2.93 | 0.62 | 0.81 | 0.33–2.02 | 0.66 | 0.49 | 0.19–1.26 | 0.14 |
| jhp0945 | 2.58 | 1.06–6.27 | 0.04 | 2.06 | 0.82–5.20 | 0.12 | 1.67 | 0.70–4.02 | 0.25 |
| jhp0947 | 2.17 | 0.48–9.80 | 0.31 | 0.43 | 0.04–4.38 | 0.48 | 0.92 | 0.17–4.96 | 0.92 |
| jhp0949 | 0.85 | 0.38–1.91 | 0.90 | 0.55 | 0.24–1.25 | 0.15 | 0.72 | 0.33–1.56 | 0.40 |
Gastric ulcer, n = 32; duodenal ulcer, n = 68; gastric cancer, n = 58.
Gastric ulcer, n = 49; duodenal ulcer, n = 44; gastric cancer, n = 65.
In Western isolates, the status of jhp0940 was significantly associated with H. pylori-related disease and with significantly lower risks of gastric ulcer and duodenal ulcer (OR [95% CI], 0.21 [0.05 to 0.94] and 0.31 [0.12 to 0.78], respectively) (Table 6). In contrast, the jhp0945-positive isolates significantly increased the risk for gastric ulcer, duodenal ulcer, and gastric cancer compared with jhp0945-negative isolates (OR [95% CI], 2.27 [1.04 to 4.98], 1.86 [1.03 to 3.34], and 1.92 [1.03 to 3.56], respectively). The carriage of jhp0949 was also related to an increased risk of gastric ulcer (OR, 2.28; 95% CI, 1.02 to 5.07).
Multivariant analysis, including age, sex, jhp0940, jhp0945, jhp0947, and jhp0949 status, cagA status, and vacA s, m, or i region genotype, was performed to determine which factor(s) was related to clinical outcome (Table 7). The jhp0945 and cagA status for gastric ulcer and the vacA m1 genotype for gastric cancer significantly increased the risks of a clinical outcome. In contrast, the jhp0940 status significantly decreased the risk for duodenal ulcer (OR, 0.49; 95% CI, 0.24 to 0.99).
Table 7.
Multivariant analysis for the risk of peptic ulcer and gastric cancer in Westerna isolates
| Disease | Parameter | OR | 95% CI | P value |
|---|---|---|---|---|
| Gastric ulcer | jhp0945 | 2.18 | 1.16–4.10 | 0.02 |
| cagA | 27.69 | 1.79–428.7 | 0.02 | |
| Duodenal ulcer | jhp0940 | 0.49 | 0.24–0.99 | 0.05 |
| Gastric cancer | vacA m1 | 5.06 | 1.05–24.48 | 0.04 |
No significant parameters remained for East Asian strains.
DISCUSSION
There has been increasing interest in strain-specific H. pylori genes transferred from other species that are located outside the cag PAI, especially genes within the plasticity regions (6, 15–17, 19, 20, 23). The plasticity regions were a large region of 45 kb in H. pylori J99 and 68 kb in H. pylori 26695, and the plasticity region is encoded by 38 genes in J99, of which 33 are absent in 26695. The gene content of H. pylori is variable because of different combinations of genes within plasticity regions (2). This gene variability is thought to possibly be responsible for the difference in virulence among different H. pylori strains that then may result in different risks of specific clinical outcomes (6, 15–17, 19, 20, 23). ORFs in the plasticity region—e.g., jhp0917 and jhp0918 [dupA (virB4)], jhp0919, jhp0920, and jhp0931; genes for DNA topoisomerase I involved in DNA replication (jhp0921-jhp0924); genes for DNA transformation competence ComB8 [jhp0921 (virB8)], ComB9 [jhp0923 (virB9)], or ComB10 [jhp0924 (virB10)]; and others (jhp0928, jhp0931, jhp0935, jhp0941, and jhp0951)—share similarity with genes encoding functional proteins, but overall, the biological function of most ORFs in the plasticity region remains unclear. Recently, jhp0940, jhp0945, jhp0947, and jhp0949 in H. pylori have been reported to be associated with an increase in inflammatory cytokines, enhancement of the NF-κB signaling pathway, and gastroduodenal disease (6, 15–17, 19, 20, 23). It has been suggested that jhp0940, jhp0945, jhp0947, and jhp0949 may be related to H. pylori-related disease pathogenesis and may be a marker for risk of peptic ulcer disease (Table 8).
Table 8.
