Abstract
The blaOXA-51-like gene, originally intrinsic to Acinetobacter baumannii, had been detected in two clones of Acinetobacter nosocomialis and one clone of Acinetobacter genomic species “Close to 13TU.” These blaOXA-51-like genes, all preceded by ISAba1, were located on plasmids that might have originated with A. baumannii. The plasmid-borne ISAba1--blaOXA-51-like confers a high level of carbapenem resistance and affects the accuracy of using blaOXA-51-like detection as a tool for differentiating A. baumannii from other Acinetobacter species.
TEXT
The most common mechanism of carbapenem resistance in Acinetobacter species is the production of carbapenem-hydrolyzing class D β-lactamases (CHDLs) (16). Among these CHDL genes, the blaOXA-51-like gene is intrinsic to Acinetobacter baumannii and originally was confined on the chromosome of this species (7, 14, 17). Therefore, its detection has been used as a method of A. baumannii identification (17). However, the genetic structure ISAba1–blaOXA-51-like has integrated into plasmids, probably via a transposition event (2, 9). The plasmids carrying ISAba1–blaOXA-51-like had disseminated into A. baumannii isolates in Taiwan (2). In addition, the plasmid-borne blaOXA-51-like gene (blaOXA-138) had also been detected in an Acinetobacter nosocomialis (formerly Acinetobacter genomic species 13TU) isolate (12). In this study, we aim to characterize the non-Acinetobacter baumannii species carrying blaOXA-51-like genes.
Among the nonduplicate bacteremic Acinetobacter calcoaceticus-A. baumannii (Acb) complex isolates collected from Taipei Veterans General Hospital (TVGH) from January 1996 through December 2007, 676 isolates were identified as non-A. baumannii species by a multiplex PCR method (3) and 6 (0.9%) of them had blaOXA-51-like genes. Among 74 other nonduplicate isolates of non-A. baumannii species that were consecutively collected from various clinical specimens from 10 medical centers (up to 40 isolates from each center) in Taiwan during the period from July through October 2007 (2), 4 (5.5%) isolates had blaOXA-51-like genes.
The clinical characteristics of the patients who carried non-A. baumannii species harboring blaOXA-51-like genes are summarized in Table 1. Nine of these Acinetobacter isolates were pathogens of nosocomial infection (infection developed more than 48 h after hospitalization), and 8 of them were isolated from patients during their stay in different intensive care units in TVGH. The 10 Acinetobacter isolates were identified as A. nosocomialis or Acinetobacter genomic species “Close to 13TU” by amplified ribosomal DNA restriction analysis (13) (Table 2). The A. nosocomialis isolates belonged to two clones (pulsotypes B and C), and all the Acinetobacter genomic species “Close to 13TU” isolates belonged to a single clone (pulsotype A), as determined by pulsed-field gel electrophoresis (10). Three isolates (one from each pulsotype) were selected for multilocus sequence typing (MLST) (6), and they fell into sequence type 74 (ST74) and ST90, corresponding to A. nosocomialis and Acinetobacter genomic species “Close to 13TU,” respectively (13). All of them were nonsusceptible to imipenem or meropenem, accounting for 11.0% and 13.9% of imipenem- and meropenem-resistant non-A. baumannii isolates collected in the same period, respectively.
Table 1.
Clinical characteristics of patients who carried non-Acinetobacter baumannii species harboring blaOXA-51-like genesa
| Case no. | Hospital | Ward/dayb | Age (yr)/sex | APACHE II score | Underlying diseases | Invasive devices | Source/infectionc | Concomitant isolated | Treatment/appropriate antimicrobial therapye | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TVGH | NSCU/11 | 77/M | 22 | Traumatic ICH, SDH, SAH | Tracheostomy, CVC, Foley, ventilator | Blood/I | Pseudomonas aeruginosa | Ticarcillin/clavulanate for 18 days/no | Survived |
| 2 | TVGH | RCU/25 | 75/F | 21 | COPD, DM, hypertension, recent CVA | Tracheostomy, CVC, Foley, ventilator | Blood/I | None | Cefmetazole for 2 days, piperacillin-tazobactam for 3 days, and imipenem for 13 days/no | Survived |
| 3 | TVGH | ICUB/7 | 21/M | 19 | Pulmonary contusion with pulmonary hemorrhage and hemothorax | Swan-Ganz catheter, CVC, chest tube, ventilator | Blood/I | None | Ciprofloxacin for 14 days/yes | Survived |
| 4 | TVGH | CCU/14 | 87/M | 18 | Recent myocardial infarction | None | Blood/I | None | Flomoxef plus netilmycin for 9 days/no | Survived |
