Abstract
Host defense peptides are naturally occurring molecules that play essential roles in innate immunity to infection. Based on prior structure-function knowledge, we tested two synthetic peptides (RP-1 and AA-RP-1) modeled on the conserved, microbicidal α-helical domain of mammalian CXCL4 platelet kinocidins. These peptides were evaluated for efficacy against Leishmania species, the causative agents of the group of diseases known as leishmaniasis. In vitro antileishmanial activity was assessed against three distinct Leishmania strains by measuring proliferation, metabolic activity and parasite viability after exposure to various concentrations of peptides. We demonstrate that micromolar concentrations of RP-1 and AA-RP-1 caused dose-dependent growth inhibition of Leishmania promastigotes. This antileishmanial activity correlated with rapid membrane disruption, as well as with a loss of mitochondrial transmembrane potential. In addition, RP-1 and AA-RP-1 demonstrated distinct and significant in vivo antileishmanial activities in a mouse model of experimental visceral leishmaniasis after intravenous administration. These results establish efficacy of RP-1 lineage synthetic peptides against Leishmania species in vitro and after intravenous administration in vivo and provide further validation of proof of concept for the development of these and related systemic anti-infective peptides targeting pathogens that are resistant to conventional antibiotics.
INTRODUCTION
Leishmaniasis, a group of vector-borne diseases caused by the protozoan parasite Leishmania, is one of the 13 core tropical diseases that affect the poorest people of the world (17). Infection is associated with at least 50,000 deaths per year and 2.4 million disability-adjusted life years lost (8, 41). There are three basic forms of clinical disease, primarily determined by the infecting species: self-limiting, yet deforming cutaneous leishmaniasis; mucocutaneous leishmaniasis, which destroys mucosal tissue; and visceral leishmaniasis, which can be fatal if left untreated. Leishmania parasites exist in two distinct forms during their life cycle: motile, rod-shaped forms called promastigotes, and nonmotile spherical forms called amastigotes. Promastigotes exist in the midgut of infected sandflies and are inoculated into the mammalian host through the skin during a blood meal. Infective metacyclic promastigotes are phagocytosed by macrophages in the host, where they transform into amastigotes, and subsist intracellularly inside parasitophorous vacuoles to establish infection and evade host immune responses (13). Disease expression and response to treatment are influenced by variables unique to both the infecting species and the human host, and include nutrition, age, and immunogenetic variations influencing immune responses (14, 32). Current treatments include pentavalent antimonials, liposomal amphotericin B, and miltefosine, but all suffer from some combination of high toxicity, prohibitive cost, and/or parasite resistance.
Host defense peptides (HDPs) are naturally occurring, ribosomally derived molecules that contribute to first-line immune defense against invading pathogens (18). A predominant mechanism of HDP action is via interaction with microbial plasma membranes. Peptide structure, amino acid composition, cationic charge, and amphipathicity affect specific interactions with target membranes and mediate antimicrobial activity (48). HDPs can also induce pathogen membrane alteration and destabilization to allow intracellular entry of HDPs and other factors, leading to the collapse of metabolic and bioenergetic pathways and culminating in autophagic, necrotic, or apoptotic cell death (26). Development of microbial resistance to HDPs is considered to be uncommon or less likely than to conventional antibiotics, ostensibly reflecting the significant changes in structure and phospholipid composition that would be necessary to prevent peptide interactions (18). The diversity of HDPs and their targets has been well documented, and many specific peptides have been demonstrated to have both in vitro and in vivo antiprotozoan activity in prior studies (1, 29, 30), including plant HDPs (3), various animal-derived HDPs (4, 9, 15, 22, 26, 33, 34), human salivary HDPs (24), and defensin-, magainin-, and cathelicidin-type HDPs (12, 21, 25).
Platelet microbicidal proteins (PMPs) are an unusual class of HDPs released directly into the bloodstream from activated platelets in response to tissue injury or infection. They have been identified in humans and rabbits and exhibit rapid and potent in vitro microbicidal activity against Gram-positive bacteria, Gram-negative bacteria, and fungi (38, 39, 47–49). In addition to direct antimicrobial activity, certain PMPs have been identified as chemokines (e.g., CXCL4 or platelet factor-4). Such microbicidal chemokines have been termed kinocidins. Kinocidins can initiate the migration of neutrophils, granulocytes, and monocytes to injury sites and interact with other blood-derived molecules to regulate defense processes (11). Their basic structure consists of an N-terminal extended domain containing the chemokine motif, a γ-core structural domain, and a C-terminal α-helical domain, the latter of which is primarily responsible for microbicidal activity.
We previously designed a synthetic peptide, called RP-1, modeled in part on α-helical domains of mammalian CXCL4 kinocidins (46). RP-1 and congener peptides such as AA-RP-1 exert potent microbicidal efficacy in complex blood and blood-derived matrices, with minimal cytotoxicity against erythrocytes and endothelial cells (5, 46). We describe here the antiprotozoan activity of RP-1 and its analogue AA-RP-1 evaluated for specific leishmanicidal activity, mechanisms of action, and efficacy in a mouse model of experimental visceral leishmaniasis. To our knowledge, this is the first report of any synthetic kinocidin or PMP derivative molecule with direct antimicrobial activity against Leishmania spp. The results presented here validate the antileishmanial efficacy of RP-1 lineage peptides after intravenous administration in vivo and support the development of these and related systemic anti-infective peptides targeting pathogens resistant to traditional antibiotics.
MATERIALS AND METHODS
Parasites.