Literature survey of plasticity region genes
| Gene | Source | Yr | Diseasea | No. isolated | No. (%) of positive patients |
|---|---|---|---|---|---|
| jhp0940 | Occhialini et al. (16) | 2000 | Gastritis | 26 | 0 (0%) |
| GC | 17 | 7 (41%) | |||
| Santos et al. (23) | 2003 | Gastritis | 68 | 1 (2%) | |
| DU | 53 | 1 (2%) | |||
| GC | 79 | 1 (1%) | |||
| Yakoob et al. (30) | 2010 | Gastritis | 36 | 14 (39%) | |
| GU | 22 | 17 (77%) | |||
| GC | 29 | 22 (76%) | |||
| DU | 27 | 18 (67%) | |||
| jhp0945 | de Jonge et al. (6) | 2004 | Gastritis | 26 | 11 (42%) |
| DU | 19 | 7 (39%) | |||
| jhp0947 | Occhialini et al. (16) | 2000 | Gastritis | 26 | 9 (35%) |
| GC | 17 | 11 (65%) | |||
| Santos et al. (23) | 2003 | Gastritis | 68 | 13 (44%) | |
| DU | 53 | 42 (79%) | |||
| GC | 79 | 68 (86%) | |||
| de Jonge et al. (6) | 2004 | Gastritis | 26 | 5 (19%) | |
| DU | 19 | 10 (53%) | |||
| Proenca Modena et al. (17) | 2007 | Gastritis | 39 | 11 (38%) | |
| GU | 24 | 15 (63%) | |||
| DU | 22 | 14 (64%) | |||
| Yakoob et al. (30) | 2010 | Gastritis | 36 | 13 (39%) | |
| GU | 22 | 8 (36%) | |||
| GC | 29 | 22 (76%) | |||
| DU | 27 | 23 (85%) | |||
| Siavoshi et al. (24) | 2011 | Gastritis | 143 | 83 (58%) | |
| jhp0949 | de Jonge et al. (6) | 2004 | Gastritis | 26 | 5 (19%) |
| DU | 19 | 10 (53%) | |||
| Lehours et al. (15) | 2004 | Gastritis | 39 | 14 (36%) | |
| ML | 43 | 17 (40%) |
Abbreviations: DU, duodenal ulcer; GC, gastric cancer; GU, gastric ulcer; ML, malignant lymphoma.
The jhp940 gene is widely prevalent geographically (India, France, Spain, Peru, Japan, South Africa, and Costa Rica), and the lowest prevalence of jhp940 was seen in the Spanish (5%) and Costa Rican (30%) isolates, followed by the Japanese (40%) and Peruvian (60%) isolates (19). In an animal model, jhp940 is strongly expressed in response to the interaction of H. pylori with the gerbil gastric mucosa (10). Although as of recently the roles of most regions remain unknown, the recombinant Jhp0940 protein has been shown to elicit tumor necrosis factor alpha (TNF-α) and interleukin-8 (IL-8) from activated human macrophages as well as enhanced translocation of NF-κB in cultured macrophages (19). Moreover, Kim et al. (14) reported that JHP940 is catalytically active as a protein kinase and translocates into cultured human cells and that the kinase activity is indispensable for indirectly upregulating phosphorylation of NF-κB p65 at Ser276. These observations might suggest that jhp0940 may play a role enhancing the severity of gastric mucosal inflammation and inflammation-related outcomes such as gastric cancer (7, 9, 12). In support of this hypothesis, Occhialini et al. (16) reported jhp0940 positivity in 41.2% of isolates from gastric cancer patients versus none of the isolates from patients with gastritis (P < 0.0006). However, other studies showed no significant relationship between the presence of jhp0940 and any clinical outcomes (23) or even a decreased risk of gastric cancer (20). For example, we previously demonstrated that in Western isolates the presence of jhp0940 was associated with a significant negative association with gastric ulcer or duodenal ulcer. The prevalence of jhp0940 status was also found to be low in areas of high gastric cancer incidence, such as East Asian and Latin America, compared with low-incidence areas, such as Africa, South Asia, and Europe (19, 20, 23). These recent results might suggest that jhp0940 has a preventive effect on gastroduodenal diseases. At best, one can surmise that no consistent effect of jhp0940 has yet been demonstrated.
Previous studies of the prevalence of jhp0945 status were limited (Table 8). Small studies using fewer than 20 isolates from Turkey, Costa Rica, and Netherlands found that the prevalence of jhp0945 status was similar between H. pylori from patients with peptic ulcer and that from patients with gastritis (6, 16, 22). In this study, we used about 300 H. pylori isolates and showed a significant association between jhp0945-positive isolates and gastric ulcer, duodenal ulcer, and gastric cancer (OR, 1.86 to 2.27) compared with jhp0945-negative isolates cultured from Western patients. In East Asian isolates, there was a significant correlation with jhp0945 status and gastric ulcer. Because jhp940 status had no significant association with cagA status or with vacA s, m, and i region genotypes, the overall conclusion from our study is that among genes we examined, jhp0945 was the best candidate for a disease marker, especially in Western isolates.