| 5 | TVGH | ICUA/7 | 24/F | 13 | Systemic lupus erythematosus | Arterial line, CVC, Foley, ventilator | Sputum/C | Pneumocystis jirovecii | —/—f | Died of other causes |
| 6 | TVGH | ICUA/13 | 81/F | 28 | Parkinson's disease, DM, hypertension | Arterial line, CVC, Foley, ventilator | Sputum/I | Stenotrophomonas maltophilia | Piperacillin-tazobactam for 21 days/no | Died of other causes |
| 7 | TVGH | ICUC/5 | 69/M | 39 | Multiple myeloma, old CVA | Arterial line, CVC, Foley, ventilator | Sputum/I | None | Imipenem for 14 days/no | Died of infection |
| 8 | NTUH | NA | NA | NA | NA | NA | Blood/I | NA | NA | NA |
| 9 | TVGH | CCU/26 | 80/F | 33 | Congestive heart failure, asthma, DM, hypertension | Arterial line, CVC, Foley, ventilator, HD via FVC | Blood/I | None | Levofloxacin for 10 days/yes | Died of other causes |
| 10 | TVGH | ER | 62/M | 11 | Lung cancer, chemotherapy | None | Blood/I | Chryseobacterium meningosepticum | Cefoperazone plus sulbactam for 5 days/no | Survived |
Abbreviations: TVGH, Taipei Veterans General Hospital; NTUH, National Taiwan University Hospital; NSCU, neurosurgical care unit; RCU, respiratory care unit; ICU, intensive care unit; CCU, cardiac care unit; NA, data not available; ER, emergency room; ICH, intracerebral hemorrhage; SDH, subdural hemorrhage; SAH, subarachnoid hemorrhage; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; CVA, cerebrovascular accident; CVC, central venous catheter; HD, hemodialysis; FVC, femoral vein catheter; Foley, Foley catheter; I, infection; C, colonization.
Ward at which the patient resided and days of admission when the first isolate of non-A. baumannii species harboring blaOXA-51-like genes was collected.
Infection (I) or colonization (C).
Organisms isolated or identified from the same site and at the same time with non-A. baumannii species.
Appropriate antimicrobial therapy was defined as therapy with at least one antimicrobial agents that had in vitro activity against the causative pathogen and administrated within 48 h after the acquisition of index clinical sample for culture.
—/—, since the patient was colonized with non-A. baumannii species, antimicrobial therapy for acinetobacters was not warranted.
Table 2.
Characteristics of non-Acinetobacter baumannii species harboring blaOXA-51-like genesa
| Isolate | Date of isolation | ARDRA profiles | Identification by ARDRA | MLSTb | Pulsotype | Plasmid pattern | blaOXA-51-like allele | Amino acid at positionc: |
Other carbapenemase gened | MIC,e μg/ml |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 7 | 69 | 167 | 192 | 214 | 264 | IPM | MEM | CAZ | CFP | TZP | SUL | COL | TGC | |||||||||
| Reference | K | A | L | L | L | F | I | T | ||||||||||||||||
| 1059 | Nov. 2004 | 31113 | “Close to 13TU” | A | I | blaOXA-138 | T | V | None | 32 | 64 | 64 | 128 | 64 | 2 | 2 | 1 | |||||||
| 1075 | Jan. 2005 | 21111 | A. nosocomialis | B | III | blaOXA-194 | F | V | blaOXA-58 | 32 | 32 | 8 | 4 | >128 | 8 | 4 | 0.75 | |||||||
| 1104 | Mar. 2005 | 31113 | “Close to 13TU” | A | I | blaOXA-195 | F | V | S | M | None | 16 | 64 | 64 | 128 | 64 | 2 | 2 | 1.5 | |||||
| 2311 | Nov. 2006 | 31113 | “Close to 13TU” | ST90 | A | II | blaOXA-194 | F | V | None | 32 | 64 | 64 | 128 | 128 | 4 | 2 | 1.5 | ||||||
| 1890 | July 2007 | 31113 | “Close to 13TU” | A | II | blaOXA-194 | F | V | None | 64 | 16 | 64 | 128 | >128 | 8 | 2 | 3 | |||||||
| 1892 | Aug. 2007 | 31113 | “Close to 13TU” | A | II | blaOXA-196 | Q | H | V | None | 64 | 64 | 64 | 128 | >128 | 8 | 2 | 3 | ||||||
| 1897 | Aug. 2007 | 31113 | “Close to 13TU” | A | II | blaOXA-197 | F | V | A | None | 32 | 64 | 64 | 128 | >128 | 8 | 2 | 3 | ||||||
| 2019 | Aug. 2007 | 21113 | A. nosocomialis | ST74 | C | IV | blaOXA-194 | F | V | None | 8 | 64 | 8 | 4 | >128 | 16 | 1 | 0.25 | ||||||
| 1522 | Dec. 2007 | 31113 | “Close to 13TU” | A | I | blaOXA-82 | V | None | 32 | 64 | 32 | 128 | >128 | 1 | 2 | 1 | ||||||||
| 1704 | Dec. 2007 | 21113 | A. nosocomialis | ST74 | B | III | blaOXA-138 | T | V | blaOXA-58 | 32 | 64 | 8 | 4 | >128 | 32 | 1 | 0.38 | ||||||
Abbreviations: ARDRA, amplified ribosomal DNA restriction analysis; A. nosocomialis, Acinetobacter nosocomialis; MLST, multilocus sequence typing; IPM, imipenem; MEM, meropenem; CAZ, ceftazidime; CFP, cefepime; TZP, piperacillin-tazobactam; SUL, sulbactam; COL, colistin; TGC, tigecycline.