Three species of Leishmania were evaluated in the present study: Leishmania major, Leishmania braziliensis, and Leishmania chagasi, now thought to be the same species as Leishmania infantum (27) and referred to here as L. infantum chagasi. Luciferase-expressing L. major promastigotes (a gift from Stephen Beverley, Washington University, St. Louis, MO) were maintained at 26°C in medium 199 (powder; Sigma) supplemented with 1 M HEPES (pH 7.4), 10 mM adenine, 0.1% biotin, 0.25% hemin, 1% penicillin-streptomycin, 10% fetal bovine serum, and 0.25 mg of biopterin/ml. For all infection experiments, stationary-phase L. major promastigotes grown for 5 days in culture were used. Virulent luciferase-expressing L. infantum chagasi promastigotes (strain MHOM/BR/00/1669; a gift from Mary Wilson, University of Iowa) were maintained by successive passage in golden hamsters. Isolated parasites were cultured at 26°C for 7 days to reach stationary phase in hemoflagellate-modified minimal essential medium supplemented with 10 mg of VAT antibiotic/ml, sodium pyruvate, glucose, sodium bicarbonate, sodium hydroxide, para-amino benzoic acid, 1 M HEPES, 10% fetal bovine serum, hemin, folic acid, adenosine, biopterin, and biotin. L. (Viannia) braziliensis parasites (isolated from a cutaneous leishmaniasis patient from Rio de Janeiro, Brazil) were maintained by successive passage in golden hamsters. Isolated parasites were cultured at 26°C for 5 days to obtain stationary-phase parasites in Schneider's medium (Gibco) supplemented with 20% fetal bovine serum, 2% human urine, and 0.1% penicillin-streptomycin.
Synthetic host defense peptides.
The synthetic host defense peptide RP-1 is an 18-amino-acid peptide (N-ALYKK5-FKKKL10-LKSLK15-RLG18-C; mass, 2,163 Da) modeled in part upon α-helical C-terminal microbicidal domains of mammalian CXCL4 kinocidins (5). AA-RP-1 is an anthryl-alanine-substituted (position 2) congener of the parent peptide RP-1. Peptides were synthesized with a Symphony multiplex synthesizer (Rainin, Woburn, MA) and authenticated by mass spectroscopy and amino acid analysis (5, 46). Purified peptides were lyophilized and resuspended in sterile phosphate-buffered saline (PBS) for use in the assays. A “scrambled” peptide of equivalent mass and sequence length but different composition was used as a negative control for preliminary experiments. Peptide stocks were prepared at a concentration of 1 mg/ml and stored at 20°C.
In vitro Leishmania quantitation assays. (i) Direct enumeration.
L. braziliensis promastigotes were incubated at 26°C in the presence of various doses of 10 to 200 μg of AA-RP-1 and RP-1/ml or 100 μg of scrambled peptide/ml as a control. After 72 h, viable parasite counts were determined using a hemocytometer following trypan blue dye exclusion.
(ii) Luciferase assay.
Logarithmic-phase L. major and L. infantum chagasi promastigotes were washed in PBS, resuspended in their respective media to a final concentration of 2 × 105/ml, and plated in 96-well flat-bottom plates. Triplicate wells were treated at 26°C with RP-1 and AA-RP-1 at concentrations ranging from 1 to 50 μg/ml or at 100 μg of scrambled peptide/ml. There were also untreated control wells. Aliquots of 50 μl from each well were collected each day and assessed for luciferase activity as described in the manufacturer's protocol (Promega). Luminescence was read using a Synergy2 Multimode Microplate Reader (Biotek, Winooski, VT) and recorded in relative light units (RLU).
(iii) MTT viability assay.
Late-logarithmic-phase L. major promastigotes were resuspended in modified, phenol red-free Dulbecco modified Eagle medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum, 400 mM l-glutamine, and 5% penicillin-streptomycin (10). Parasites were seeded in sterile culture tubes and exposed to increasing concentrations of RP-1 and AA-RP-1 (10 to 100 μg/ml). The tubes were incubated at 26°C for 4 h, centrifuged at 3,500 rpm for 15 min, resuspended in 100 μl of fresh medium, and treated with 10 μl of 5 mg of MTT (Molecular Probes, CA)/ml according to the manufacturer's protocol. Absorbance was read at 540 nm using a Synergy2 Multimode microplate reader to determine the amount of formazan production, which correlates with relative cell viability.
Microscopy.
Logarithmic-phase L. infantum chagasi promastigotes were exposed to 100 μg of RP-1 or AA-RP-1/ml or PBS. At various times postexposure, 10 μl of each sample was air dried on a glass slide, fixed with methanol, and stained with Giemsa.
Annexin V/PI staining.
The ApoScreen annexin V apoptosis kit (Southern Biotech, AL) is an assay optimized to determine the frequency of apoptotic cells. Exponential-phase L. major promastigotes were incubated at 37°C humidified air with peptides for different time intervals. After each time point, the parasites were washed twice, centrifuged (3,500 rpm for 15 min), and then resuspended in binding buffer. Cells were labeled with annexin V-fluorescein isothiocyanate (FITC) and/or propidium iodide (PI) according to the manufacturer's protocol. Flow cytometry analysis was performed using a LSRII flow cytometer (BD Bioscience, San Jose, CA) using single laser excitation at 488 nm. The data were analyzed by using FCS Express V3 software (De Novo Software, Los Angeles, CA).
Mitochondrial membrane potential.