In previous studies, jhp0947 was found to be homologous to jhp0938 (hp0990) and jhp253 (hp1333), which all encode hypothetical proteins, and was considered to be the most sensitive H. pylori-related-disease plasticity region marker (16, 23). Although jhp0917 and jhp0918 (dupA) are known to be homologous with virB4 as cag PAI markers in the plasticity zone, the 5′ region of jhp0947 is also homologous to that of jhp0477 (hp0528), which is part of the cag PAI (virB9 homologue) and has been identified as an important structural component of the type IV secretion system (16, 23). In 2000, Occhialini et al. (16) reported that jhp0947 was found more frequently in isolates from gastric cancer patients (64.7%) than in those from gastritis patients (34.6%) (16). Moreover, Santos et al. (23) reported that in multivariate analysis the presence of the jhp0947 remained associated only with gastric cancer (OR, 2.94; 95% CI, 1.86 to 4.64) and with duodenal ulcer disease (4.84, 2.13 to 10.96). The cagA and jhp0947 genes were independently associated with development of duodenal ulcer, and among the 140 H. pylori strains harboring jhp0947, 127 (90.7%) were also cagA positive (16). The presence of cagA and jhp0947 in the H. pylori strains was associated with the severity of gastritis in the subset of patients without duodenal ulcer or gastric cancer (16). Moreover, Yakoob et al. (30) reported that jhp0947 was significantly associated with chronic active inflammation, and we also showed that the jhp0947 status was associated with a significantly increased risk of corpus mucosal atrophy. The presence of jhp0947 was completely linked with that of jhp0949 and was roughly associated with that of jhp0945 (6). Disruption of the jhp0945/jhp0947/jhp0949 genes, such as in H. pylori 26695, significantly decreased IL-12 production from mononuclear cells (THP-1 cells) in vitro; IL-12 directs native T cell development into inflammatory T1 cells (6), and prolonged activation of Th1 cells might result in severe tissue damage. However, this study and other reports have not confirmed that jhp0947 and jhp0949 status is associated with peptic ulcer and gastric cancer (17). Importantly, the prevalence of jhp0947 in East Asian isolates was only 5.5%. Moreover, there was no association with jhp0947 and jhp0949 status and histological evaluation of inflammatory cell infiltration and atrophy in Brazil (23), Colombia, the United States, or East Asian countries. Overall, these data are not consistent with the notion that jhp0947 and jhp0949 constitute a sensitive plasticity region marker for H. pylori-related diseases in East Asia.
It is well known that atrophic gastritis is positively associated with both gastric ulcer and gastric cancer whereas antral predominant gastritis is associated with development of duodenal ulcer. The high-activity and high-producer genotype of H. pylori virulence factors and the cagA-positive, vacA s1, m1, and i1 genotypes have been associated with enhanced gastric mucosal inflammation and mucosal atrophy (18). Although the 90.7% of H. pylori isolates with the jhp0947 status in Brazil were reported to be associated with cagA (23), this relationship was not observed in Indian (19), Dutch (6), or other Brazilian groups (17). In this study, we showed that the jhp0940, jhp0945, jhp0947, and jhp0949 status had no significant associations with vacA s1, m1, and i1 genotypes or with cagA positivity in either Western or East Asian populations. We found that in multivariance analysis jhp0940 and jhp0945 status was related to peptic ulcer and gastric cancer and to gastric mucosal inflammation and atrophy, suggesting it may be a new virulent marker for H. pylori-related diseases. However, none of the associations were significant.
In conclusion, we concluded that the jhp0940 and jhp0945 genotype was a marker of peptic ulcer and gastric cancer development irrespective of severe inflammation and gastric mucosal atrophy, as is the current genotyping of vacA s, m, and i regions and cagA status.
ACKNOWLEDGMENTS
This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center. Y. Yamaoka is supported in part by NIH grant DK 62813. This report is also partially based on work supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (22390085 and 22659087), and the Special Coordination Funds for Promoting Science and Technology from the MEXT of Japan.
We thank Oscar Gutierrez (Universidad Nacional de Colombia, Bogota, Colombia) for providing clinical samples in Colombia.