The sequences of the STs are available at www.pasteur.fr/mlst.
The reference amino acids and their positions are from blaOXA-66.
Including blaOXA-58-like, blaOXA-24-like, blaOXA-23-like, blaOXA-143-like, blaIMP-like, blaVIM-like, blaSIM-like, blaGIM-like and blaSPM-like.
In vitro testing of susceptibilities to imipenem and meropenem was done using Etest (AB BIODISK, Solna, Sweden). In vitro testing of susceptibilities to other antibiotics was done using agar dilution methods. The results were interpreted according to the recommendations made by the Clinical and Laboratory Standards Institute (5) or the Food and Drug Administration (for TGC, breakpoints used for the Enterobacteriaceae).
The blaOXA-24-like, blaOXA-23-like, blaOXA-143-like, blaIMP-like, blaVIM-like, blaSIM-like, blaGIM-like, and blaSPM-like genes were not detected in the isolates (8, 11). Two isolates of pulsotype B carried blaOXA-58 genes, which were flanked by IS1006–ΔISAba3-like (upstream) and ISAba3 (downstream) (1). The genetic structure IS1006–ΔISAba3-like–blaOXA-58–ISAba3 has been described for an A. nosocomialis isolate (1). Most of the blaOXA-51-like genes were blaOXA-194 (n = 4), followed by blaOXA-138 (n = 2). The blaOXA-51-like alleles were different from each other in one to three amino acids (Table 2).
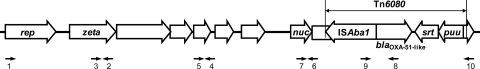
PCR mapping with different primer sets showed that all of the 10 isolates had a similar genetic arrangement around ISAba1--blaOXA-51-like (Fig. 1). This genetic structure has been found in pAbSK-OXA-82, which is a widely disseminated plasmid carrying ISAba1--blaOXA-51-like in A. baumannii in Taiwan (2). Although the isolates had different plasmid patterns, a Southern blot analysis revealed that they had a plasmid of similar size carrying blaOXA-51-like genes. The size of the plasmids was approximately 50 kb, comparable to that of pAbSK-OXA-82 (data not shown).
Fig 1.
Schematic representation of the surrounding structures of plasmid-borne blaOXA-51-like. Genes and their corresponding transcription orientations are indicated by horizontal white arrows. The black horizontal arrows indicate the locations of primers used for PCR mapping. rep, gene encoding a plasmid replicase; zeta, gene encoding a zeta toxin family protein; nuc, gene encoding a thermonuclease protein; srt, gene encoding a sortase; puu, gene encoding a transcriptional regulator. The putative transposon Tn6080 contained ISAba1--blaOXA-51-like srt-puu. Other details of the genetic structures are described under GenBank accession no. GQ352402.
The non-A. baumannii species carrying plasmid-borne ISAba1–blaOXA-51-like genes may have emerged in three ways. First, they may have acquired the plasmids carrying ISAba1-blaOXA-51-like genes from different A. baumannii strains independently. Second, the plasmids may have disseminated among different clones of non-A. baumannii species, since the plasmids were similar in size and had similar genetic structures surrounding the blaOXA-51-like allele. Third, clonal propagation of Acinetobacter may have also participated in the emergence of isolates carrying plasmid-borne ISAba1--blaOXA-51-like genes, especially for isolates 1890, 1892, and 1897. These three Acinetobacter genomic species “Close to 13TU” isolates were isolated in nearby intensive care units (ICUs) (Table 1) in the same period, belonged to clone A, and had similar plasmid patterns.