To detect changes in the mitochondrial transmembrane potential (ΔΨm), a MitoPT JC-1 mitochondrial permeability transition detection kit (ImmunoChemistry Technologies, MN) was used. JC-1 (5,5′,6,6′-tetracholor-1,1′,3,3′-tetraethylbenzamidazolocarbocyanin iodide) is a lipophilic dye that forms aggregates inside healthy cells at high concentrations and emits red fluorescence at 585 nm. When mitochondrial membrane potential collapses, JC-1 de-aggregates, disperses throughout the cell, and loses red fluorescence. Cells were labeled with JC-1, incubated for 1 h at 37°C, and washed according to the manufacturer's protocol. The data were acquired using a LSRII flow cytometer and was analyzed using FCS Express V3 software.
In vivo therapeutic antileishmanial activity.
Female BALB/c mice, 6 to 8 weeks old, were purchased from the Jackson Laboratory (Sacramento, CA) and maintained in a regulated animal care facility. All studies were done with the approval of the Los Angeles Biomedical Research Institute Institutional Animal Care and Use Committee. Three groups of 10 age-matched mice were inoculated intravenously in the tail vein with 5 × 107 stationary-phase L. infantum chagasi promastigotes. After 7 days, each group was treated every other day with synthetic peptide RP-1 or AA-RP-1 or with PBS. Mice received 0.25 mg of peptide in sterile PBS, which was equivalent to ∼12.5 mg/kg per mouse. A total of seven doses were administered to each mouse. On day 20, all mice were euthanized. Whole spleens and livers were removed and homogenized, and genomic DNA was isolated using an UltraClean tissue DNA isolation kit (MoBio Laboratories Inc., Carlsbad, CA). Parasitemia was determined by quantitative PCR using a 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA) by measuring levels of the Leishmania gene GP63. GP63 cycle threshold (CT) values were converted to absolute parasite counts by using previously determined standard curves and were normalized to the amount of tissue DNA in each sample as determined by RPLP0 (single-copy mouse gene) CT values, as previously described (6). The data were analyzed using SDS v2.4 software.
Statistical analysis.
Results and graphs are presented as means ± the standard deviations (SD) or means ± the standard error of the mean (SEM), as indicated. Differences among control and peptide-treated samples were analyzed with an unpaired Student t test using GraphPad Prism v5 (San Diego, CA). P values of <0.05 were considered significant.
RESULTS
Inhibition of in vitro growth of Leishmania by synthetic peptides.
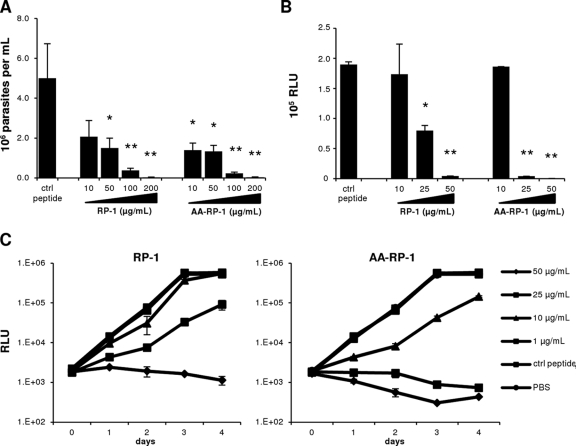
Synthetic PMP-based peptides such as RP-1 and analogues have previously been shown to have antimicrobial effects against bacteria and fungi in vitro, ex vivo, and in vivo (5, 46). We investigated the effects of RP-1 and AA-RP-1 on the in vitro growth of three different species of Leishmania. L. major is mainly associated with cutaneous leishmaniasis in the Old World, and L. braziliensis and L. infantum chagasi are representative New World strains that cause predominantly mucocutaneous and visceral disease, respectively (16). We cultured promastigotes at 26°C in the absence or presence of various concentrations of synthetic peptides in the growth media. Both RP-1 and AA-RP-1 caused a dose-dependent decrease in the number of L. braziliensis parasites after 72-h exposure, with significant inhibition of parasite growth at 10 μg/ml for AARP-1 and at 50 μg/ml for RP-1 and maximal inhibition at 200 μg/ml for both peptides (Fig. 1A). We used transgenic, luciferase-expressing strains of L. infantum chagasi and L. major to assess the sensitivity of these species to the study peptides. Exposure of L. infantum chagasi parasites to increasing concentrations of RP-1 and AA-RP-1 resulted in a dose-dependent decrease of luciferase activity after 72 h of culture (Fig. 1B). Luciferase expression of L. major parasites similarly declined over the course of 4 days of culture. Both RP-1 and AA-RP-1 (Fig. 1C) exhibited dose-dependent effects compared to a scrambled control peptide, which was equivalent to PBS treatment. AA-RP-1 consistently exhibited a more pronounced decrease in measured luciferase activity of L. major compared to RP-1 at doses of 10 μg/ml and higher.
Fig 1.
RP-1 and AA-RP-1 inhibit the growth of promastigotes of Leishmania species in vitro. (A) L. braziliensis promastigotes were cultured in media with various concentrations of peptide (or scrambled peptide as a control) as indicated, and the numbers of viable parasites were determined after 72 h. Bars represent the means ± the SD of triplicate wells. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01. (B) Luciferase-expressing L. infantum chagasi promastigotes were cultured in media with various concentrations of peptide (or scrambled peptide as a control) as indicated. Luciferase activity (indicated in relative light units [RLU]) was measured after 72 h. Bars represent the mean ± the SD of duplicate samples. ∗, P ≤ 0.005; ∗∗, P ≤ 0.0005. (C) Luciferase-expressing L. major promastigotes were cultured in media with various concentrations of peptide (or 100 μg of scrambled control peptide/ml or PBS) as indicated, and the luciferase activity (in RLU) was measured at various time points. Bars represent the means ± the SD of duplicate samples. All experiments were performed at least twice with similar results.