No conflicts of interest exist for this paper.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1. Alm RA, et al. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176–180 [DOI] [PubMed] [Google Scholar]
- 2. Alm RA, Trust TJ. 1999. Analysis of the genetic diversity of Helicobacter pylori: the tale of two genomes. J. Mol. Med. 77:834–846 [DOI] [PubMed] [Google Scholar]
- 3. Atherton JC, et al. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771–17777 [DOI] [PubMed] [Google Scholar]
- 4. Atherton JC, Peek RM, Jr, Tham KT, Cover TL, Blaser MJ. 1997. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112:92–99 [DOI] [PubMed] [Google Scholar]
- 5. Covacci A, et al. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. U. S. A. 90:5791–5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Jonge R, et al. 2004. The Helicobacter pylori plasticity region locus jhp0947-jhp0949 is associated with duodenal ulcer disease and interleukin-12 production in monocyte cells. FEMS Immunol. Med. Microbiol. 41:161–167 [DOI] [PubMed] [Google Scholar]
- 7. El-Omar EM, et al. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398–402 [DOI] [PubMed] [Google Scholar]
- 8. el-Zimaity HM, et al. 1996. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum. Pathol. 27:35–41 [DOI] [PubMed] [Google Scholar]
- 9. Furuta T, et al. 2002. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology 123:92–105 [DOI] [PubMed] [Google Scholar]
- 10. Graham JE, Peek RM, Jr, Krishna U, Cover TL. 2002. Global analysis of Helicobacter pylori gene expression in human gastric mucosa. Gastroenterology 123:1637–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gressmann H, et al. 2005. Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet. 1:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hwang IR, et al. 2002. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology 123:1793–1803 [DOI] [PubMed] [Google Scholar]
- 13. Kersulyte D, et al. 2000. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 182:3210–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim DJ, et al. 2010. Helicobacter pylori proinflammatory protein up-regulates NF-kappaB as a cell-translocating Ser/Thr kinase. Proc. Natl. Acad. Sci. U. S. A. 107:21418–21423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lehours P, et al. 2004. Identification of a genetic marker of Helicobacter pylori strains involved in gastric extranodal marginal zone B cell lymphoma of the MALT-type. Gut 53:931–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Occhialini A, et al. 2000. Distribution of open reading frames of plasticity region of strain J99 in Helicobacter pylori strains isolated from gastric carcinoma and gastritis patients in Costa Rica. Infect. Immun. 68:6240–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Proenca Modena JL, et al. 2007. Association between Helicobacter pylori genotypes and gastric disorders in relation to the cag pathogenicity island. Diagn. Microbiol. Infect. Dis. 59:7–16 [DOI] [PubMed] [Google Scholar]
- 18. Rhead JL, et al. 2007. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133:926–936 [DOI] [PubMed] [Google Scholar]
- 19. Rizwan M, Alvi A, Ahmed N. 2008. Novel protein antigen (JHP940) from the genomic plasticity region of Helicobacter pylori induces tumor necrosis factor alpha and interleukin-8 secretion by human macrophages. J. Bacteriol. 190:1146–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romo-Gonzalez C, et al. 2009. Differences in genome content among Helicobacter pylori isolates from patients with gastritis, duodenal ulcer, or gastric cancer reveal novel disease-associated genes. Infect. Immun. 77:2201–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salama N, et al. 2000. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. U. S. A. 97:14668–14673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salih BA, Abasiyanik MF, Ahmed N. 2007. A preliminary study on the genetic profile of cag pathogenicity-island and other virulent gene loci of Helicobacter pylori strains from Turkey. Infect. Genet. Evol. 7:509–512 [DOI] [PubMed] [Google Scholar]
- 23. Santos A, et al. 2003. New pathogenicity marker found in the plasticity region of the Helicobacter pylori genome. J. Clin. Microbiol. 41:1651–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siavoshi F, et al. 2011. Helicobacter pylori genotypes and types of gastritis in first-degree relatives of gastric cancer patients. Int. J. Med. Microbiol. 301:506–512 [DOI] [PubMed] [Google Scholar]
- 25. Sugimoto M, et al. 2007. Role of angiotensinogen gene polymorphism on Helicobacter pylori infection-related gastric cancer risk in Japanese. Carcinogenesis 28:2036–2040 [DOI] [PubMed] [Google Scholar]
- 26. Sugimoto M, Yamaoka Y. 2009. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin. Microbiol. Infect. 15:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tummuru MK, Cover TL, Blaser MJ. 1993. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect. Immun. 61:1799–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uemura N, et al. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784–789 [DOI] [PubMed] [Google Scholar]
- 29. van Doorn LJ, et al. 1998. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology 115:58–66 [DOI] [PubMed] [Google Scholar]
- 30. Yakoob J, et al. 2010. Associations between the plasticity region genes of Helicobacter pylori and gastroduodenal diseases in a high-prevalence area. Gut Liver 4:345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamaoka Y. 2008. Roles of the plasticity regions of Helicobacter pylori in gastroduodenal pathogenesis. J. Med. Microbiol. 57:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamaoka Y, Kodama T, Kashima K, Graham DY, Sepulveda AR. 1998. Variants of the 3′ region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J. Clin. Microbiol. 36:2258–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamaoka Y, Kwon DH, Graham DY. 2000. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 97:7533–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamaoka Y, et al. 2002. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 517:180–184 [DOI] [PubMed] [Google Scholar]