The plasmids carrying ISAba1-blaOXA-138 (from isolate 1704, susceptible to cefepime) or ISAba1-blaOXA-194 (from isolate 2019, susceptible to kanamycin) were both self-transferable to an A. baumannii 218 isolate (susceptible to carbapenem but resistant to cefepime and kanamycin), as demonstrated in mating-out assays (15), in which the transconjugants were selected on agar plates containing imipenem (4 μg/ml) plus either cefepime (16 μg/ml) or kanamycin (50 μg/ml). The plasmids carrying ISAba1-blaOXA-138 and ISAba1-blaOXA-194 conferred an increase in the imipenem MIC (from 0.5 μg/ml to 64 μg/ml and 8 μg/ml, respectively) to A. baumannii transconjugants, respectively. To determine the contribution of plasmid-borne ISAba1–blaOXA-51-like, without other possible carbapenem resistance determinants in the original plasmids, to carbapenem resistance, a recombinant plasmid carrying ISAba1-blaOXA-138 was constructed and electrotransformed into an A. nosocomialis reference strain, ATCC 17903, using previously described methods (4). The transformants demonstrated an increase in the imipenem MIC (from 0.12 to 32 μg/ml). Taken together, these results indicated that non-A. baumannii species can be a reservoir for the dissemination of the carbapenem resistance determinant, the plasmid-borne ISAba1--blaOXA-51-like.
In conclusion, plasmids carrying ISAba1–blaOXA-51-like emerged in carbapenem-resistant isolates of A. nosocomialis and the Acinetobacter genomic species “Close to 13TU.” The emergence of these plasmids is due primarily to plasmid propagation between different clones of non-A. baumannii species and dissemination of the Acinetobacter clones. The plasmid-borne ISAba1–blaOXA-51-like in non-A. baumannii species not only contributes to a high level of carbapenem resistance but also affects the accuracy of using blaOXA-51-like detection as a tool for differentiating A. baumannii from other Acinetobacter species.
Nucleotide sequence accession numbers.
The nucleotide sequences of blaOXA-194 to blaOXA-197 were assigned accession numbers HQ425492 to HQ425495 in the GenBank database.
ACKNOWLEDGMENTS
The isolates used in this study form part of a collection of the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) Program, 2007. All of the contributors of the isolates are highly appreciated.
This study was supported by grants from the Taipei Veterans General Hospital (V100C-025 and V10E4-005), the National Science Council (NSC98-2314-B-010-010-MY3), and the Yen Tjing Ling Medical Foundation (CI-99-18).
Footnotes
Published ahead of print 14 November 2011
REFERENCES
- 1. Chen TL, et al. 2010. Contribution of a plasmid-borne blaOXA-58 gene with its hybrid promoter provided by IS1006 and an ISAba3-like element to β-lactam resistance in Acinetobacter genomic species 13TU. Antimicrob. Agents Chemother. 54:3107–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen TL, et al. 2010. Emergence and distribution of plasmids bearing the blaOXA-51-like gene with an upstream ISAba1 in carbapenem-resistant Acinetobacter baumannii isolates in Taiwan. Antimicrob. Agents Chemother. 54:4575–4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen TL, et al. 2007. Comparison of one-tube multiplex PCR, automated ribotyping and intergenic spacer (ITS) sequencing for rapid identification of Acinetobacter baumannii. Clin. Microbiol. Infect. 13:801–806 [DOI] [PubMed] [Google Scholar]
- 4. Chen TL, Wu RC, Shaio MF, Fung CP, Cho WL. 2008. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:2573–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document M100-S21 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951 [DOI] [PubMed] [Google Scholar]
- 8. Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H. 2009. OXA-143, a novel carbapenem-hydrolyzing class D beta-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:5035–5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu WS, et al. 2007. An OXA-66/OXA-51-like carbapenemase and possibly an efflux pump are associated with resistance to imipenem in Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:3844–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang LY, et al. 2008. Dissemination of multidrug-resistant, class 1 integron-carrying Acinetobacter baumannii isolates in Taiwan. Clin. Microbiol. Infect. 14:1010–1019 [DOI] [PubMed] [Google Scholar]
- 11. Lee YT, et al. 2009. Differences in phenotypic and genotypic characteristics among imipenem-non-susceptible Acinetobacter isolates belonging to different genomic species in Taiwan. Int. J. Antimicrob. Agents 34:580–584 [DOI] [PubMed] [Google Scholar]
- 12. Lee YT, et al. 2009. First identification of blaOXA-51-like in non-baumannii Acinetobacter spp. J. Chemother. 21:514–520 [DOI] [PubMed] [Google Scholar]
- 13. Nemec A, et al. 2011. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res. Microbiol. 162:393–404 [DOI] [PubMed] [Google Scholar]
- 14. Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poirel L, Guibert M, Bellais S, Naas T, Nordmann P. 1999. Integron- and carbenicillinase-mediated reduced susceptibility to amoxicillin-clavulanic acid in isolates of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 from French patients. Antimicrob. Agents Chemother. 43:1098–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poirel L, Nordmann P. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 12:826–836 [DOI] [PubMed] [Google Scholar]
- 17. Turton JF, et al. 2006. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 44:2974–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]