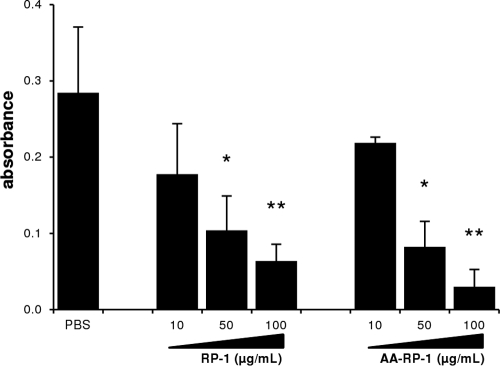
To determine the effect of peptides on Leishmania promastigote metabolic activity, we used the MTT assay to assess the number of metabolically active cells in culture (31). These assays demonstrated a concentration-dependent effect of both RP-1 and AA-RP-1 on L. major viability at similar doses (Fig. 2). Similar dose-response experiments with longer incubation times (24 and 48 h) showed similar results (data not shown).
Fig 2.
RP-1 and AA-RP-1 inhibit metabolic activity of Leishmania promastigotes in vitro. L. major promastigotes were cultured in media with various concentrations of peptide (or PBS) as indicated. MTT assays were performed after 4 h to determine metabolic activity. Absorbance readings representing formazan production are shown. Bars represent the mean ± the SD of triplicate samples. Experiment shown is representative of at least three independent experiments. ∗, P ≤ 0.005; ∗∗, P ≤ 0.0005.
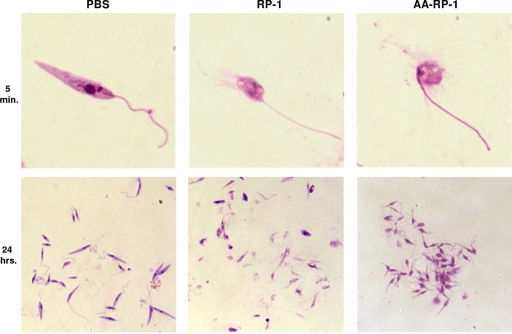
Peptide-induced morphological changes of promastigotes.
To visualize the effects of RP-1 and AA-RP-1 on Leishmania promastigotes, we used direct microscopy. Treatment of promastigotes with these peptides led to immediate and profound morphological changes. Giemsa-staining of healthy, live L. infantum chagasi promastigotes normally reveals thin, elongated cells with a unipolar flagellum, and a clearly visible nucleus and kinetoplast. As early as 5 min after exposure to either peptide, a majority of promastigotes exhibited rounding and swelling of cell bodies, with clear disruption of the cellular membrane accompanied by a release of cytoplasmic contents into the culture medium. (Fig. 3). After 24 h of exposure, nearly 100% of the RP-1-treated promastigotes had lost their elongated form and appeared in various states of disintegration, with high levels of amorphous staining that was likely released cellular material (Fig. 3, lower middle panel). AA-RP-1-treated promastigote cultures, however, consistently had less background staining but displayed clumping patterns in which many parasites clustered together (Fig. 3, lower right panel). Therefore, both RP-1 and AA-RP-1 induced rapid, obvious morphological changes in promastigotes but also exhibited discernible differences in their effects on target organisms in vitro.
Fig 3.
RP-1 and AA-RP-1 exposure causes morphological changes in Leishmania promastigotes. Untreated or peptide-treated L. infantum chagasi promastigotes were Giemsa stained after 5 min or 24 h exposure to 100 μg of peptide/ml. The magnification is ×1,000 for the top row and ×400 for the bottom row.
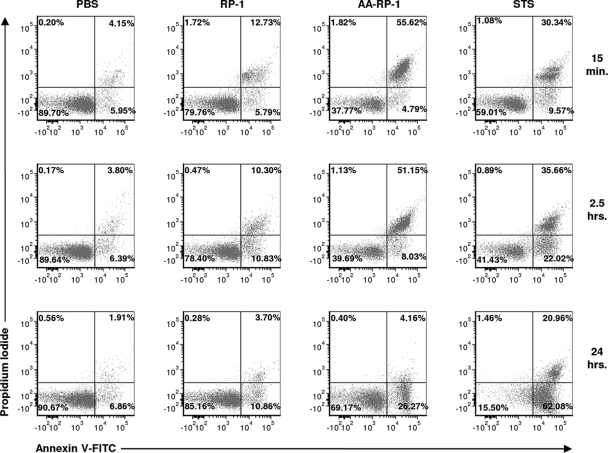
Membrane perturbation induced by peptide treatment.
