Abstract
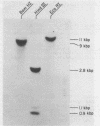
The gene coding for the common alpha subunit of the bovine pituitary glycoprotein hormones was isolated from a bovine genomic library. The gene spans roughly 16.5 kbp, contains three intervening sequences, and codes for a message of approximately 730 nucleotides. The complete coding region of the gene was sequenced as well as 315 nucleotides of 5' flanking sequence and the entire intron C. Only a single base difference was found when the sequence of the gene was compared with that of the cDNA. Genomic blotting experiments suggest the presence of a single alpha subunit gene. Comparison of the bovine and human alpha subunit genes indicated that the high level of homology observed in the coding regions has been maintained throughout the 5' and 3' untranslated regions, and at least 90 nucleotides of the 5'flanking regions. Additionally, there is an 18 base pair sequence present in both the 5' flanking and 5' untranslated regions of the gene that is homologous to a region of the chick ovalbumin gene. This ovalbumin sequence has been suggested as a binding site for the progesterone receptor-complex.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. C., Miller W. L. Regulation of ovine follicle-stimulating hormone beta-chain mRNA by 17 beta-estradiol in vivo and in vitro. J Biol Chem. 1982 Mar 10;257(5):2282–2286. [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Boorstein W. R., Vamvakopoulos N. C., Fiddes J. C. Human chorionic gonadotropin beta-subunit is encoded by at least eight genes arranged in tandem and inverted pairs. Nature. 1982 Dec 2;300(5891):419–422. doi: 10.1038/300419a0. [DOI] [PubMed] [Google Scholar]
- Compton J. G., Schrader W. T., O'Malley B. W. DNA sequence preference of the progesterone receptor. Proc Natl Acad Sci U S A. 1983 Jan;80(1):16–20. doi: 10.1073/pnas.80.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Molitch M., Pierce J., Odell W. D. Secretion of alpha and beta subunits of TSH by the anterior pituitary. Clin Endocrinol (Oxf) 1975 Sep;4(5):525–530. doi: 10.1111/j.1365-2265.1975.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Fetherston J., Boime I. Synthesis of bovine lutropin in cell-free lysates containing pituitary microsomes. J Biol Chem. 1982 Jul 25;257(14):8143–8147. [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. The gene encoding the common alpha subunit of the four human glycoprotein hormones. J Mol Appl Genet. 1981;1(1):3–18. [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Fontaine Y. A., Burzawa-Gerard E. Esquisse de l'evolution des hormones gonadotropes et thyreotropes des vertébrés. Gen Comp Endocrinol. 1977 Jul;32(3):341–347. doi: 10.1016/0016-6480(77)90213-1. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Kaplan S. L., Grumbach M. M., Aubert M. L. alpha and beta glycoprotein hormone subunits (hLH, hFSH, hCG) in the serum and pituitary of the human fetus. J Clin Endocrinol Metab. 1976 May;42(5):995–998. doi: 10.1210/jcem-42-5-995. [DOI] [PubMed] [Google Scholar]
- Kourides I. A., Weintraub B. D., Ridgway E. C., Maloof F. Pituitary secretion of free alpha and beta subunit of human thyrotropin in patients with thyroid disorders. J Clin Endocrinol Metab. 1975 May;40(5):872–885. doi: 10.1210/jcem-40-5-872. [DOI] [PubMed] [Google Scholar]
- Landefeld T. D., Kepa J., Karsch F. J. Regulation of alpha subunit synthesis by gonadal steroid feedback in the sheep anterior pituitary. J Biol Chem. 1983 Feb 25;258(4):2390–2393. [PubMed] [Google Scholar]
- Landefeld T. D., Kepa J. The cell free synthesis of bovine lutropin beta subunit. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1111–1118. doi: 10.1016/0006-291x(79)91150-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyata T., Yasunaga T., Nishida T. Nucleotide sequence divergence and functional constraint in mRNA evolution. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7328–7332. doi: 10.1073/pnas.77.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson J. H., Thomason A. R., Cserbak M. T., Moncman C. L., Woychik R. P. Nucleotide sequence of a cDNA for the common alpha subunit of the bovine pituitary glycoprotein hormones. Conservation of nucleotides in the 3'-untranslated region of bovine and human pre-alpha subunit mRNAs. J Biol Chem. 1983 Apr 25;258(8):4679–4682. [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Prentice L. G., Ryan R. J. LH and its subunits in human pituitary, serum and urine. J Clin Endocrinol Metab. 1975 Feb;40(2):303–312. doi: 10.1210/jcem-40-2-303. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor W. L., Collier K. J., Deschenes R. J., Weith H. L., Dixon J. E. Sequence analysis of a cDNA coding for a pancreatic precursor to somatostatin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6694–6698. doi: 10.1073/pnas.78.11.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Changing placental concentrations of human chorionic gonadotropin and its subunits during gestation. J Clin Endocrinol Metab. 1974 May;38(5):755–760. doi: 10.1210/jcem-38-5-755. [DOI] [PubMed] [Google Scholar]