Unicellular protozoa can undergo phenotypic changes similar to those observed during mammalian cell apoptosis (programmed cell death), including phosphatidylserine (PS) exposure and mitochondrial membrane depolarization (2, 7, 35, 36, 50). We utilized several biochemical approaches to further characterize the changes induced by study peptides in Leishmania. The externalization of PS from the inner side to the outer layer of the membrane is a key early step that signals the initiation of apoptosis (7). We costained peptide-treated L. major promastigotes with annexin V-FITC (a Ca2+-dependent phospholipid-binding protein with affinity for PS), and PI. PI permeates cells and binds to cellular DNA only if a loss of plasma membrane integrity occurs, such as during necrosis or late-stage apoptosis. Staurosporine (STS) is a protein kinase inhibitor that induces an apoptosis-like programmed death in L. major (2, 35) and was used as a control reference. Similar staining patterns indicating immediate loss of plasma membrane integrity were observed after only 1 min of either RP-1 or AA-RP-1 exposure (data not shown). However, much higher levels of annexin V+ and PI+ staining (upper right quadrant) were evident following 15 min of exposure to AA-RP-1 compared to either RP-1 or staurosporine (Fig. 4). After 24 h, the number of parasites staining positive for annexin V but negative for PI (lower right quadrant) was significantly increased compared to control after treatment with either AA-RP-1 or STS, but much less so following RP-1 treatment. These various levels of annexin V+ PI− cells, which are more typical of early-stage apoptosis, demonstrate that RP-1 and AA-RP-1 have distinct and qualitatively discernible effects on L. major promastigotes in terms of membrane permeability and PS accessibility.
Fig 4.
RP-1 and AA-RP-1 exposure leads to increased membrane permeability and phosphatidylserine exposure. L. major promastigotes were cultured in medium with or without 100 μg of RP-1 or AA-RP-1/ml or 1 μg of staurosporine (STS)/ml for the various times indicated. Cells were then stained with annexin V-FITC and propidium iodide and analyzed by flow cytometry. The results are representative of at least three independent experiments.
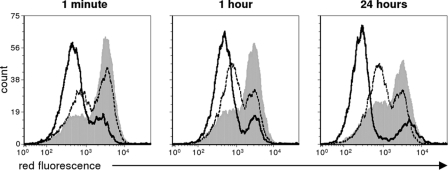
We next characterized the effects of study peptides on promastigotes by measuring the loss of mitochondrial membrane potential (ΔΨm). In many models of apoptosis, a collapse in the electrochemical gradient across the mitochondrial membrane is a key indicator of the initiation of programmed cell death (7, 36, 40, 50). JC-1 is a unique fluorescent cationic dye that fluoresces red upon aggregation within intact, negatively charged mitochondria. Once the transmembrane potential collapses, the dye disperses throughout the cell and loses its red fluorescence. As with membrane perturbation, exposure of JC-1-stained L. major promastigotes to either RP-1 or AA-RP-1 for as little as 1 min led to a decrease in red fluorescence intensity compared to untreated promastigotes (Fig. 5). This finding suggests that RP-1 and AA-RP-1, to various degrees, both induce a rapid loss of membrane electrochemical gradient in the mitochondria. However, this shift was more pronounced in AA-RP-1-treated promastigotes compared to RP-1-treated promastigotes for all of the time points analyzed.
Fig 5.
RP-1 and AA-RP-1 exposure leads to depolarization of the mitochondrial membrane. L. major promastigotes were cultured in media with 100 μg of RP-1 (dotted line), AA-RP-1 (solid line), or dimethyl sulfoxide/ml as a negative control (gray shaded) for the various times indicated. Cells were stained with JC-1 dye (which emits red fluorescence when aggregated in intact, polarized mitochondria) and analyzed by flow cytometry. The results are representative of two independent experiments.
Antileishmanial activity of RP-1 and AA-RP-1 in vivo.
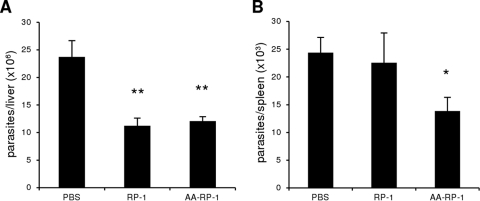
Ultimately, any therapy targeting Leishmania spp. must be effective against the parasite in live mammals without causing significant toxicity. For visceral leishmaniasis, the therapy must be delivered systemically to reach parasites in the bloodstream, liver, bone marrow, and spleen. Thus, we evaluated the ability of RP-1 and AA-RP-1 to alter parasite burden in established leishmaniasis in vivo. BALB/c mice were injected intravenously with stationary-phase L. infantum chagasi promastigotes to establish systemic infections. Experimental visceral leishmaniasis in BALB/c mice results in asymptomatic infection, most closely modeling the subclinical form of human disease. Parasite loads in the liver and spleen are commonly used as correlates of protection (42). Starting 1 week after challenge, mice were administered RP-1 or AA-RP-1 by intravenous injections every other day for a total of seven doses. After 2 weeks of treatment, the spleens and livers were harvested to determine the parasite loads in each organ. We observed significant hepatomegaly and splenomegaly upon organ dissection as expected, but we detected no significant differences in liver and spleen weights among the different groups (data not shown). Mice treated with either RP-1 or AA-RP-1 had significantly fewer parasites in the liver compared to control mice treated with PBS (Fig. 6A). In the spleen, where mice typically harbor a lower parasite load than in the liver after 3 weeks of infection, treatment with AA-RP-1, but not RP-1, led to fewer numbers of parasites (Fig. 6B). These results demonstrate that both RP-1 and AA-RP-1 can lead to a significant reduction of parasite load in an in vivo model of visceral disease. These findings also suggest the anthryl-alanine substitution in the parental RP-1 amino acid sequence is associated with enhanced antileishmanial activity in vivo.
Fig 6.
RP-1 and AA-RP-1 display therapeutic efficacy against established visceral leishmaniasis in mice. BALB/c mice infected with L. infantum chagasi for 1 week were therapeutically administered sterile PBS, RP-1, or AA-RP-1 for an additional 2 weeks, every other day. Total DNA was then isolated from livers (A) and spleens (B), and quantitative PCR was performed to determine the amount of parasites/organ. Bars represent the means ± the SEM of 10 mice/group. ∗, P = 0.01; ∗∗, P < 0.005. The results are representative of two independent experiments.
DISCUSSION
In this study we demonstrate the intrinsic antileishmanial activity of two synthetic peptides based in part on the α-helical domain of CXCL4 family platelet kinocidins. These peptides were designed to recapitulate the antimicrobial properties of this class of mammalian host defense protein. The addition of either of these synthetic peptides to in vitro promastigote cultures led to dose-dependent inhibition of three distinct species of Leishmania (L. braziliensis, L. infantum chagasi, and L. major), each of which is generally associated with a different manifestation of disease in humans. We observed small but significant differences in the efficacy of the two peptides, as AA-RP-1 consistently demonstrated growth inhibition at slightly lower concentrations than RP-1. In addition to growth patterns, we quantified metabolic activity of peptide-treated parasites, using both direct and indirect methods. Inhibitory effects of the peptides correlated with changes induced in promastigotes at the membrane level, including increased permeability, phosphatidylserine externalization, and the collapse of mitochondrial membrane potential. These studies revealed specific differences between RP-1- and AA-RP-1-induced effects, suggesting that the two molecules have distinct impacts on target promastigote structures and functions. In addition, in vivo experiments demonstrated significant therapeutic efficacy of both RP-1 and AA-RP-1 against experimental visceral leishmaniasis in mice, with only AA-RP-1 treatment significantly impacting the small numbers of parasites measured in the spleen after 3 weeks of infection. The single amino acid difference (at position 2) between AA-RP-1 (anthryl-alanine) and RP-1 (alanine) thus correlated with subtle differences in antileishmanial activities, both in vitro and in vivo; however, the structure/function basis for these differences is not currently understood.
In general, PMPs, kinocidins, and derivatives thereof—specifically RP-1—have been shown to have minimal toxicity to mammalian cells (48). Even so, our data suggest that the eukaryotic membranes of Leishmania promastigotes are susceptible to these peptides. The surfaces of promastigotes of all Leishmania species are characterized by a thick anionic glycocalyx made up of glycoconjugates and glycosylphosphatidylinositol-anchored proteins, the most predominant being lipophosphoglycan. Another major molecule on the promastigote surface is a metalloproteinase called GP63, or leishmanolysin, that may be protective against naturally occurring HDPs by proteolytic degradation (28). Leishmanolysin knockout mutants of L. major were more susceptible to various alpha- and theta-defensins, magainins, and cathelicidins and underwent pexiganan-mediated apoptosis more readily than wild-type parasites (20). Leishmanolysin is known to be expressed by all pathogenic Leishmania species (44), including the strains in our study, which suggests that kinocidin-derived peptides maintain antileishmanial activity even in the presence of leishmanolysin on the surface. There are, however, at least three different known isoforms of leishmanolysin expressed at different stages of parasite development and at various expression levels (37, 45). Further characterization of the interaction between leishmanolysin and other parasite peptidases and this class of host defense peptides will be interesting to pursue in future studies.
Many studies demonstrating the in vitro activity of HDPs against Leishmania species have focused on molecules from species that are not natural hosts of the parasite, such as plants, frogs, aquatic animals, and certain insects (23, 28, 30). The synthetic peptides in the present study were engineered to recapitulate certain properties of mammalian kinocidins, including enhanced antimicrobial activity in physiologically relevant settings, such as blood (46). RP-1 has previously demonstrated amplified activity against E. coli within human blood, plasma, and serum biomatrices, compared to artificial media (43, 46). RP-1 also has staphylocidal activity characterized by membrane permeabilization and inhibition of DNA and RNA synthesis (43). Although we did not examine the leishmanicidal activities of RP-1 and AA-RP-1 in blood matrices ex vivo, each peptide had significant efficacy against established visceral infections, suggesting that intracellular parasites in their amastigote form are susceptible to peptide action in vivo. These in vivo effects on organ parasite load may reflect significant antileishmanial properties of these peptides distinct from their in vitro properties, since established visceral infections are composed predominantly of intracellular amastigotes, as opposed to extracellular promastigotes. It is possible, based on prior observations, that peptide efficacy in physiologic settings might be influenced and/or amplified by blood components that potentiate antileishmanial effects. Such an outcome could contribute to synergy with other immune mechanisms such as phagocytosis, other endogenous HDPs or free radicals (34). Further studies will help to define whether the mechanisms of peptide-mediated antipromastigote activity are relevant to the in vivo mechanisms that reduce amastigote burdens.
The binding of annexin V to exposed phosphatidylserine is a tool commonly used to identify mammalian cells undergoing apoptosis. Both staurosporine (which induces apoptosis-like cell death in protozoans) (35) and AA-RP-1 rapidly induced a large subpopulation of promastigotes that stain positively for annexin V. Initially (15 min to 2.5 h after treatment), these promastigotes also stained positive for PI, signifying that their membranes had been permeabilized to allow DNA staining. Interestingly, by 24 h the majority of annexin V+ cells stained negative for PI, thus resembling a more typical “early apoptotic” stage in which PS residues are exposed, and the plasma membrane is not permeabilized. Whether these annexin V+ PI− cells initially stained positively for PI, or whether they arose from the double-negative population, is unknown. In contrast to AA-RP-1, treatment with RP-1 led to a relatively small population of annexin V-staining promastigotes compared to PBS controls. This dichotomy suggests a qualitative difference in the mechanisms by which each peptide acts on promastigotes, a difference that was also apparent using mitochondrial membrane potential (ΔΨm) as a readout of peptide-induced changes. In such experiments herein, we also observed an increased activity of AA-RP-1 compared to RP-1 with regard to the levels of ΔΨm. This mechanistic difference caused by the substitution of an anthryl-alanine single amino acid is currently being explored.
In summary, the present findings establish that RP-1 and AA-RP-1 have significant antileishmanial effects against three different Leishmania species. Both peptides triggered immediate and rapid effects in vitro, manifesting changes in membrane permeability and phospholipid orientation, mitochondrial membrane depolarization, and parasite morphology. These observations suggest a complex structural basis behind the antileishmanial effects of these peptides. In addition, the ability of RP-1 and AA-RP-1 to reduce established visceral Leishmania infection in vivo is suggestive of mechanisms beyond direct microbicidal activity against promastigotes. Future experiments will address the effects of these peptides on infected macrophages and dissect the basis for the in vivo reduction in parasite number that we report here. Ultimately, effective treatment of leishmaniasis in human patients may involve not only leishmanicidal activity but also modulation of the host immune system. The abilities of such synthetic peptides to act directly against target organisms and potentiate endogenous immune mechanisms, such as host cell migration and the control of pro- and anti-inflammatory cytokine release, may reflect the diverse roles that endogenous antimicrobial peptides play in host defense (19). The mechanisms by which peptides, both endogenous and engineered, can reduce established Leishmania burdens will be important to understand as we develop translatable novel therapies for this important class of neglected tropical diseases.
ACKNOWLEDGMENTS
This study was supported in part by grants R01-AI-39001 and R01-AI-48031 (M.R.Y.) from the National Institutes of Health (NIAID).
Footnotes
Published ahead of print 28 November 2011
REFERENCES
- 1. Alberola J, et al. 2004. Safety and efficacy of antimicrobial peptides against naturally acquired leishmaniasis. Antimicrob. Agents Chemother. 48: 641–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnoult D, et al. 2002. On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ. 9: 65–81 [DOI] [PubMed] [Google Scholar]
- 3. Berrocal-Lobo M, Molina A, Rodriguez-Palenzuela P, Garcia-Olmedo F, Rivas L. 2009. Leishmania donovani: thionins, plant antimicrobial peptides with leishmanicidal activity. Exp. Parasitol. 122: 247–249 [DOI] [PubMed] [Google Scholar]
- 4. Borges A, et al. 2006. In vitro leishmanicidal activity of Tityus discrepans scorpion venom. Parasitol. Res. 99: 167–173 [DOI] [PubMed] [Google Scholar]
- 5. Bourbigot S, et al. 2009. Antimicrobial peptide RP-1 structure and interactions with anionic versus zwitterionic micelles. Biopolymers 91: 1–13 [DOI] [PubMed] [Google Scholar]
- 6. Bruhn KW, et al. 2010. LXR deficiency confers increased protection against visceral Leishmania infection in mice. PLoS Negl. Trop. Dis. 4: e886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Debrabant A, Lee N, Bertholet S, Duncan R, Nakhasi HL. 2003. Programmed cell death in trypanosomatids and other unicellular organisms. Int. J. Parasitol. 33: 257–267 [DOI] [PubMed] [Google Scholar]
- 8. Desjeux P. 2004. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27: 305–318 [DOI] [PubMed] [Google Scholar]
- 9. Diaz-Achirica P, Ubach J, Guinea A, Andreu D, Rivas L. 1998. The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem. J. 330(Pt 1): 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dutta A, Bandyopadhyay S, Mandal C, Chatterjee M. 2005. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol. Int. 54: 119–122 [DOI] [PubMed] [Google Scholar]
- 11. Flad HD, Brandt E. 2010. Platelet-derived chemokines: pathophysiology and therapeutic aspects. Cell Mol. Life Sci. 67: 2363–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haines LR, et al. 2009. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Negl. Trop. Dis. 3: e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Handman E, Bullen DV. 2002. Interaction of Leishmania with the host macrophage. Trends Parasitol. 18: 332–334 [DOI] [PubMed] [Google Scholar]
- 14. Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis: evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169: 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hernandez C, et al. 1992. Functional and structural damage in Leishmania mexicana exposed to the cationic peptide dermaseptin. Eur. J. Cell Biol. 59: 414–424 [PubMed] [Google Scholar]
- 16. Herwaldt B. L. 1999. Leishmaniasis. Lancet 354: 1191–1199 [DOI] [PubMed] [Google Scholar]
- 17. Hotez PJ, Fenwick A, Savioli L, Molyneux DH. 2009. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 373: 1570–1575 [DOI] [PubMed] [Google Scholar]
- 18. Jenssen H, Hamill P, Hancock RE. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19: 491–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kulkarni MM, et al. 2011. Mammalian antimicrobial peptide influences control of cutaneous Leishmania infection. Cell Microbiol. 13: 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kulkarni MM, et al. 2006. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 62: 1484–1497 [DOI] [PubMed] [Google Scholar]
- 21. Kulkarni MM, McMaster WR, Kamysz W, McGwire BS. 2009. Antimicrobial peptide-induced apoptotic death of Leishmania results from calcium-dependent, caspase-independent mitochondrial toxicity. J. Biol. Chem. 284: 15496–15504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lofgren SE, Miletti LC, Steindel M, Bachere E, Barracco MA. 2008. Trypanocidal and leishmanicidal activities of different antimicrobial peptides (AMPs) isolated from aquatic animals. Exp. Parasitol. 118: 197–202 [DOI] [PubMed] [Google Scholar]
- 23. Luque-Ortega JR, Rivas L. 2010. Characterization of the leishmanicidal activity of antimicrobial peptides. Methods Mol. Biol. 618: 393–420 [DOI] [PubMed] [Google Scholar]
- 24. Luque-Ortega JR, van't Hof Veerman WEC, Saugar JM, Rivas L. 2008. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 22: 1817–1828 [DOI] [PubMed] [Google Scholar]
- 25. Lynn MA, et al. 2011. Effect of BMAP-28 antimicrobial peptides on Leishmania major promastigote and amastigote growth: role of leishmanolysin in parasite survival. PLoS Negl. Trop. Dis. 5: e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mangoni ML, et al. 2005. Temporins, small antimicrobial peptides with leishmanicidal activity. J. Biol. Chem. 280: 984–990 [DOI] [PubMed] [Google Scholar]
- 27. Mauricio IL, Stothard JR, Miles MA. 2000. The strange case of Leishmania chagasi. Parasitol. Today 16: 188–189 [DOI] [PubMed] [Google Scholar]
- 28. McGwire BS, Kulkarni MM. 2010. Interactions of antimicrobial peptides with Leishmania and trypanosomes and their functional role in host parasitism. Exp. Parasitol. 126: 397–405 [DOI] [PubMed] [Google Scholar]
- 29. McGwire BS, Olson CL, Tack BF, Engman DM. 2003. Killing of African trypanosomes by antimicrobial peptides. J. Infect. Dis. 188: 146–152 [DOI] [PubMed] [Google Scholar]
- 30. Mor A. 2009. Multifunctional host defense peptides: antiparasitic activities. FEBS J. 276: 6474–6482 [DOI] [PubMed] [Google Scholar]
- 31. Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65: 55–63 [DOI] [PubMed] [Google Scholar]
- 32. Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366: 1561–1577 [DOI] [PubMed] [Google Scholar]
- 33. Perez-Cordero JJ, Lozano JM, Cortes J, Delgado G. 2011. Leishmanicidal activity of synthetic antimicrobial peptides in an infection model with human dendritic cells. Peptides 32: 683–690 [DOI] [PubMed] [Google Scholar]
- 34. Roch P, Beschin A, Bernard E. 2004. Antiprotozoan and antiviral activities of non-cytotoxic truncated and variant analogues of mussel defensin. Evidence Based Complement Alternat. Med. 1: 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaha C. 2006. Apoptosis in Leishmania species and its relevance to disease pathogenesis. Indian J. Med. Res. 123: 233–244 [PubMed] [Google Scholar]
- 36. Soeiro MN, Souza EM. 2008. Programmed cell death and trypanosomatids: a brief review, p 24–38 In Martin JP. (ed), Programmed cell death in protozoa. Landes Bioscience/Springer Science/Business Media, Rio de Janeiro, Brazil [Google Scholar]
- 37. Streit JA, Donelson JE, Agey MW, Wilson ME. 1996. Developmental changes in the expression of Leishmania chagasi gp63 and heat shock protein in a human macrophage cell line. Infect. Immun. 64: 1810–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang YQ, Yeaman MR, Selsted ME. 2002. Antimicrobial peptides from human platelets. Infect. Immun. 70: 6524–6533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trier DA, et al. 2008. Platelet antistaphylococcal responses occur through P2X1 and P2Y12 receptor-induced activation and kinocidin release. Infect. Immun. 76: 5706–5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wanderley JL, Barcinski MA. 2010. Apoptosis and apoptotic mimicry: the Leishmania connection. Cell Mol. Life Sci. 67: 1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. WHO 2010. WHO technical report series: control of the leishmaniases. Document 949 World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 42. Wilson ME, Jeronimo SM, Pearson RD. 2005. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb. Pathog. 38: 147–160 [DOI] [PubMed] [Google Scholar]
- 43. Xiong YQ, Bayer AS, Elazegui L, Yeaman MR. 2006. A synthetic congener modeled on a microbicidal domain of thrombin-induced platelet microbicidal protein 1 recapitulates staphylocidal mechanisms of the native molecule. Antimicrob. Agents Chemother. 50: 3786–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao C, Donelson JE, Wilson ME. 2003. The major surface protease (MSP or GP63) of Leishmania sp. biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 132: 1–16 [DOI] [PubMed] [Google Scholar]
- 45. Yao C, Luo J, Storlie P, Donelson JE, Wilson ME. 2004. Multiple products of the Leishmania chagasi major surface protease (MSP or GP63) gene family. Mol. Biochem. Parasitol. 135: 171–183 [DOI] [PubMed] [Google Scholar]
- 46. Yeaman MR, Gank KD, Bayer AS, Brass EP. 2002. Synthetic peptides that exert antimicrobial activities in whole blood and blood-derived matrices. Antimicrob. Agents Chemother. 46: 3883–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeaman MR, Norman DC, Bayer AS. 1992. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob. Agents Chemother. 36: 1665–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yeaman MR, Yount NY. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55: 27–55 [DOI] [PubMed] [Google Scholar]
- 49. Yount NY, et al. 2004. Platelet microbicidal protein 1: structural themes of a multifunctional antimicrobial peptide. Antimicrob. Agents Chemother. 48: 4395–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zangger H, Mottram JC, Fasel N. 2002. Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death. Differ. 9: 1126–1139 [DOI] [PubMed] [Google Scholar]